Abstract
Macrophages play a crucial role in the defense against pathogens. Distinct macrophage populations can be defined by the expression of restricted cell surface proteins. Resident tissue macrophages, encompassing Kupffer cells of the liver and red pulp macrophages of the spleen, characteristically express the F4/80 molecule, a cell surface glycoprotein related to the seven transmembrane-spanning family of hormone receptors. In this study, gene targeting was used to simultaneously inactivate the F4/80 molecule in the germ line of the mouse and to produce a mouse line that expresses the Cre recombinase under the direct control of the F4/80 promoter (F4/80-Cre knock-in). F4/80-deficient mice are healthy and fertile. Macrophage populations in tissues can develop in the absence of F4/80 expression. Functional analysis revealed that the generation of T-cell-independent B-cell responses and macrophage antimicrobial defense after infection with Listeria monocytogenes are not impaired in the absence of F4/80. Interestingly, tissues of F4/80-deficient mice could not be labeled with anti-BM8, another macrophage subset-specific marker with hitherto undefined molecular antigenic structure. Recombinant expression of a F4/80 cDNA in heterologous cells confirmed this observation, indicating that the targets recognized by the F4/80 and BM8 monoclonal antibodies are identical.
Macrophages and their precursors, blood monocytes, represent widely distributed populations of myeloid cells. Resident tissue macrophages display extensive functional and phenotypic heterogeneity shaped in large part by the tissue microenvironment, as do monocytes recruited to local sites in response to inflammatory and immune stimuli (16, 26). Immunohistochemical analyses of normal tissue sections and various tissues from infected or immunologically challenged mice have utilized panels of monoclonal antibodies which recognize phenotypic markers expressed by macrophage subpopulations, such as macrosialin (the mouse ortholog of CD68), sialoadhesin (mouse CD169), scavenger receptor (SRA-I and SRA-II), complement receptor type 3 (CR3, CD11b/CD18), and the F4/80 glycoprotein (3, 5, 10, 16, 26, 35, 39, 43). Perhaps the most extensively utilized reagent for detection of mature tissue macrophages is the monoclonal antibody (MAb) F4/80, which recognizes the F4/80 molecule expressed at high levels on the surface of a range of cells including Kupffer cells in the liver, splenic red pulp macrophages, brain microglia, gut lamina propria, and Langerhans cells in the skin, as well as resident macrophages found throughout connective tissue and many other organs including the heart, kidney, and the reproductive and neuroendocrine systems (3, 21-23, 31, 36).
The isolation of cDNA clones encoding the mouse F4/80 glycoprotein resulted in the molecular characterization of its unusual structure and led to the further identification of related leukocyte cell surface proteins (4, 6, 27, 31, 44, 45). The F4/80 molecule contains seven-transmembrane (TM7) regions, which anchor the protein in the cell membrane, and thereby shows similarity in this region to G-protein-coupled receptors. The F4/80 molecule shares overall structural homology to other members of the epidermal growth factor (EGF)-TM7 family, which includes EMR1 (the human ortholog of F4/80), CD97, EMR2, EMR3, and FIRE, which are differentially expressed on populations of leukocytes, as well as ETL, which is expressed by cardiomyocytes (4, 6, 19, 32, 44, 45). The EGF-TM7 gene family cluster has been mapped to human chromosome 19p and syntenic regions within the mouse genome (7, 27, 44). These EGF-TM7 molecules are a subset of a larger group of TM7 molecules termed LNB-TM7, which are defined by amino acid sequence similarity within the TM7 region to members of G-protein-coupled receptor family B, yet have large N-terminal extracellular regions often containing a complex array of protein modules (44). The presence of such large and composite structures also suggests specialized functions for these molecules, possibly in addition to peptide receptor activities.
To date, the function of F4/80 has not been determined, although speculation has suggested its involvement in macrophage adhesion events, either to matrix molecules or a ligand expressed on other cell types, a role in cell migration or as a G protein-coupled signaling component of macrophages (30, 44). Indeed, one member of the EGF-TM7 family, CD97, has been shown to bind CD55 (decay accelerating factor), a glycosylphosphatidylinositol-linked cell-surface receptor previously shown to regulate complement activation by catalyzing the dissociation of components of the C3 convertases (30, 44). This interaction was specific for a particular isoform of CD97 containing three EGF domains (EGF1, EGF2, and EGF5), suggesting that EGF-TM7 gene-splicing mechanisms regulate receptor-ligand pairing (20). In fact, this represented the first demonstration of a TM7 molecule binding a cellular ligand, although the functional significance of this interaction has yet to be established. In the absence of information relating to the function of F4/80 and its ligand(s), we sought to address the functional consequences of deleting the gene in a mouse germ line. In parallel, we aimed to generate a mouse line that specifically expresses Cre recombinase (38) within F4/80+ macrophage populations, thereby allowing cell-specific targeted deletion of candidate loxP-flanked genes in offspring.
MATERIALS AND METHODS
Targeting vector and structure of the genomic F4/80 locus.
A partial murine F4/80 genomic clone [1J9(P1)], isolated from 129Sv mice and containing the first coding exon, was cloned into pBluescript (Stratagene, Amsterdam, The Netherlands) and completely sequenced. Sequence comparison with the F4/80 mRNA (31) revealed that within 5.9 kb of genomic sequence 1J9(P1) contained the first coding exon (designated exon 1) and one downstream exon (exon 2). The sequenced exons were identical to the corresponding murine F4/80 mRNA sequence (GenBank/EMBL accession no. X93328). The ATG initiation codon was located in exon 1. To inactivate the F4/80 gene, a targeting vector (TV) was constructed (Fig. 1A). A short arm of homology was amplified by PCR with a genomic DNA template from E14.1 embryonic stem (ES) cells with primers F4/80SANotI (5′-AAA AAA AAG CGG CCG CTA GAC TTC CTC CTT TCT AAT TAG-3′) and F4/80SANcoI (5′-AAA GTC GAC CCA TGG TAC TGT GGC AGT CAT TCA-3′) subcloned into pBluescript, and the fragment was control sequenced. A modified Cre expression cDNA cassette (a kind gift of H. Gu and K. Rajewsky) (17) was inserted as a NcoI/XhoI fragment and ligated. The neomycin resistance gene cassette was introduced following XhoI digestion into the XhoI/SalI-digested vector backbone. Thereafter, the long homology arm was amplified by PCR with primers F4/80LAXhoI (5′-AAA CTC GAG GAG GTT GAA TGG GGC ATG-3′) and F4/80LAKpnI (5′-AAA GGT ACC GCA GCA TCC AGT AAC AAG-3′), cut with XhoI and KpnI, and ligated. The targeting vector F4/80TV was completed by inserting a herpes simplex virus (HSV)-tk cassette into the KpnI site of F4/80TV. The orientations and integrity of the fragments and expression cassettes were verified by restriction analysis and partial sequencing.
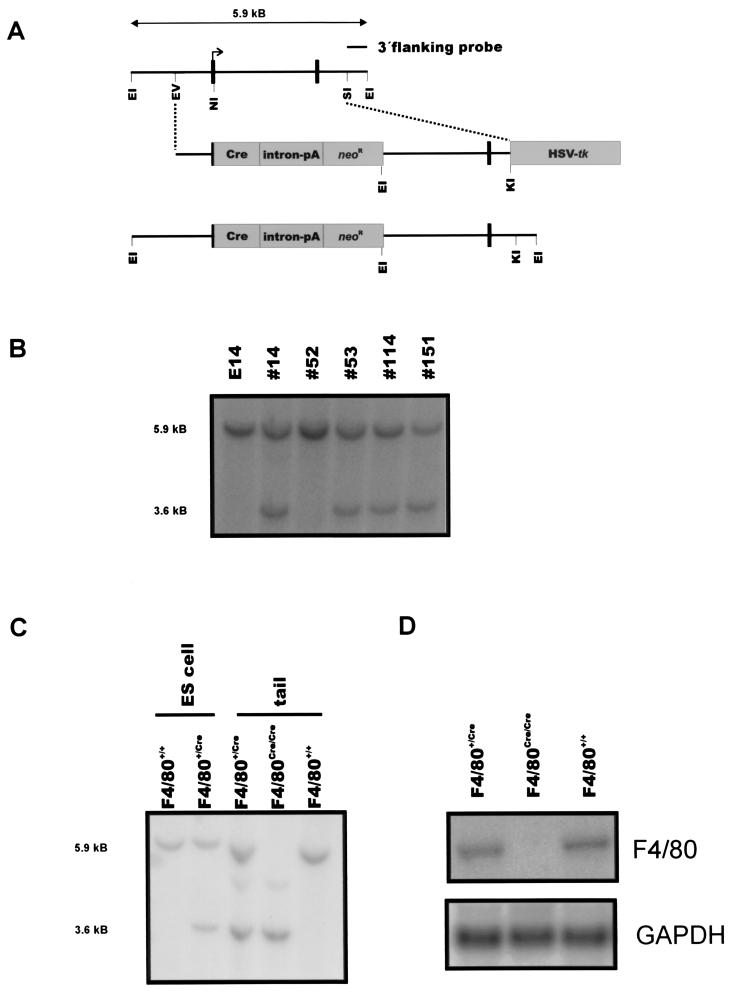
FIG. 1.
Generation of F4/80-deficient mice. (A) Targeting strategy used for inactivation of the murine F4/80 gene. EI, EcoRI; EV, EcoRV; NI, NcoI; SI, StuI; KI, KpnI. Parts of the first coding exon were replaced by insertion of a Cre recombinase cDNA cassette at the ATG initiation codon and a neomycin resistance gene cassette. (B) Southern blot analysis of genomic DNA from targeted ES cell clones. EcoRI digest of genomic DNA from E14.1 wild-type and targeted ES cells. Hybridization with the flanking probe yields a 5.9-kb fragment for the wild-type allele and a 3.6-kb fragment for the targeted allele. Sizes are indicated at the left in kilobases (kb). (C) Southern blot analysis of genomic DNA from mouse tail biopsies and, for comparison, from one targeted ES cell clone. EcoRI digest of genomic DNA from E14.1 wild-type and targeted ES cells and mouse tails. For fragment sizes see panel B. (D) Northern blot analysis verifying the lack of F4/80 RNA in mice homozygous for the targeted F4/80 allele. Total RNA from F4/80+/+, F4/80+/Cre, and F4/80Cre/Cre (i.e., F4/80−/−) livers was hybridized with a full-length F4/80 cDNA and a GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA (loading control).
Targeting of the F4/80 locus and generation of F4/80 mutant mice.
To enable direct screening for correct recombination of the F4/80TV, the vector was linearized on the short arm homology site with NotI and transfected into E14.1 ES cells. The transfection, culture, and selection of ES cells was performed as described previously (33, 37). G418- and ganciclovir-resistant ES cell colonies were picked and screened for integration of the targeting vector by PCR with the external primer F4/80scr1 (5′-GTT GTT TAT CAG CTG TAG GAG −3′) and the internal primer Crerev.1 (5′-GCA TGC ACC GGT AAT GCA GGC-3′). From five PCR-positive clones, the correct recombination event could be verified for four clones by blotting ES cell DNA after digestion with EcoRI and hybridization with the 3′ flanking probe. Single copy integration was verified by Southern probing of the EcoRI-digested ES cell DNA with a part of the neor cassette (data not shown). Chimeric mice were generated from F4/80+/Cre ES cells by injection of C57BL/6 blastocysts. Chimeric mice obtained from F4/80+/Cre ES cell lines were crossed with C57BL/6 wild-type mice. Germ line transmission of the targeted allele was identified by Southern blot analysis. Mice were housed in a specific-pathogen-free animal facility with barrier conditions. For genotyping of mice by PCR, the primers F4/80scr1 and Crerev.1 were used for the targeted allele and primers F4/80scr1 and F4/80exon1 (5′-AGA GGA GCA GCC AAA AGC CCC-3′) was used for the wild-type allele.
RNA analysis.
Total RNA was extracted as described previously (8). For Northern blotting, 20 μg of RNA was separated on a formaldehyde agarose gel, transferred onto a Genescreen Plus nylon membrane (DuPont), and hybridized with a full-length murine F4/80 cDNA probe and GAPDH as a loading control.
Immunocytochemistry.
Spleens and livers of control and F4/80-deficient mice were embedded in OCT Tissue Tec media (Leica, Bensheim, Germany). Eight-micrometer sections were cut by using a Leica Cryotom, and immunohistochemical labeling was performed as described previously (15). The antibodies used were anti-F4/80 (hybridoma supernatant) and biotin-conjugated BM8 (Diagnostic International BMA, Hamburg, Germany); second-step reagents were peroxidase-coupled F(ab′)2 murine anti-rat immunoglobulin G (IgG) (Dianova, Hamburg, Germany) and peroxidase-coupled Extravidin (Sigma, Deisenhofen, Germany), respectively.
Flow cytometry.
Heparinized peripheral blood was obtained by puncture of the posterior aorta. Erythrocyte-lysed single-cell suspensions were pretreated with Fc-block (anti-CD16/CD32; PharMingen, Beckton Dickinson, Heidelberg, Germany) and then incubated with specific antibodies. The antibodies used were fluorescence dye-coupled anti-CD3ɛ, anti-CD4, anti-CD8, DX5, anti-CD19, anti-B220, and anti-NK1.1 MAb (all PharMingen). Biotinylated antibodies were detected with streptavidine-PerCP (PharMingen). Ten to fifty thousand events were analyzed in a live gate as determined by forward and side scatter profiles obtained with a FACSCalibur cytometer (Beckton Dickinson).
T-cell-independent antibody responses.
Mice (n = 4 per group) were immunized intravenously with 10 μg of the Ti-2 antigen NP32-AECM-Ficoll (NP-Ficoll; Biosearch Technologies Inc., Novato, Calif.) dissolved in 200 μl of phosphate-buffered saline solution (Seromed, Berlin, Germany). Mice were bled 1 day prior to immunization and on days 6 and 10 postimmunization. Concentrations of NP-specific serum antibodies were determined by enzyme-linked immunosorbent assay by using NP12-bovine serum albumin as antigen and alkaline phosphatase conjugated rat anti-mouse IgM and IgG3 (PharMingen) antibodies for detection.
Infection with Listeria.
Infection with Listeria was performed as described previously (13). Briefly, cultures of Listeria monocytogenes (ATCC strain 43251) were grown in brain heart infusion (Difco, Detroit, Mich.) overnight, adjusted to an optical density of 0.75 at 460 nm, and serially diluted (10 times) in medium. Titrated numbers of Listeria were inoculated intraperitoneally into mice in a volume of 500 μl. The doses of bacteria were monitored by plating serially diluted aliquots on Columbia blood agar.
Transfection of F4/80 cDNA.
For determination of the molecules detected by BM8 MAb and F4/80 MAb, 293T cells (2 × 106) were transiently transfected (240 V, 960 μF; Bio-Rad Gene Pulser; Bio-Rad, Munich, Germany) in complete medium with a full-length F4/80 cDNA cloned into the pcDNA3 expression vector (10 μg) and plated onto glass slides in the presence of complete medium. After 24 h cells were washed and fixed with 4% paraformaldehyde (Merck) in phosphate-buffered saline. Blocking of nonspecific antibody binding was performed with bovine serum albumin (Merck) and avidin-biotin blocking kit (Vector Labs, Burlingame, Calif.). Then, incubation with F4/80 or BM8-biotin was performed. Detection of the primary antibodies was achieved with mouse anti-rat peroxidase and peroxidase-extravidin, respectively. The substrate for peroxidase was aminoethylcarbazol (Sigma). Cell nuclei were counterstained with hematoxylin (Merck).
Detection of Cre-mediated recombination.
F4/80+/Cre mice were crossed to a mouse line with a floxed ikba locus (IκBα2loxP/2loxP; R. Rupec and K. Pfeffer, unpublished data) to detect site-specific Cre recombination activity. The floxed allele (2loxP) and the wild-type allele were discriminated by PCR with primer set lox1 (5′-GTG GAG TCA GAT GTA GCA CG-3′) and lox2 (5′-AGA AAG GGA TAA GCC ATG GAG-3′); expected sizes of the PCR products are 363 bp for the 2loxP allele and 275 bp for the wild-type allele. The recombined allele (1loxP) was detected with the primer set 1loxP1 (5′-CAT ACT TCC AAG CAG AGA CGT3′) and 1loxP2 (5′-TAC GTT CGG AGT TTA AGA CTC −3′) (expected size, 526 bp), whereas the wild-type allele (wt) was amplified by using the primers 2loxP1 (5′-GAA GTC ATT GGT CAG GTG AAG −3′) and wt2 (5′-GCA AAC AGG AGG CCC TAG GT −3′) (expected size, 257 bp). The sequence of primer wt2 is located upstream and downstream of the inserted loxP site in the 2loxP IκBα allele; hence, only the wild-type allele can be detected by the combination of primers 2loxP1 and wt2.
Nucleotide sequence accession number.
The gene sequence for the genomic clone isolated in this study was deposited in the GenBank/EMBL database under accession number AJ295275.
RESULTS
Generation of mice targeted for inactivation of F4/80.
In order to inactivate the F4/80 molecule in the germ line of the mouse and to enable Cre-mediated deletion of loxP-flanked target DNA sequences (“floxed” alleles [17, 38]) in F4/80-expressing cells, an approach was chosen to replace the 3′ region of the first coding exon of F4/80 directly following the ATG translation start codon with the coding sequence for the P1 phage-derived Cre recombinase. Therefore, a F4/80 targeting vector was constructed and used for homologous recombination (for details, see Materials and Methods) (Fig. 1A and B). Correct gene targeting (Fig. 1B) and germ line transmission (Fig. 1C) of the F4/80 Cre knock-in allele were verified by Southern blotting. Hybridization with a neo-specific probe yielded only one band, consistent with a single integration of the targeting vector (data not given). F4/80+/Cre offspring were interbred to obtain homozygous F4/80Cre/Cre animals, which are thereby deficient for F4/80 expression. Mice with the F4/80 inactivation were born at the expected Mendelian frequency, appeared healthy, and proved to be fertile. To verify proper inactivation of the F4/80 molecule, Northern blot analysis was performed. As depicted in Fig. 1D, F4/80 mRNA was readily found in F4/80+/+ and F4/80+/Cre animals, whereas F4/80 mRNA was not detected in F4/80Cre/Cre (i.e., F4/80−/−) animals.
Lymphocyte populations and T-cell-independent immune response in F4/80 deficient animals.
To determine the functional consequences of the inactivation of the F4/80 gene, lymphocyte populations were analyzed by flow cytometry. No significant differences in T-cell, B-cell, and natural killer cell populations could be detected in F4/80-deficient animals (data not given).
While it has been suggested that splenic marginal zone macrophage populations are involved in T-cell-independent antibody responses (1, 2, 25), no data about macrophages expressing the F4/80 molecule are available. Thus, the immunological response of F4/80-deficient mice to T-cell-independent antigens was investigated by immunization of F4/80-deficient and control mice with nitrophenyl (NP)-coupled Ficoll and subsequent monitoring of the immune response towards the hapten NP on days 6 and 10. In the sera of F4/80-deficient animals slightly reduced levels of specific anti-NP antibodies of the IgM and IgG3 subclasses were detected; however, the differences appeared not to be significant (data not shown). This result indicates that the presence of peripheral lymphocyte populations and T-cell-independent antibody responses are not affected by the absence of the F4/80 molecule from mature tissue macrophage populations.
Infection with Listeria in the absence of F4/80.
Neutrophils and macrophages are important cell populations required for the control of listeria replication during infection (12-14, 47). To investigate whether in particular the macrophage-expressed F4/80 molecule is involved in the defense against intracellular bacteria or if the F4/80 molecule participates in the localization or activation of infected macrophages, F4/80-deficient and control mice were infected with titrated amounts of L. monocytogenes. The survival of infected mice was monitored up to day 15 postinfection. However, no significant differences in the defense against Listeria could be detected; all F4/80-deficient animals succumbed to a 10× 50% lethal dose (LD50) inoculation of Listeria, whereas all mice survived a challenge dose of 0.1× LD50 (data not shown). This clearly indicates that F4/80-targeted animals were neither immunodeficient nor better protected than normal F4/80-expressing littermates in response to a Listeria infection.
The F4/80 molecule is also the antigen detected by the BM8 MAb.
Macrophage subpopulations can be characterized by the differential expression of specific markers (16, 26, 48). Therefore, the expression of macrophage-associated molecules in F4/80-deficient animals was analyzed in detail. When cryosections of spleens and livers were incubated with the F4/80 (anti-F4/80) MAb, as expected, no staining could be detected in the tissues of F4/80-deficient tissues whereas the characteristic labeling of Kupffer cells in the livers and red pulp macrophages in the spleens of normal mice was readily obtained.
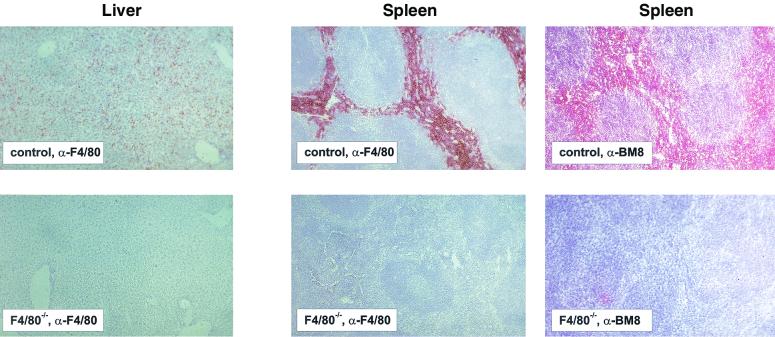
Surprisingly, labeling with the BM8 MAb (29) was also found to be negative in the absence of F4/80 expression, suggesting that the hitherto unidentified target structure of the BM8 MAb is also the F4/80 molecule (Fig. 2). Conventional histology of liver tissue from F4/80-deficient mice revealed that Kupffer cells are still present, as revealed by the distinct morphology of the nuclei of Kupffer cells (data not shown), indicating that the differentiation of Kupffer cells was not affected by the absence of F4/80. In the spleens of F4/80-deficient mice, labeling with anti-CR3 (CD11b/CD18) (M1/70 MAb), antisialoadhesin (Ser4, 3D6), and anti-MOMA1 was detectable and not altered compared to the labeling observed with control mice, indicating that the selective absence of the F4/80 molecule does not influence the presence of these other macrophage cell surface markers (data not shown), nor indeed does it influence the development of the resident tissue macrophage populations that express these lineage-restricted molecules.
FIG. 2.
Absence of F4/80 and BM8 antigen in organs of F4/80-deficient mice. Immunohistochemical staining of liver and spleen sections from control mice (top panels) and F4/80-deficient mice (lower panels) is shown. Cryosections were labeled with MAbs as indicated. Original magnification, ×100.
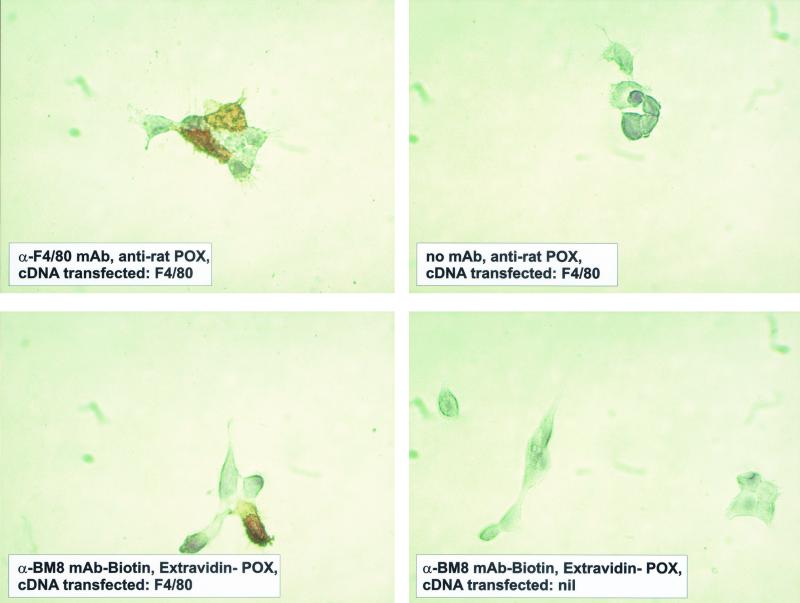
To prove that the BM8 MAb detects the F4/80 molecule, 293T cells were transiently transfected with a F4/80 cDNA expression construct and labeled with BM8 and F4/80 MAbs. As shown in Fig. 3, nontransfected 293T cells cannot be labeled by anti-BM8 or anti-F4/80 whereas after transfection of the F4/80 cDNA, anti-BM8 and anti-F4/80 labeling of 293T cells could be readily demonstrated. This clearly proves that the hitherto-undefined BM8 MAb detects the F4/80 glycoprotein.
FIG. 3.
The antigen detected by F4/80 and BM8 antibodies is identical. 293T cells were transiently transfected with a pcDNA3-based expression vector containing the complete coding sequence of F4/80. Cells were labeled with the respective MAbs as indicated.
Cre-driven recombination in F4/80+/Cre mice.
For the examination of tissue- and/or cell lineage-specific gene functions, the Cre/loxP recombination system has been established (17, 34, 38). Hereby, as a prerequisite, Cre-expressing mouse lines that express Cre recombinase under the control of lineage or cell type-specific promoters have to be generated. These mouse lines can then be crossed to another mouse line, harboring a modified (loxP motif flanked) sequence of the gene of particular interest (2loxP allele). One approach to achieving specific expression of Cre recombinase activity is the targeted insertion (knock-in) of the Cre-cDNA sequence into the locus of a gene expressed by a specific cell type.
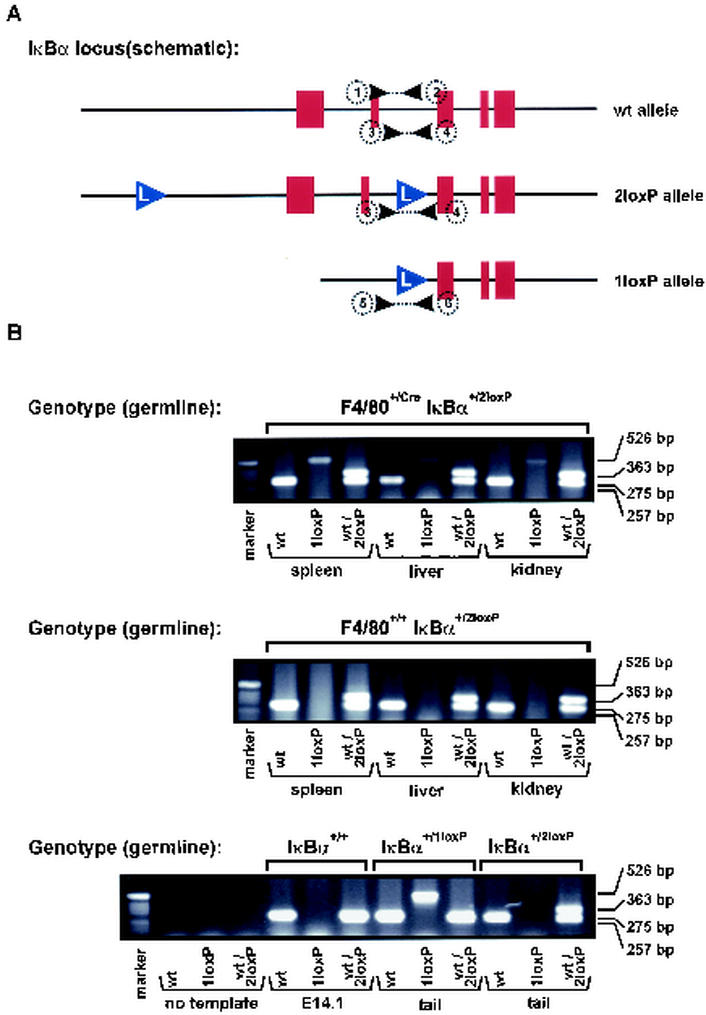
To achieve Cre expression in F4/80+ cells, the Cre cDNA sequence was used to replace the coding sequence of the first exon of F4/80, directly downstream of the ATG initiation codon (Fig. 1A to D). To verify Cre-mediated recombination, the F4/80+/Cre mouse line was crossed to an IκBα+/2loxP line as a reporter. PCR amplification utilizing specific primers to detect the wild-type IκBα, the 2loxP-flanked IκBα allele, and the Cre-recombined 1loxP IkBα allele was performed (Fig. 4 ). While in (F4/80+/+ × IκBα+/2loxP)F1 mice no recombination of the IκBα 2loxP allele could be detected, clearly an amplified PCR product specific for the IkBα 1loxP allele was present in (F4/80+/Cre × IκBα+/2loxP)F1 mice, indicating that Cre recombinase activity expressed under the control of the F4/80 promoter was present. Importantly, when utilizing the primer sets that detect the IκBα 1loxP allele, no amplified product could be found in the tail DNA of (F4/80+/Cre × IκBα+/2loxP)F1 mice, indicating that F4/80 is not ubiquitously expressed during ontogeny and that F4/80-Cre mice can hopefully be employed in the future to analyze gene functions in this specific macrophage subset.
FIG. 4.
Cre-mediated recombination in F4/80+/Cre mice crossed with IκBα+/2loxP mice. (A) Schematic structure of the wild-type and floxed murine ikba locus; exons (red), loxP motifs (blue triangle), and PCR primers (number and black triangle, see Materials and Methods) are indicated. (B) Spleen, livers, and kidneys from (F4/80+/Cre × IκBα+/2loxP)F1 (upper panel) and (F4/80+/+ × IkBα+/2loxP)F1 (middle panel) mice wereanalyzed for Cre recombinase activity. In DNA isolated from spleen, liver, and kidney tissues of (F4/80+/Cre × IκBα+/2loxP)F1 mice, a PCR product that is characteristic for the Cre-mediated deletion at the ikba locus was amplified, whereas in DNA isolated from spleen, liver, and kidney tissues of (F4/80+/+ × IκBα+/2loxP)F1 mice only the germ line configuration of the IkBα alleles can be detected. Results of control PCRs using template DNA derived from wild-type ES cells or tail DNA from F4/80+/+ IκBα+/1loxP and F4/80+/+ IκBα+/2loxP mice are shown in the lower panel as indicated.
DISCUSSION
Macrophages play a crucial role in the innate and adaptive immune response. A number of specialized cell surface molecules have been identified and proposed to participate in macrophage-initiated functional responses orchestrating the initiation and effector phases of host immunity (16, 26, 29). A prototypic macrophage membrane molecule, highly restricted to mature macrophage subpopulations residing in tissue, is the F4/80 glycoprotein (3, 31). Gene targeting now reveals that the F4/80 molecule is also recognized by the MAb BM8 (24, 29), reported to label a concordant set of macrophages, the target antigen for which, however, remained elusive until now. Surprisingly, expression of F4/80 is dispensable for host effector programs following infection with Listeria and the generation of T-cell-independent B-cell responses. However, introduction of the Cre recombinase cDNA into the first coding exon of F4/80 now enables the lineage-specific ablation of loxP motif-flanked (floxed) target genes in mature macrophages. The strategy of inactivation of a gene product restricted to tissue combined with the simultaneous expression of Cre recombinase activity (Cre knock-in strategy [38]) is instrumental in addressing gene functions in defined cellular subpopulations in vivo.
Macrophage subsets have been characterized by the expression of restricted cell surface molecules. The macrophage mannose receptor, the macrophage scavenger receptors (SRA-I, SRA-II), the CR3 (CD11b/CD18), the 7/4 glycoprotein, the sialoadhesin molecule, and the FA.11 antigen have been widely applied as discriminatory markers for the characterization of macrophage subsets (16, 26, 35). The assignment of specific biological functions for this distinct group of cell surface glycoprotein antigens in vivo has proven difficult; the ligands of these molecules show promiscuous binding patterns or are, in most cases, still undefined (16, 44, 45). In vivo administration of antibodies directed against cell surface molecules for elucidation of their functions is not a feasible approach, since antibodies in most cases are cytotoxic to the antigen-expressing cell population due to complement activation in vivo. Thus, inactivation of the gene of interest, which is expressed by the macrophage subset via gene targeting, appears to be the most straightforward way for the characterization of the specific function of the marker molecule of interest. Gene targeting of the 7/4 glycoprotein, which is expressed on polymorphonuclear cells as well as on activated macrophages, of sialoadhesin, expressed on stromal macrophages, and of FA.11, expressed on macrophages and dendritic cells (16), has not yet been reported. The inactivation of CD18, however, has been published (41). The phenotype is described as leukocyte adhesion deficiency I and is not restricted to macrophage dysfunction, since the CD18 molecule is not only expressed on macrophages but also found on other leukocyte populations and thus most likely reflects a composite deficiency, encompassing lack of T-cell responses (41). The specific functions of the CD18 molecule on macrophages remain to be addressed; for example, by specifically deleting the CD18 gene in macrophages employing a Cre/loxP-mediated approach by using F4/80-Cre mice (see below) with CD182loxP/2loxP mice; a mouse line will have to be generated for this purpose in the future. Mice deficient in CD11b reveal a moderate defect in adhesion of neutrophils to fibrinogen and neutrophil degranulation; however, the phenotype is very discrete compared to that of mice deficient in CD18, which can form heterodimers not only with CD11b but also with CD11a, CD11c, and CD11d (11, 28). Since these molecules are not restricted to macrophages, the F4/80 glycoprotein appeared to be primarily the most attractive candidate for functional characterization and the generation of a Cre-expressing line for macrophage-restricted gene ablation.
Experimental infection with L. monocytogenes, a gram-positive facultative intracellular bacterium, has been employed extensively as a model system for the investigation of host defense mechanisms (12-14, 37, 40, 47). Activated macrophages are essential for the control of Listeria replication. However, no gross alteration of the resistance towards Listeria in F4/80-deficient mice was found, indicating that the F4/80 molecule is not essential for survival after challenge with Listeria. Furthermore, the formation of Listeria-induced granulomatous foci in the livers of F4/80-deficient animals was not altered in comparison to that observed in control mice, indicating that the recruitment, activation, and maturation of peripheral blood circulation-derived CD11b+ neutrophils and macrophages into tissues of infected organs can occur even in the absence of F4/80 (data not shown).
Macrophages have also been implicated in the generation of T-cell-independent B-cell immune responses (1, 18, 25). The role of the F4/80 molecule on splenic macrophages which are located in the red pulp, directly surrounding the marginal zone, in T-cell-independent B-cell responses was not known. Based on the results obtained in this study, a major role of the F4/80 glycoprotein can be ruled out, since immunization with NP-Ficoll in the absence of F4/80 resulted in normal generation of specific anti-NP antibodies of the T-cell-independent IgM and IgG3 subclasses.
The murine F4/80 glycoprotein, as a member of the EGF-TM7 family (30, 44), shares sequence and structural homology with another EGF-TM7 family members (6, 27, 45). Due to the expression pattern of the EGF-TM7 family members, which include macrophages, it might be speculated that a single deficiency of an EGF-TM7 member does not yet lead to a functional deficiency, since other members of the family may readily compensate for the loss. In order to verify this hypothesis in the future, a systematic targeting approach of the other EGF-TM7 members has to be performed and the gene-targeted mouse lines have to be crossed. Alternatively, since at least some genes of the family members are located in close proximity (on human chromosome 19p13.1) (7, 27), a targeting strategy removing most members within the syntenic region of the mouse genome might be feasible via a Cre/loxP-mediated excision after flanking the EGF-TM7 loci with loxP motifs via gene targeting (38, 49).
The study of the function of gene products in discrete cell types or cell lineages is of great interest for dissecting the roles of distinct molecules in a specific cellular microenvironment. This approach will lead to a better understanding of gene functions, in contrast to the global deficiencies that are generated by conventional gene-targeting techniques. In particular, a conditional inactivation is of crucial importance for cases in which global genetic deficiencies would result in lethality due to functional checkpoints during ontogeny. However, these conditional gene ablation strategies essentially depend on the availability of mouse lines harboring lineage-restricted expression of heterologous site-specific recombinases. In this respect, mainly the introduction of the bacteriophage P1-derived Cre recombinase into the mouse genome has been pioneered (17, 34). Recently, Cre-expressing mouse lines enabling conditional gene ablation in myeloid cell types (LysM-Cre mice) or in neutrophils (Elastase-Cre) have been reported (9, 46). For studying gene functions in mature macrophages, however, no Cre-expressing line has been available. By targeting the F4/80 locus via the introduction of the Cre cDNA into the first coding exon of the F4/80 gene, enabling F4/80 promoter controlled Cre expression, this goal has now been achieved. A knock-in strategy was used to maintain the F4/80 locus-specific regulation of the inserted Cre recombinase. After crossing the F4/80+/Cre line with a mouse line harboring a loxP motif-flanked locus (IκBα2loxP/2loxP), F1 mice with the genotype F4/80+/Cre IκBα+/2loxP showed a recombined band characteristic of the Cre/loxP-mediated deletion of the ikba locus. However, the loxP motif-flanked (2loxP) IκBα allele was still readily detectable in all investigated tissues, indicating that the F4/80 molecule is not expressed ubiquitously at some point during ontogeny, which would be characteristic for a “deleter” mouse line (42), resulting in the sole presence of a 1loxP IκBα allele. Thus, the F4/80-Cre knock-in mouse line will hopefully prove useful for addressing gene functions in mature macrophages in the future.
Acknowledgments
We thank Karin Mink, Jennifer Meinecke, Agnes Fütterer, Birgit Eckelt, Angela Servatius, and Nadja Sandholzer for excellent technical help, Heinrich Flaswinkel for support with flow cytometry, as well as Jens Würthner and Sandra Beer for critical reading of the manuscript. The continuous and generous support of H. Wagner is highly appreciated.
This work was supported by the DFG (SFB 391, 576, Pf259/2-5/6, RU601/3-1) and by the Medical Research Council, United Kingdom.
REFERENCES
- 1.Amlot, P. L., D. Grennan, and J. H. Humphrey. 1985. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur. J. Immunol. 15:508-512. [DOI] [PubMed] [Google Scholar]
- 2.Amlot, P. L., and A. E. Hayes. 1985. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet i:1008-1011. [DOI] [PubMed]
- 3.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. [DOI] [PubMed] [Google Scholar]
- 4.Baud, V., S. L. Chissoe, E. Viegas-Pequignot, S. Diriong, V. C. N′Guyen, B. A. Roe, and M. Lipinski. 1995. EMR1, an unusual member in the family of hormone receptors with seven transmembrane segments. Genomics 26:334-344. [DOI] [PubMed] [Google Scholar]
- 5.Beller, D. I., T. A. Springer, and R. D. Schreiber. 1982. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J. Exp. Med. 156:1000-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caminschi, I., K. M. Lucas, M. A. O'Keeffe, H. Hochrein, Y. Laabi, F. Kontgen, A. M. Lew, K. Shortman, and M. D. Wright. 2001. Molecular cloning of F4/80-like-receptor, a seven-span membrane protein expressed differentially by dendritic cell and monocyte-macrophage subpopulations. J. Immunol. 167:3570-3576. [DOI] [PubMed] [Google Scholar]
- 7.Carver, E. A., J. Hamann, A. S. Olsen, and L. Stubbs. 1999. Physical mapping of EMR1 and CD97 in human Chromosome 19 and assignment of Cd97 to mouse Chromosome 8 suggest an ancient genomic duplication. Mamm. Genome 10:1039-1040. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.Clausen, B. E., C. Burkhardt, W. Reith, R. Renkawitz, and I. Forster. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265-277. [DOI] [PubMed] [Google Scholar]
- 10.Crocker, P. R., and S. Gordon. 1989. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J. Exp. Med. 169:1333-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunne, J. L., C. M. Ballantyne, A. L. Beaudet, and K. Ley. 2002. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood 99:336-341. [DOI] [PubMed] [Google Scholar]
- 12.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425-431. [DOI] [PubMed] [Google Scholar]
- 13.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7:419-432. [DOI] [PubMed] [Google Scholar]
- 14.Falkow, S., R. R. Isberg, and D. A. Portnoy. 1992. The interaction of bacteria with mammalian cells. Annu. Rev. Cell Biol. 8:333-363. [DOI] [PubMed] [Google Scholar]
- 15.Futterer, A., K. Mink, A. Luz, M. H. Kosco-Vilbois, and K. Pfeffer. 1998. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity 9:59-70. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, S., L. Lawson, S. Rabinowitz, P. R. Crocker, L. Morris, and V. H. Perry. 1992. Antigen markers of macrophage differentiation in murine tissues. Curr. Top. Microbiol. Immunol. 181:1-37. [DOI] [PubMed] [Google Scholar]
- 17.Gu, H., J. D. Marth, P. C. Orban, H. Mossmann, and K. Rajewsky. 1994. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 265:103-106. [DOI] [PubMed] [Google Scholar]
- 18.Guinamard, R., M. Okigaki, J. Schlessinger, and J. V. Ravetch. 2000. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 1:31-36. [DOI] [PubMed] [Google Scholar]
- 19.Hamann, J., C. van Zeventer, A. Bijl, C. Molenaar, K. Tesselaar, and R. A. van Lier. 2000. Molecular cloning and characterization of mouse CD97. Int. Immunol. 12:439-448. [DOI] [PubMed] [Google Scholar]
- 20.Hamann, J., B. Vogel, G. M. van Schijndel, and R. A. van Lier. 1996. The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J. Exp. Med. 184:1185-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hume, D. A., and S. Gordon. 1983. Mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Identification of resident macrophages in renal medullary and cortical interstitium and the juxtaglomerular complex. J. Exp. Med. 157:1704-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hume, D. A., J. F. Loutit, and S. Gordon. 1984. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J. Cell Sci. 66:189-194. [DOI] [PubMed] [Google Scholar]
- 23.Hume, D. A., A. P. Robinson, G. G. MacPherson, and S. Gordon. 1983. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J. Exp. Med. 158:1522-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraal, G., M. Rep, and M. Janse. 1987. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. An immunohistochemical study. Scand. J. Immunol. 26:653-661. [DOI] [PubMed] [Google Scholar]
- 25.Kraal, G., H. Ter Hart, C. Meelhuizen, G. Venneker, and E. Claassen. 1989. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur. J. Immunol. 19:675-680. [DOI] [PubMed] [Google Scholar]
- 26.Leenen, P. J., M. F. de Bruijn, J. S. Voerman, P. A. Campbell, and W. van Ewijk. 1994. Markers of mouse macrophage development detected by monoclonal antibodies. J. Immunol. Methods 174:5-19. [DOI] [PubMed] [Google Scholar]
- 27.Lin, H. H., M. Stacey, J. Hamann, S. Gordon, and A. J. McKnight. 2000. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics 67:188-200. [DOI] [PubMed] [Google Scholar]
- 28.Lu, H., C. W. Smith, J. Perrard, D. Bullard, L. Tang, S. B. Shappell, M. L. Entman, A. L. Beaudet, and C. M. Ballantyne. 1997. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J. Clin. Investig. 99:1340-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malorny, U., E. Michels, and C. Sorg. 1986. A monoclonal antibody against an antigen present on mouse macrophages and absent from monocytes. Cell Tissue Res. 243:421-428. [DOI] [PubMed] [Google Scholar]
- 30.McKnight, A. J., and S. Gordon. 1996. EGF-TM7: a novel subfamily of seven-transmembrane-region leukocyte cell-surface molecules. Immunol. Today 17:283-287. [DOI] [PubMed] [Google Scholar]
- 31.McKnight, A. J., A. J. Macfarlane, P. Dri, L. Turley, A. C. Willis, and S. Gordon. 1996. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J. Biol. Chem. 271:486-489. [DOI] [PubMed] [Google Scholar]
- 32.Nechiporuk, T., L. D. Urness, and M. T. Keating. 2001. ETL, a novel seven-transmembrane receptor that is developmentally regulated in the heart. ETL is a member of the secretin family and belongs to the epidermal growth factor-seven-transmembrane subfamily. J. Biol. Chem. 276:4150-4157. [DOI] [PubMed] [Google Scholar]
- 33.Neubauer, H., A. Cumano, M. Muller, H. Wu, U. Huffstadt, and K. Pfeffer. 1998. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93:397-409. [DOI] [PubMed] [Google Scholar]
- 34.Orban, P. C., D. Chui, and J. D. Marth. 1992. Tissue- and site-specific DNA recombination in transgenic mice. Proc. Natl. Acad. Sci. USA 89:6861-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peiser, L., S. Mukhopadhyay, and S. Gordon. 2002. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14:123-128. [DOI] [PubMed] [Google Scholar]
- 36.Perry, V. H., D. A. Hume, and S. Gordon. 1985. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15:313-326. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 38.Rajewsky, K., H. Gu, R. Kuhn, U. A. Betz, W. Muller, J. Roes, and F. Schwenk. 1996. Conditional gene targeting. J. Clin. Investig. 98:600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen, H., and S. Gordon. 1987. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J. Exp. Med. 166:1685-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 41.Scharffetter-Kochanek, K., H. Lu, K. Norman, N. van Nood, F. Munoz, S. Grabbe, M. McArthur, I. Lorenzo, S. Kaplan, K. Ley, C. W. Smith, C. A. Montgomery, S. Rich, and A. L. Beaudet. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwenk, F., U. Baron, and K. Rajewsky. 1995. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23:5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Springer, T., G. Galfre, D. S. Secher, and C. Milstein. 1979. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur. J. Immunol. 9:301-306. [DOI] [PubMed] [Google Scholar]
- 44.Stacey, M., H. H. Lin, S. Gordon, and A. J. McKnight. 2000. LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors. Trends Biochem. Sci. 25:284-289. [DOI] [PubMed] [Google Scholar]
- 45.Stacey, M., H. H. Lin, K. L. Hilyard, S. Gordon, and A. J. McKnight. 2001. Human epidermal growth factor (EGF) module-containing mucin-like hormone receptor 3 is a new member of the EGF-TM7 family that recognizes a ligand on human macrophages and activated neutrophils. J. Biol. Chem. 276:18863-18870. [DOI] [PubMed] [Google Scholar]
- 46.Tkalcevic, J., M. Novelli, M. Phylactides, J. P. Iredale, A. W. Segal, and J. Roes. 2000. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity 12:201-210. [DOI] [PubMed] [Google Scholar]
- 47.Unanue, E. R. 1997. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr. Opin. Immunol. 9:35-43. [DOI] [PubMed] [Google Scholar]
- 48.Yamada, Y., T. Doi, T. Hamakubo, and T. Kodama. 1998. Scavenger receptor family proteins: roles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. 54:628-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, Y., and A. Bradley. 2001. Engineering chromosomal rearrangements in mice. Nat. Rev. Genet. 2:780-790. [DOI] [PubMed] [Google Scholar]