Abstract
Ligand activation of Notch receptors leads to release of the intracellular receptor domain (Notch IC), which translocates to the nucleus and interacts with the DNA-binding protein RBP-Jκ to control expression of specific target genes. A number of proteins have been shown to interact with Notch ICs and to modulate target gene activation, but the precise function of and interplay between these factors is not known. This report investigates the Notch IC-interacting proteins, p300, PCAF, and Mastermind-like 1 (MAML1), in an in vitro transcription system with purified factors and naked DNA or chromatin templates. MAML1, RBP-Jκ, and Notch IC are all required for optimal transcription from DNA, whereas transcription from chromatin requires, in addition, p300, which interacts with MAML1. The transcriptional activity of p300 requires acetyl coenzyme A, indicating that it functions as a histone acetyltransferase when mediating Notch IC function. PCAF is unable to promote transcription on its own but enhances Notch IC-mediated transcription from chromatin in conjunction with p300. These data define a critical role for p300 in the potentiation of Notch IC function by MAML1 and PCAF, provide the first evidence for cooperativity between PCAF and p300 in Notch IC function, and also indicate direct effects of RBP-Jκ, Notch IC, and MAML1 on the general transcription machinery.
The Notch signaling pathway is an evolutionarily conserved system for cell-cell communication, and exists in most, if not all, multicellular organisms (reviewed in reference 3). Vertebrates contain four Notch receptors (Notch 1 to 4) and Notch signaling controls cell fate decisions in many cell types that include those of the nervous system, muscle, pancreas, and the hematopoietic system (reviewed in reference 24). The Notch receptor is a single transmembrane-spanning protein that undergoes a set of complex proteolytic processing events. The first cleavage occurs at the prospective extracellular side during intracellular trafficking in the Golgi complex and results in a bipartite protein, composed of most of the extracellular domain linked to the transmembrane and intracellular portions. Interaction with Delta or Serrate ligands on juxtaposed cells results in a second cleavage at the extracellular side, close to the plasma membrane. This cleavage results in a third and final cleavage in the plasma membrane, which releases the intracellular domain of the receptor (Notch IC) (10, 18, 25, 29, 33). The Notch IC constitutes the active form of the receptor and translocates to the nucleus, where it controls transcription of specific target genes through its interaction with the DNA-binding protein RBP-Jκ {CSL; Suppressor of Hairless [Su(H)] in Drosophila melanogaster} (39). Notch IC is modular and composed of at least four distinct domains: the RAM domain, the ankyrin repeats, the RE/AC domain and a C-terminal transactivation domain (6, 21). The best-characterized target genes are the HES (Hairy/Enhancer of split) genes, which are homologs of the Drosophila Enhancer of Split [E(Spl)] genes (4, 22).
In addition to ligand-mediated activation, Notch signaling can be further modulated by interactions of Notch IC with a number of other proteins (16). These include p300 and PCAF (21, 31), two coactivators with histone acetyltransferase activity (30, 42) that have been found to potentiate Notch signaling in transient transfection experiments. This suggests that histone acetylation may be involved in Notch-mediated activation. This is of particular interest, given that RBP-Jκ acts as a transcriptional repressor through interactions with the corepressor SMRT and that the formation of the SMRT-RBP-Jκ complex is disrupted by Notch IC (17). Direct physical interactions between Notch ICs and both p300 (31) and PCAF (6, 21) have been demonstrated. In the case of p300 the interaction is dependent on a three-amino-acid motif (31) in the Notch IC RE/AC domain, which is critical for activation (6). PCAF interacts predominantly with the Notch IC ankyrin repeat region and to some extent with the C-terminal transactivation domain.
An additional coactivator interacting with Notch, MAML1 (Mastermind-like 1), was recently cloned (41) on the basis of homology to the Drosophila gene Mastermind. MAML1 encodes a 130-kDa protein that localizes primarily to nuclear bodies and displays no apparent homology to other proteins. In Drosophila, Mastermind, like Notch, is a neurogenic gene, and is genetically linked to Notch function (9, 23, 34, 43). MAML1 is a potentiator of Notch signaling for all four Notch receptors, binds to the ankyrin repeat region of Notch IC, and is believed to stabilize the interaction between Notch IC and RBP-Jκ (41).
Despite the identification of several proteins that interact with Notch IC, relatively little is known about the exact functions of these proteins and whether they act individually or jointly in Notch IC-activated transcription. To investigate these questions, we have analyzed the functions of p300, PCAF, MAML1, and Notch IC in an in vitro transcription system reconstituted with purified factors and either naked DNA or chromatin templates. We also compared the effects of Notch1 IC and Notch3 IC, since Notch3 IC is a very weak activator and able to repress Notch1 IC-mediated activation of HES promoters in various cell types (2, 5).
MATERIALS AND METHODS
Plasmids.
Mouse Notch1 IC, Notch3 IC, and mouse RBP-Jκ coding sequences were subcloned into baculovirus expression plasmid pVL1392 after the FLAG-sequence. Human MAML1 coding sequence was subcloned into pVL1393 baculovirus expression plasmid after the FLAG-sequence. The BacVector 3000 system from Novagen was used to create baculoviruses from the above-described vectors. The 12RBP-Jκ template was constructed by inserting 12 response elements for RBP-Jκ into the p2085S plasmid (without Gal4 binding sites) upstream of the E4 promoter.
Protein expression and purification.
Full-length derivatives of FLAG-tagged Notch1 IC, Notch3 IC, MAML1, RBP-Jκ, p300, and PCAF were separately expressed in Sf9 cells via baculoviruses and purified from extracts of infected cells. Infected Sf9 cells were collected, resuspended in lysis buffer (20 mM Tris-HCl [pH 7.9], 500 mM NaCl, 4 mM MgCl2, 0.4 mM EDTA, 20% glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 2 mM dithiothreitol [DTT]), and disrupted with a Dounce homogenizer. After removal of cell debris by centrifugation, the supernatant was adjusted to 300 mM NaCl (by dilution with 20 mM Tris-HCl and 10% glycerol) and 0.1% NP-40 and incubated with M2-agarose beads. Beads with the bound proteins were washed extensively with wash buffer (20 mM Tris-HCl [pH 7.9], 150 mM NaCl, 2 mM MgCl2, 0.2 mM EDTA, 15% glycerol, 0.1% NP-40, 1 mM PMSF, and 1 mM DTT) and immediately used in pull-down assays. Alternatively, the proteins were eluted with FLAG peptide and used in transcription experiments. His-tagged full-length p300 was also expressed in Sf9 cells via baculovirus and purified as described previously (19).
Chromatin assembly.
The purification method for HeLa core histones was adapted from Côte et al. (12). Drosophila S190 extract, kindly provided by Elena Lymar (28), was used to assemble the 12RBP-Jκ plasmid into chromatin with addition of purified HeLa core histones as described previously (7). The S190-assembled chromatin was analyzed by DNA supercoiling and micrococcal nuclease digestion analyses (11). Procedures for chromatin assembly with the recombinant ACF system and subsequent analysis by DNA supercoiling and micrococcal nuclease digestion assays were adapted from Ito et al. (15) and have been described elsewhere (27). Drosophila Acf-1 and ISWI (Flag-tagged) were expressed in Sf9 cells via baculoviruses and purified from extracts of infected cells. His-tagged mouse NAP-1 was expressed in, and purified from, Escherichia coli (20).
In vitro transcription.
Transcription reactions were carried out as schematized in Fig. 1B and as described previously (14, 36, 37). Typically, 70 ng of the naked DNA or chromatin-assembled 12RBP-Jκ template was preincubated with 10 ng of Notch1 IC or Notch3 IC, 50 ng of RBP-Jκ, 100 ng of MAML1, 200 ng of p300, 200 ng of PCAF, or 3 μM acetyl coenzyme A (acetyl-CoA) as indicated in the figures. Approximately 50 μg of HeLa nuclear extract was then added per reaction mixture, and transcription was initiated by addition of 0.4 mM nucleoside triphosphates (final concentration). For primer extension analysis of transcripts, a 32P-labeled probe (25,000 to 50,000 cpm) spanning the region from +86 to +110 of the adenovirus E4 gene was used in each reaction. Purified reverse transcriptase products were analyzed on 8% polyacrylamide gels containing 7 M urea and quantitated with a PhosphorImager (Molecular Dynamics).
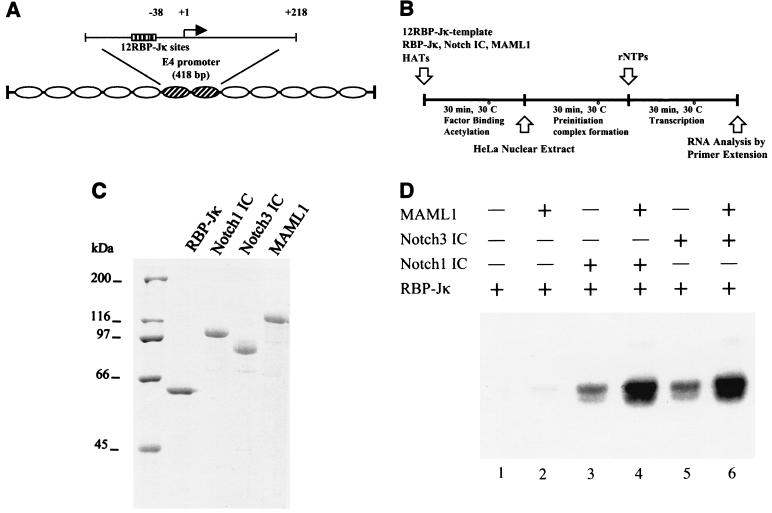
FIG. 1.
MAML1 stimulates Notch IC-driven transcription from a DNA template. (A) Diagram showing the 12RBP-Jκ template. (B) Schematic representation of the in vitro transcription assays indicating the order in which the reagents were added. (C) Coomassie blue staining of purified FLAG-tagged proteins used in the transcription assays. (D) The DNA template was transcribed following RBP-Jκ and Notch1 IC or Notch3 IC binding in the presence or absence of MAML1, as indicated.
Protein interaction assay.
Twenty-microgram amounts of purified FLAG-tagged MAML1, Notch1 IC, and Notch3 IC proteins bound to 20 μl of M2-agarose were separately incubated with 1 μg of purified His-tagged p300 in buffer A (150 mM NaCl, 50 mM HEPES [pH 7.5], 10% glycerol, 0.1% Tween 20, 0.2 mg of bovine serum albumin/ml, 0.5 mM DTT, 1 mM PMSF). After centrifugation of the suspension, the supernatant was removed and the beads were washed five times with 100 μl of buffer A. The proteins were eluted from the beads and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (9% acrylamide). After transfer to nitrocellulose membrane, the samples were incubated with a polyclonal rabbit anti-p300 antibody from Santa Cruz Biotechnology. The Western blot was developed by using ECL from Amersham.
RESULTS
MAML1 potentiates Notch IC-mediated transcription from naked DNA templates.
MAML1 has been shown to potentiate Notch IC-mediated transcription in transient transfection experiments (41). It was also shown to stabilize the interaction between Notch IC and RBP-Jκ, but it is not clear whether this reflects its main function and/or whether it has additional roles in derepressing chromatin. To investigate the function of MAML1 as a coactivator for Notch ICs, we first studied its ability to stimulate Notch1 and Notch3 IC-mediated transcription from a naked DNA template in vitro. The template contained a minimal adenovirus E4 promoter and 12 RBP-Jκ binding sites (referred to as the 12RBP-Jk template) (Fig. 1A) and was incubated with highly purified RBP-Jκ, Notch1 IC, Notch 3 IC, and MAML1 (Fig. 1C). In vitro transcription assays were subsequently performed by using unfractionated HeLa nuclear extract as a source of general transcription factors (Fig. 1B).
Addition of RBP-Jκ protein alone (Fig. 1D, lane 1) only gave a low basal level of transcription equivalent to that observed in the absence of RBP-Jκ (data not shown). This activity was stimulated significantly (circa threefold) by either Notch1 IC (lane 3) or Notch3 IC (lane 5), but not by MAML1 (lane 2), consistent with the known interaction of RBP-Jk with Notch ICs but not with MAML1 (41). However, MAML1 further (circa threefold) enhanced the Notch1 IC (lane 4 versus lane 3) and Notch3 IC (lane 6 versus lane 5) activities, consistent with the demonstrated interaction of MAML1 with Notch ICs. We conclude from these experiments that MAML1 can potentiate Notch IC-mediated transcription from a naked DNA template.
Both p300 and MAML1 are required for Notch IC-dependent transcriptional activation from reconstituted chromatin templates.
Given the ability of MAML1 to potentiate Notch IC-mediated transcription on a naked DNA template, we tested whether MAML1 would have the same effect on a reconstituted chromatin template. To this end, we first reconstituted a 12RBP-Jk containing chromatin template with Drosophila S190 extract and purified histones. Analysis of the chromatin template by both supercoiling (Fig. 2A) and micrococcal nuclease digestion (Fig. 2B) assays revealed that the 12RBP-Jκ template was regularly reconstituted with histones. Subsequent transcription assays revealed that, in contrast to what was observed on the naked DNA template (Fig. 1 and 2D, lanes 10 and 11), neither Notch1 IC nor Notch3 IC (in conjugation with RBP-Jκ) could significantly activate transcription from the chromatin template when added alone (data not shown) or when supplemented with MAML1 (Fig. 2D, lanes 4 and 7).
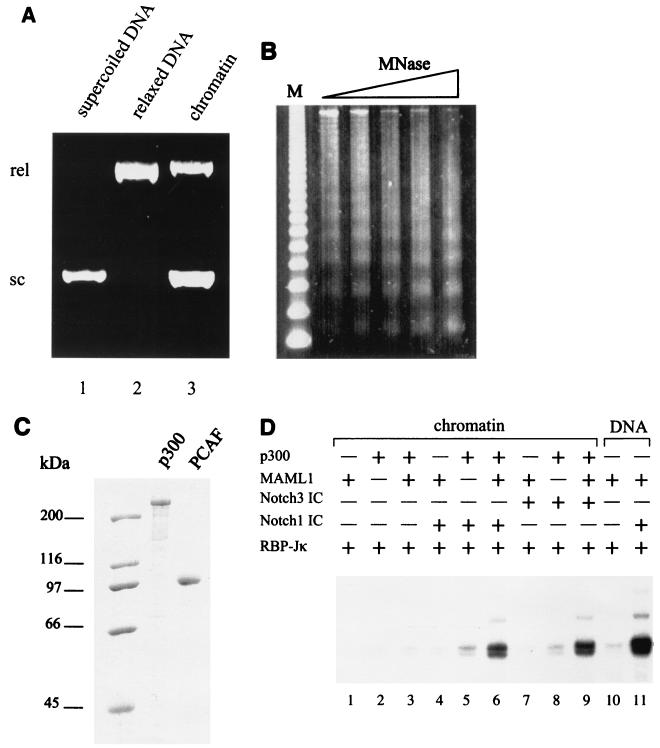
FIG. 2.
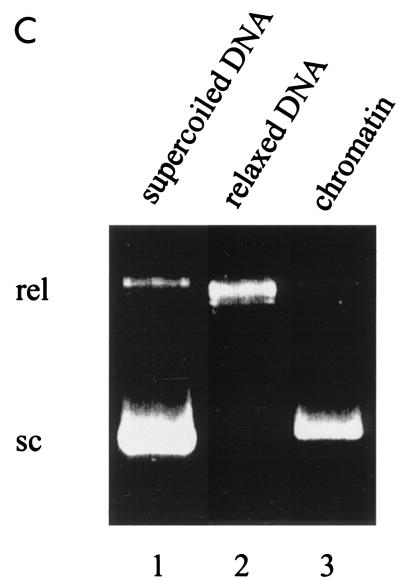
Notch function on a chromatin template is dependent on both MAML1 and p300. (A) DNA supercoiling assay for chromatin assembly. Chromatin was assembled on relaxed circular plasmids, and the resulting superhelicity was checked on a 1% agarose gel which was visualized by ethidium bromide staining. Lanes contain CsCl purified supercoiled (sc) plasmid (lane 1), topoisomerase 1-relaxed (rel) plasmid used for assembly (lane 2), and plasmid purified after chromatin assembly with S190 extract (lane 3). (B) Micrococcal nuclease (MNase) analysis of assembled chromatin on a 1.2% agarose gel. A 123-bp DNA ladder was used as size marker (M). (C) A Coomassie blue-stained SDS-PAGE gel shows the purified p300 and PCAF proteins used in the transcription assays. (D) Transcription from chromatin (lanes 1 to 9) or DNA (lanes 10 and 11) templates. The chromatin templates were incubated with RBP-Jk, Notch1 IC or Notch3 IC, MAML1, and p300 as indicated. Acetyl-CoA was subsequently added to all lanes.
A direct comparison at equimolar template levels (Fig. 2D, lane 11 versus lane 4) indicates that DNA assembly into chromatin restricts the function of Notch ICs and MAML1 and led us to investigate possible cooperative functions of chromatin-modifying factors. We first tested the histone acetyltransferase p300, which previously was shown to potentiate Notch IC signaling and to physically interact with Notch IC (31). Addition of purified p300, together with RBP-Jκ and either Notch1 IC or Notch3 IC, resulted in only a minimal increase in transcription from the chromatin template (Fig. 2D, lanes 5 and 8). In contrast, the simultaneous addition of p300 and MAML1, together with RBP-Jκ and either Notch1 IC or Notch3 IC, resulted in a much more significant level of transcription than that observed with either alone (Fig. 2D, lane 6 versus lanes 4 and 5 and lane 9 versus lanes 7 and 8). As expected, p300 and MAML1, either alone or together, failed to activate transcription significantly in the absence of Notch1 IC or Notch3 IC (Fig. 2D, lanes 1 to 3). From direct comparison with the DNA template activity (Fig. 2D, lanes 10 and 11), the levels of Notch IC-mediated transcription from the chromatinized template are estimated to be almost 60% of the level from the DNA template (Fig. 2D, compare lanes 6 and 9 to lane 11). These data argue strongly that p300 and MAML1 are both required in order to activate Notch IC-mediated transcription from chromatin templates, while MAML1 alone is sufficient on a naked DNA template.
The histone acetyltransferase activity of p300 is important for Notch IC-mediated activation on chromatin templates.
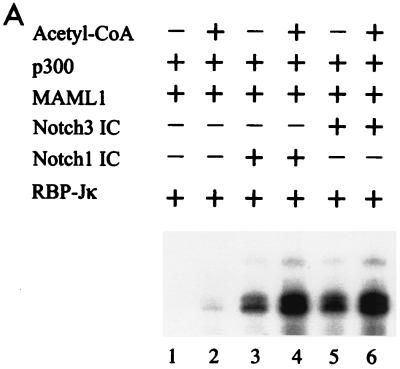
The experiments described above suggest a role for p300 in overcoming the chromatin barrier to Notch IC and MAML1 function. Since p300 is a histone acetyltransferase and since acetylation of histones often correlates with transcriptionally active chromatin (reviewed in reference 38), it is probable that p300 executes its function in Notch IC-mediated transcription through its histone acetyltransferase activity. To investigate this possibility, and since the experiments in Fig. 2 were performed in the presence of acetyl-CoA, the assays were repeated with the variable addition of acetyl-CoA (Fig. 3). When p300 was present with Notch1 IC or Notch3 IC, RBP-Jκ, and MAML1, a significant level of transcription was observed even in the absence of added acetyl-CoA (Fig. 3A, lanes 3 and 5). However, addition of acetyl-CoA increased the level of transcription approximately 1.5-fold (Fig. 3A, lanes 4 and 6). The activation observed when acetyl-CoA was omitted was probably due to the presence of endogenous acetyl-CoA in the S190 extract used to reconstitute the chromatin template, which is in agreement with earlier publications showing p300-mediated transcriptional activation from S190-assembled templates without exogenous acetyl-CoA (19).
FIG. 3.
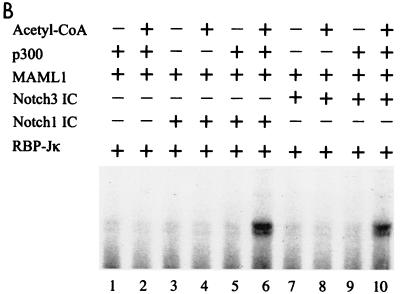
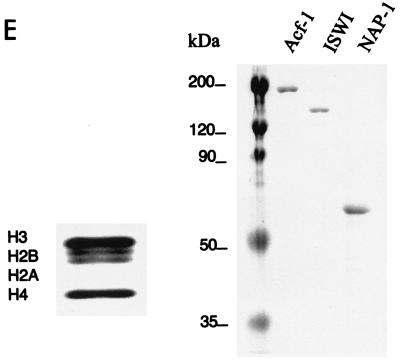
p300-mediated transcription is dependent on acetyl-CoA. (A) In vitro transcription of chromatin templates assembled with S190 extract. (B) In vitro transcription of chromatin templates assembled with the recombinant ACF system. (C) DNA supercoiling assay for chromatin assembly. Lane 1 shows CsCl-purified supercoiled (sc) plasmid, lane 2 shows topoisomerase 1-relaxed (rel) plasmid used for assembly, and lane 3 shows plasmid purified after chromatin assembly with the recombinant ACF system. (D) Micrococcal nuclease (MNase) analysis of chromatin assembled with the recombinant ACF system. A 123-bp DNA ladder was used as size marker (M). (E) Coomassie blue-stained SDS-PAGE gels show the purified HeLa histones, Acf-1, ISWI, and NAP-1 proteins used for assembly.
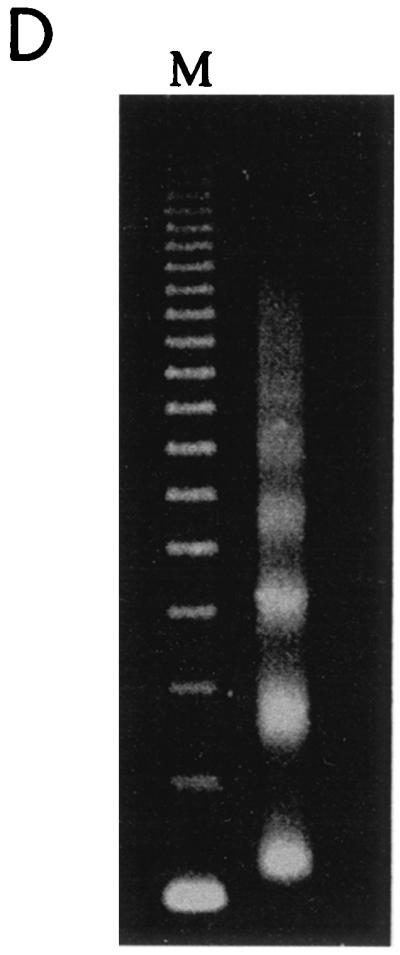
To investigate the acetyl-CoA dependence for p300 more stringently, we reconstituted an 12RBP-Jκ containing chromatin template by using the ACF-based assembly system (14). This system utilizes purified NAP-1, Acf-1, ISWI, and HeLa cell histones (Fig. 3E) and contains no endogenous acetyl-CoA. DNA supercoiling (Fig. 3C) and micrococcal nuclease digestion (Fig. 3D) assays confirmed that the 12RBP-Jκ template was fully assembled into chromatin with regularly spaced nucleosomes. In transcription assays with ACF-assembled chromatin, high levels of activity were seen in the presence of RBP-Jκ, Notch1 IC or Notch3 IC, MAML1, p300, and acetyl-CoA (Fig. 3B, lanes 6 and 10). However, this activity was almost completely lost when acetyl-CoA alone (lanes 3 and 7) or Notch IC proteins (lane 2) were omitted. Thus, these results with more purified templates clearly show that acetyl-CoA and associated acetylation events are necessary for p300 to mediate Notch IC- and MAML1-driven transcription from chromatin templates. They further show a strong cooperativity between Notch ICs, MAML1, and p300.
MAML1 can interact directly with p300.
The ability of activators to recruit histone acetyltransferases such as p300 to promoter regions in chromatin is known to be an important mechanism for gene activation (reviewed in references 32 and 38). It was recently demonstrated that Notch1 IC can physically interact with purified p300 and endogenous p300 in cell extracts (31). To test whether MAML1 also could physically interact with p300, we performed pull-down experiments that used Notch1 IC and Notch3 IC as controls. MAML1, Notch1 IC, and Notch3 IC were expressed separately in Sf9 cells via baculovirus vectors and purified through their FLAG-tags. The FLAG-tagged proteins were coupled to M2-agarose beads, and the concentrations were determined via SDS-PAGE and Coomassie blue staining. Equal amounts of the immobilized FLAG-tagged proteins were incubated with purified His-tagged p300. After pelleting and washing the M2-agarose beads, the proteins were eluted and levels of p300 associated with Notch1 IC, Notch3 IC, and MAML1 were determined by Western blotting with an antibody against p300.
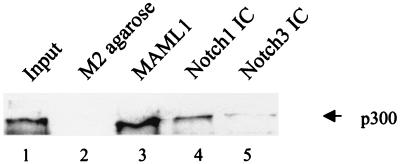
The data in Fig. 4 show very significant binding of p300 (10% of the input) to M2-agarose-immobilized MAML1 (lane 3) but no binding to M2-agarose alone (lane 2). The interaction of p300 with MAML1 is much more prominent than the interaction with Notch1 IC (lane 4) and, especially, Notch3 IC (lane 5). Thus, this analysis demonstrates a robust physical interaction between p300 and MAML1, and therefore suggests that MAML1 may be more important than Notch IC proteins in directly recruiting p300 to the promoter.
FIG. 4.
Binding of p300 to MAML1, Notch1 IC, and Notch3 IC. FLAG-tagged proteins bound to M2-agarose were tested for interaction with purified p300 in an in vitro protein interaction assay. p300 was detected in a Western blot by using an antibody (Santa Cruz Biotechnology) against the N-terminal domain of p300. The input represents 10% of the p300 protein used in each binding reaction. The negative control is M2-agarose without any bound FLAG-tagged protein.
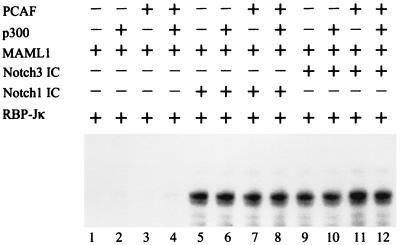
The function of PCAF as a coactivator for Notch1 and Notch3 ICs on chromatin templates is dependent on p300 and MAML1.
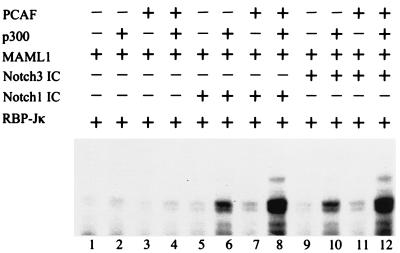
PCAF previously has been shown to have histone acetyltransferase activity (42) and to potentiate transcriptional activation by both Notch1 IC (21) and Notch3 IC (6) in transient-transfection assays. We decided to analyze whether PCAF could act alone on naked or chromatin-reconstituted DNA templates to potentiate Notch IC-mediated activation or whether it, like MAML1, would require the presence of p300. When PCAF was added to the chromatin template together with RBP-Jκ, MAML1, and Notch1 IC or Notch3 IC, the level of activation was barely over baseline (Fig. 5, lanes 7 and 11 versus lanes 5 and 9). This contrasts with the strong stimulatory effect of p300 in the same assay (Fig. 5, lanes 6 and 10 versus lanes 5 and 9; Fig. 2 and 3). However, addition of PCAF with p300 (lanes 8 and 12) resulted in a marked increase (almost threefold) in activity relative to that observed with p300 alone (lanes 6 and 10).
FIG. 5.
PCAF acts synergistically with p300 to mediate Notch IC activity from chromatin templates. Transcription assay with chromatin templates assembled with S190 extract. Acetyl-CoA, RBP-Jκ, and MAML1 were added to all reactions, and Notch1 IC, Notch3 IC, p300, and PCAF were added as indicated.
We next analyzed the effect of PCAF addition on a naked DNA template, using the same experimental setup as for the chromatin template shown in Fig. 5. As shown in Fig. 6, and as described earlier, a high level of transcription was observed with RBP-Jκ, Notch1 IC or Notch3 IC, and MAML1 on the DNA template (lanes 5 and 9). This activity was unaffected by independent addition of either PCAF (lanes 7 and 11 versus lanes 5 and 9) or of p300 (lanes 6 and 10 versus lanes 5 and 9). However, and in contrast to the results observed with the chromatin templates, the joint addition of p300 and PCAF also failed to stimulate transcription from the DNA template (lanes 8 and 12 versus lanes 5 and 9). These data support the view that p300 and MAML1 are critically required for PCAF to potentiate Notch IC-mediated transcription from a chromatin template but that PCAF, in contrast to MAML1, is unable to potentiate transcription from a naked DNA template.
FIG. 6.
Notch function on naked DNA is not affected by p300 and PCAF. The DNA templates were transcribed following RBP-Jκ, Notch1 IC or Notch3 IC, and MAML1 binding in the presence or absence of p300 and PCAF as indicated (lanes 5 to 12). p300 and PCAF do not significantly influence basal transcription (lanes 1 to 4).
DISCUSSION
In this report, we have addressed the roles of the Notch IC-interacting proteins MAML1, p300, and PCAF and how they interact to facilitate Notch IC-mediated activation. We have used purified factors and an in vitro transcription system, which allowed us to test independent versus cooperative functions of factors and to distinguish between activation from naked DNA and chromatin templates.
p300 plays a key role in facilitating the ability of MAML1 and PCAF to potentiate Notch IC-mediated transcriptional activation from chromatin templates.
The data presented in this report show that MAML1, PCAF, and p300 each play a distinct role in Notch IC-mediated activation. Together with RBP-Jκ, MAML1 can potentiate Notch IC-mediated transcription on a naked DNA template, but not on a reconstituted chromatin template. By contrast, p300 is incapable of increasing transcription with Notch IC and RBP-Jκ alone, either on naked DNA or on chromatin templates, but markedly potentiates transcription from chromatin templates in conjunction with MAML1. Finally, PCAF is unable to increase transcription in the absence of p300, but further enhances the effect of p300 and MAML1 on chromatin templates. These results define a hierarchical order of coactivator function for Notch IC-mediated transcriptional activation and show that p300 plays a key role. These observations extend previous results, based on transient transfection experiments, showing that all three factors can potentiate Notch IC activation (6, 21, 31, 41).
As p300 is a histone acetyltransferase and is critically and selectively required on chromatin templates for Notch IC-mediated activation through MAML1 and PCAF, it seems plausible that the function of p300 is to modify chromatin structure. This notion is supported by previous observations that RBP-Jκ (in the absence of Notch IC) binds the corepressor SMRT, which in turn recruits histone deacetylases, and that SMRT is displaced by Notch IC on RBP-Jκ after Notch induction (17). Furthermore, a Notch IC target gene is upregulated in Xenopus explant cultures by addition of the histone deacetylase inhibitor trichostatin A (17). Also, E1A and Twist, two histone acetyltransferase inhibitor proteins, reduce Notch IC-mediated activation from RBP-Jκ-binding elements (21). While these observations suggest a role for histone deacetylation and acetylation in the Notch signaling pathway, we provide more-direct evidence that p300 functions as a histone acetyltransferase in Notch IC-mediated transcription. This was accomplished by experimentally altering the levels of acetyl-CoA in the in vitro transcription experiments. Omission of acetyl-CoA in the S190 chromatin system significantly reduced the level of transcription, whereas omission in the ACF chromatin assembly system, which contains no endogenous acetyl-CoA, led to a total reduction in Notch IC-mediated transcription. This strongly supports a model wherein the primary function of p300 is to act as a histone acetyltransferase. This model is supported by recent studies indicating that histone acetylation events are both targeted (20) and essential (1) during p300-dependent transcriptional activation by Gal4-VP16. This notion is further supported by the observation that p300, together with Notch IC and RBP-Jκ, has no detectable effect on transcription from naked DNA templates, arguing that it is critically required only to overcome the barrier to transcription imposed by the chromatin template.
How can the cooperation between p300 and MAML1 for Notch IC-mediated activation be explained? It previously was shown that both p300 (31) and MAML1 (41) can physically interact with Notch IC. The binding sites for MAML1 and p300 are located in different parts of Notch IC (31, 41): MAML1 binds to the ankyrin repeats, and p300 binds to a three-amino-acid motif (31) in a region that has been shown to be a transactivating domain (6). The data presented here confirm the interactions between Notch IC and p300 but also show a robust interaction of p300 with MAML1 that is much stronger than the interaction of p300 with Notch IC protein. Along with our observation that Notch1 IC and Notch3 IC show different affinities for p300 but comparable levels of p300-dependent transcriptional activation, these results strongly suggest that MAML1 plays the major role in p300 recruitment for subsequent chromatin modifications and are in agreement with a recent report (13) that was published while this report was under review. That study showed (i) that acetylation of chromatin templates by p300 is strictly dependent on RBP-Jκ, Notch1 IC, and MAML1; (ii) that MAML1 mutations that abolish the binding of MAML1 to p300 in vitro correlate with impaired acetylation of the templates; and, most importantly, (iii) that Notch signaling in vivo is blocked by a MAML1 mutant which lacks the domain that interacts with p300. On the other hand, Oswald et al. (31) have described Notch1 IC mutants that show reduced binding to p300 and reduced transcription. These results are consistent with a secondary role for Notch in p300 stabilization on the promoter, although possible effects of the mutations on interactions with other components of the transcriptional machinery are not excluded.
Although the major function of MAML1 may be to recruit p300, we also have found that MAML1 can increase transcription with only Notch IC and RBP-Jκ on a naked DNA template. The latter finding is consistent with a proposed MAML1 function in the stabilization of Notch IC-RBP-Jκ-promoter interactions. However, it is equally possible that MAML1 is involved in interactions with other coactivators (e.g., Mediator) or components of the general transcription machinery that facilitate preinitiation complex assembly or function (reviewed in reference 26). The C terminus of MAML1 has been found to contain a transactivation domain that is active in transfection assays when recruited to promoters by the DNA-binding domain of Gal4 (41). This result clearly indicates a MAML1 function beyond any potential stabilization of the Notch IC-RBP-Jκ-promoter complex but is compatible with roles for the transactivation domain in the recruitment and function either of p300 or of factors acting on a DNA template. Future studies of the function of Gal4-MAML1 fusion proteins in DNA versus chromatin template assays should distinguish these functions.
The observation that p300 can facilitate PCAF function in Notch IC-mediated transcription is intriguing, since PCAF, like p300, is also a histone acetyltransferase. To our knowledge this is the first demonstration of cooperativity between p300 and PCAF in the activation of transcription in a defined in vitro system. In support of our data, p300 and PCAF recently have been shown in transient transfection assays to function cooperatively in the stimulation of FKLF2 (35). It appears that the histone acetyltransferase activity of PCAF is insufficient to derepress chromatin, as PCAF does not act cooperatively with MAML1 and thus cannot substitute for p300 in this regard. This may relate to the fact that the interaction between PCAF and Notch1 IC or Notch3 IC is weak (6) and not observed with purified PCAF (A. E. Wallberg, unpublished observation). The suggestion that p300 might be involved in recruiting PCAF is supported by the observation that PCAF interacts directly with p300 and, in fact, was isolated as a protein interacting with p300 (42). However, it cannot be excluded that PCAF has other activities in addition to being a histone acetyltransferase.
Relative activities of Notch1 and Notch3 IC domains.
In the in vitro assays described here, Notch1 IC and Notch3 IC activated transcription to essentially the same extent. In contrast, transient-transfection analysis with different cell lines revealed that Notch3 IC is a poor activator of target genes compared to Notch1 IC (5, 6). Related studies showed that Notch3 IC overexpression in central nervous system progenitor cells of transgenic mice leads to a reduction in HES5 expression (5), whereas overexpression in pancreatic progenitor cells results both in reduced HES1 expression and an expansion of exocrine cells at the expense of endocrine cells (2). However, there are also examples in which Notch3 IC overexpression leads to increased HES expression, as in the case of developing thymocytes (8) and adult central nervous system progenitor cells (40). Thus, whereas our assays in cell-free systems indicate that Notch1 and Notch3 ICs have similar intrinsic transcription activation potentials, the cellular assays indicate that these activities are differentially mediated by other factors in vivo and that these factors differ from cell type to cell type (reviewed in reference 24).
In conclusion, the data presented here begin to unveil a complex hierarchical interplay between three Notch IC-interacting proteins that allows for high levels of Notch IC-mediated transcriptional activation. In this hierarchy, p300 has a critical role in promoting Notch IC-mediated transcription from chromatin templates, as well as in facilitating PCAF function, but is nonetheless dependent on the presence of MAML1.
Acknowledgments
A.E.W. and K.P. contributed equally to this work.
We thank E. Lymar for generously sharing S190 extract, J. Kadonaga for His-tagged p300 and FLAG-tagged PCAF and ACF baculoviruses, M. Guermah for the FLAG-tagged p300 baculovirus, and S. Malik, W. An, and M. Teichman for critical reading of the manuscript.
This work was supported by HFSP and EU project QLG3-CT-2000-01471 and Swedish Cancer Society grants to U.L., by NIH grant CA42567 to R.G.R., and by a fellowship from the Swedish Cancer Society and research support from the Swedish Medical Research Council to A.E.W.
REFERENCES
- 1.An, W., V. B. Palhan, M. A. Karymov, S. H. Leuba, and R. G. Roeder. 2002. Selective requirements for histone H3 and H4 N-termini in p300-dependent transcriptional activation from chromatin. Mol. Cell 9:811-821. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist, A., H. Li, L. Sommer, P. Beatus, D. J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signalling controls pancreatic cell differentiation. Nature 400:877-881. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas, S., M. D. Rand, and R. J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science 284:770-776. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, A. M., and J. W. Posakony. 1995. Suppressor of hairless directly activates transcription of Enhancer of split complex genes in response to Notch receptor activity. Genes Dev. 9:2609-2622. [DOI] [PubMed] [Google Scholar]
- 5.Beatus, P., J. Lundkvist, C. Öberg, and U. Lendahl. 1999. The Notch3 intracellular domain represses Notch1-mediated activation through Hairy/Enhancer of split (HES) promoters. Development 126:3925-3935. [DOI] [PubMed] [Google Scholar]
- 6.Beatus, P., J. Lundkvist, C. Öberg, K. Pedersen, and U. Lendahl. 2001. The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mech. Dev. 104:3-20. [DOI] [PubMed] [Google Scholar]
- 7.Becker, P. B., T. Tsukiyama, and C. Wu. 1994. Chromatin assembly extracts from Drosophila embryos. Methods Cell. Biol. 44:207-223. [DOI] [PubMed] [Google Scholar]
- 8.Bellavia, D., A. F. Campese, E. Alesse, A. Vacca, M. P. Felli, A. Balestri, A. Stoppacciaro, C. Tiveron, L. Tatangelo, M. Giovarelli, et al. 2000. Constitutive activation of NF-κβ and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 19:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettler, D., S. Pearson, and B. Yedvobnick. 1996. The nuclear protein encoded by the Drosophila neurogenic gene mastermind is widely expressed and associates with specific chromosomal regions. Genetics 143:859-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brou, C., F. Logeat, N. Gupta, C. Bessia, O. LeBail, J. R. Doedens, A. Cumano, P. Roux, R. A. Black, and A. Israel. 2000. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol. Cell 5:207-216. [DOI] [PubMed] [Google Scholar]
- 11.Bulger, M., and J. T. Kadonaga. 1994. Biochemical reconstitution of chromatin with physiological nucleosome spacing. Methods Mol. Genet. 5:241-262. [Google Scholar]
- 12.Côte, J., R. T. Utley, and J. L. Workman. 1995. Basic analysis of transcription factor binding to nucleosomes. Methods Mol. Genet. 6:108-129. [DOI] [PubMed] [Google Scholar]
- 13.Fryer, C. J., E. Lamar, I. Turbachova, C. Kintner, and K. A. Jones. 2002. Mastermind mediates chromatin-specific transcription and turnover of the Notch enhancer complex. Genes Dev. 16:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda, K., D. Steger, A. Eberharter, and J. L. Workman. 1999. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol. Cell. Biol. 19:855-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, T., M. E. Levenstein, D. V. Fyodorov, A. K. Kutach, R. Kobayashi, and J. T. Kadonaga. 1999. ACF consists of two subunits, Acf1 and ISWI, that function cooperatively in the ATP-dependent catalysis of chromatin assembly. Genes Dev. 13:1529-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadesch, T. 2000. Notch signaling: a dance of proteins changing partners. Exp. Cell Res. 260:1-8. [DOI] [PubMed] [Google Scholar]
- 17.Kao, H.-Y., P. Ordentlich, N. Koyano-Nakagawa, Z. Tang, M. Downes, C. R. Kintner, R. M. Evans, and T. Kadesch. 1998. A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev. 12:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopan, R., E. H. Schroeter, H. Weintraub, and J. S. Nye. 1996. Signal transduction by activated mNotch: importance of proteolytic processing and its regulation by the extracellular domain. Proc. Natl. Acad. Sci. 93:1683-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraus, W. L., and J. T. Kadonaga. 1998. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 12:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundu, T. K., V. B. Palhan, Z. Wang. W. An, P. A. Cole, and R. G. Roeder. 2000. Activator-dependent transcription from chromatin in vitro involving targeted histone acetylation by p300. Mol. Cell 6:551-561. [DOI] [PubMed] [Google Scholar]
- 21.Kurooka, H., and T. Honjo. 2000. Functional interaction between the mouse Notch1 intracellular region and histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 275:17211-17220. [DOI] [PubMed] [Google Scholar]
- 22.Lecourtois, M., and F. Schweisguth. 1995. The neurogenic Suppressor of Hairless DNA-binding protein mediates the transcriptional activation of the Enhancer of split complex genes triggered by Notch signaling. Genes Dev. 9:2598-2608. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann, R. F., W. Jimenez, U. Dietrich, and J. A. Campos-Ortega. 1983. On the phenotype and development of mutants of early neurogenesis in D. melanogaster. Wilhelm Roux's Arch. Dev. Biol. 192:62-74. [DOI] [PubMed] [Google Scholar]
- 24.Lundkvist, J., and U. Lendahl. 2001. Notch and the birth of glial cells. Trends Neurosci. 9:492-494. [DOI] [PubMed] [Google Scholar]
- 25.Logeat, F., C. Bessia, C. Brou, O. LeBail, S. Jarriault, N. G. Seidah, and A. Israel. 1998. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. USA 95:8108-8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik, S., and R. G. Roeder. 2000. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25:277-283. [DOI] [PubMed] [Google Scholar]
- 27.Malik, S., A. E. Wallberg, Y. K. Kang, and R. G. Roeder. 2002. TRAP/SMCC/Mediator-dependent transcriptional activation from DNA and chromatin templates by orphan nuclear receptor HNF-4. Mol. Cell. Biol. 22:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez, E., V. B. Palhan, A. Tjernberg, E. S. Lymar, A. M. Gamper, T. K. Kundu, B. T. Chait, and R. G. Roeder. 2001. Human STAGA complex is a chromatin-acetylating transcription coactivator that interacts with pre-mRNA splicing and DNA damage-binding factors in vivo. Mol. Cell. Biol. 21:6782-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mumm, J. S., E. H. Schroeter, M. T. Saxena, A. Griesemer, X. Tian, D. J. Pan, W. J. Ray, and R. Kopan. 2000. A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol. Cell 5:197-206. [DOI] [PubMed] [Google Scholar]
- 30.Ogryzko, V. V., R. L. Schiltz, V. Russanova, B. H. Howard, and Y. Nakatani. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953-959. [DOI] [PubMed] [Google Scholar]
- 31.Oswald, F., B. Täuber, T. Dobner, S. Bourteele, U. Kostezka, G. Adler, S. Liptay, and R. M. Schmid. 2001. p300 acts as a transcriptional coactivator for mammalian Notch-1. Mol. Cell. Biol. 21:7761-7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth, S. Y., J. M. Denu, and C. D. Allis. 2001. Histone acetyltransferases. Annu. Rev. Biochem. 70:81-120. [DOI] [PubMed] [Google Scholar]
- 33.Schroeter, E. H., J. A. Kisslinger, and R. Kopan. 1998. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393:382-386. [DOI] [PubMed] [Google Scholar]
- 34.Smoller, D., C. Friedel, A. Schmid, D. Bettler, L. Lam, and B. Yedvobnick. 1990. The Drosophila neurogenic locus mastermind encodes a nuclear protein usually rich in amino acid homopolymers. Genes Dev. 4:1688-1700. [DOI] [PubMed] [Google Scholar]
- 35.Song, C.-Z., K. Keller, K. Murata, H. Asano, and G. Stamatoyannopoulos. 2002. Functional interaction between coactivators CBP/p300, PCAF and transcription factor FKLF2. J. Biol. Chem. 277:7029-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steger, D. J., A. Eberharter, S. John, P. A. Grant, and J. L. Workman. 1998. Purified histone acetyltransferase complexes stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc. Natl. Acad. Sci. USA 95:12924-12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steger, D. J., and J. L. Workman. 1999. Transcriptional analysis of purified histone acetyltransferase complexes. Methods 19:410-416. [DOI] [PubMed] [Google Scholar]
- 38.Sterner, D. E., and S. L. Berger. 2000. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 64:435-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura, K., Y. Taniguchi, S. Minoguchi, T. Sakai, T. Tun, T. Furukawa, and T. Honjo. 1995. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-Jκ/Su(H). Curr. Biol. 5:1416-1423. [DOI] [PubMed] [Google Scholar]
- 40.Tanigaki, K., F. Nogaki, J. Takahashi, K. Tashiro, H. Kurooka, and T. Honjo. 2001. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron 29:45-55. [DOI] [PubMed] [Google Scholar]
- 41.Wu, L., J. C. Aster, S. C. Blacklow, R. Lake, S. Artavanis-Tsakonas, and J. D. Griffin. 2000. MAML1, a human homologue of Drosophila Mastermind, is a transcriptional co-activator for NOTCH receptors. Nat. Genet. 26:484-489. [DOI] [PubMed] [Google Scholar]
- 42.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 43.Yedvobnick, B., D. Smoller, P. Young, and D. Mills. 1988. Molecular analysis of the neurogenic locus mastermind of Drosophila melanogaster. Genetics 118:483-497. [DOI] [PMC free article] [PubMed] [Google Scholar]