Abstract
The O acetylation of peptidoglycan occurs specifically at the C-6 hydroxyl group of muramoyl residues. Using a combination of high-performance liquid chromatography-based organic acid analysis and carbohydrate analysis by high-pH anion-exchange chromatography, we determined that strains of Entercoccus durans, E. faecalis, E. faecium, and E. hirae produce O-acetylated peptidoglycan. The levels of O acetylation ranged from 19% to 72% relative to the muramic acid content, and they were found to vary with the growth phase of the culture. Increases of 10 to 40% in O acetylation were observed with cultures entering the stationary phase. Cells of E. faecalis in the viable but nonculturable (VBNC) state had the highest levels of peptidoglycan O acetylation. The presence of this modification to peptidoglycan was shown to inhibit the action of hen egg white lysozyme in a concentration-dependent manner. Zymography using sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels containing either O-acetylated or chemically de-O-acetylated peptidoglycan was used to monitor the production of specific autolysins in E. faecalis. Differences in the expression of specific autolysins were observed with the age of the culture, and VBNC E. faecalis produced the highest levels of these enzymes. This technique also permitted classification of the enterococcal autolysins into enzymes that preferentially hydrolyze either O-acetylated or non-O-acetylated peptidoglycan and enzymes that show no apparent preference for either substrate type.
The gram-positive, gut commensal bacterium Enterococcus faecalis is the causative agent of 90% of all enterococcal infections, which include endocarditis, bacteremia, and urinary tract infections. Of these, urinary tract infections are the most common, and the majority of them are acquired nosocomially. E. faecalis infections pose problems for the clinician because of their resistance to multiple antibiotics, sometimes including vancomycin, the drug of last resort for many gram-positive infections (recently reviewed in references 14, 19, and 32). To exacerbate this situation, this bacterium has been shown to persist in a mouse model for extended periods of time in the kidney (26). This persistence is thought to result from the cell's ability to enter the viable but nonculturable (VBNC) state, a feature first described for E. faecalis by Lleo et al. (30). VBNC E. faecalis, like other VBNC pathogenic bacteria, appears to retain its pathogenicity genes and is able to resume active growth upon restoration of the optimal environmental conditions (reference 30 and references therein). While in this state, E. faecalis cells have an irregular morphology, and there are concomitant alterations in the molecular architecture of the peptidoglycan sacculus (39). However, the changes observed in the peptidoglycan composition do not readily account for the persistence associated with the VBNC enterococci. One possible explanation for the apparent resilience of VBNC E. faecalis could involve a modification to the peptidoglycan, such as its O acetylation, that has not been characterized previously.
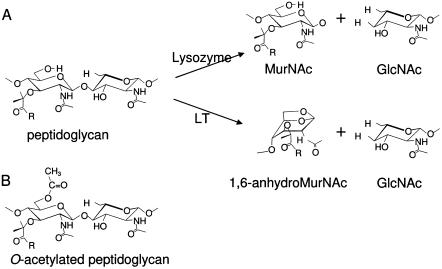
The O acetylation of peptidoglycan occurs on the C-6 hydroxyl moiety of component N-acetylmuramoyl residues, producing the corresponding N,O-diacetylmuramoyl derivatives in the bacterial cell wall heteropolymer (Fig. 1). To date, a total of 28 species of both gram-positive and gram-negative bacteria are known to modify their peptidoglycan in this manner. These species include a number of important human pathogens, such as Staphylococcus aureus, Bacillus anthracis, Campylobacter jejuni, Helicobacter pylori, Neisseria gonorrhoeae, and all species of Proteus, Providencia, and Morganella (11, 12, 44). For each species and strain examined, the extent of peptidoglycan O acetylation is not stoichiometric but instead ranges typically between 20% and 70% relative to the muramic acid content. Nonetheless, this modification has been shown to inhibit the lytic action of the muramidases (lysozymes) associated with the innate immune system in a concentration-dependent manner (6, 16, 35, 36, 41). The O acetylation of peptidoglycan has also been demonstrated to inhibit the action of the lytic transglycosylases (5), which are ubiquitous bacterial enzymes associated with peptidoglycan metabolism (both biosynthesis and turnover) (4). The latter enzymes act like muramidase to cleave the β-1,4 linkage between N-acetylmuramoyl and N-acetylglucosaminyl residues, but instead of catalyzing a hydrolytic reaction, they produce a 1,6-anhydromuramoyl product (21). Thus, the catalytic mechanism of the lytic transglycosylases requires a free C-6 hydroxyl group on the N-acetylmuramoyl substrate (Fig. 1). For this reason, O acetylation has been proposed to serve as one level of control for peptidoglycan metabolism (33).
FIG. 1.
Structure of peptidoglycans and their lysis by lysozyme and lytic transglycosylases. (A) Lysis of peptidoglycan by lysozyme and lytic transglycosylases (LT), resulting in the release of GlcNAc and either MurNAc or 1,6-anhydroMurNAc residues. (B) Structure of O-acetylated peptidoglycan. The presence of the C-6 acetyl moiety on muramoyl residues precludes the formation of 1,6-anhydromuramoyl products by lytic transglycosylases, while it also sterically hinders the binding of lysozyme. R, stem peptides associated with MurNAc residues.
In the current study, the presence and extent of peptidoglycan O acetylation were determined for four clinically important species of enterococci, E. faecalis, E. faecium, E. hirae, and E. durans. In addition, the role of O acetylation in the VBNC state of E. faecalis was investigated.
MATERIALS AND METHODS
Materials.
Ferric ammonium citrate was purchased from Fisher Scientific (Nepean, ON, Canada), and sodium azide was purchased from Acros Organics (Geel, Belgium). Tryptic soy growth medium, oxgall, esculin, and all other medium supplements were provided by Difco Laboratories (Detroit, MI), while all other chemicals and reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Bacteria and growth conditions.
Most of the bacterial strains used in this study (Table 1) were obtained from the American Type Culture Collection, Rockville, Md.; the only exception was the E. durans strain, which was obtained from the culture collection of the Department of Molecular and Cellular Biology (formerly Department of Microbiology), University of Guelph. To obtain pure viable cultures, lyophilized stocks of each species and strain were resuspended in 5 ml of tryptic soy broth (TSB), diluted 1/20 into fresh TSB, and incubated overnight at 37°C with shaking (200 rpm). Samples of each culture were then serially diluted to a dilution of 10−7, and 100-μl aliquots were spread onto selective Bacto bile esculin azide (BBEA) agar (15). Cell growth was monitored turbidometrically by determining the optical density at 600 nm (OD600) using a DU530 LifeScience UV/visible spectrophotometer (Beckman Coulter, Mississauga, ON, canada).
TABLE 1.
Extents of peptidoglcyan O acetylation in the entercocci
| Species | Strain | % Acetylationa
|
||
|---|---|---|---|---|
| Mid-exponential | Stationary | VBNC | ||
| Enterococcus faecalis | ATCC 19433 (type strain) | 22.4 ± 0.58b | 35.5 ± 1.6 | 44.1 ± 0.051 |
| NRC 799 | 19.1 ± 0.65 | 48.9 ± 2.9 | 65.1 ± 2.3 | |
| NRC 8023 | 58.3 ± 0.48 | 55.6 ± 2.1 | 72.1 ± 1.7 | |
| Enterococcus faecium | NRC 8012 | 46.3 ± 2.6 | 57.1 ± 4.4 | NAc |
| Enterococcus hirae | ATCC 9790 (type strain) | 43.7 ± 1.7 | 68.3 ± 1.0 | NA |
| ATCC 8043 | 35.2 ± 1.5 | 59.7 ± 1.2 | NA | |
| ATCC 10541 | 26.2 ± 1.5 | 67.9 ± 1.3 | NA | |
| Enterococcus durans | MCB 238 | 25.2 ± 2.0 | 37.8 ± 1.6 | NA |
Data are expressed as percentages of the total muramic acid content in peptidoglycan samples.
The values are means ± standard deviations (n ≥ 3).
NA, not applicable.
To obtain mid-exponential-phase E. durans, E. faecalis, and E. hirae cultures for cell wall analysis, overnight cultures (OD600, 1.4) were diluted 100-fold into 3 liters of fresh TSB and incubated at 37°C until the OD600 was 0.7. Stationary-phase cultures were cultured to an OD600 of 1.4, at which point they were incubated for a further 12 h to ensure uniformity. For E. faecium, which grew more slowly and to a lower density than the other species under the same conditions, mid-exponential and stationary cultures were grown to OD600 of 0.4 and 0.9, respectively.
The method of Signoretto et al. (39) was used to prepare VBNC cells of E. faecalis. Briefly, an early-exponential-phase culture (OD600, 0.3) was resuspended in filter-sterilized raw (well) water (Guelph aquifer) that had been autoclaved twice at 121°C for 20 min to obtain a final concentration of 4 × 107 to 5 × 107 CFU/ml and incubated at 4°C for 16 to 21 days. To confirm the VBNC state and to check for possible contamination, two 50-ml samples of each strain were passed through a 0.22-μm filter, and the cells recovered on the filter were placed on either BBEA or tryptic soy agar plates and incubated at 37°C for 5 days. The remaining cells were harvested by centrifugation (15,000 × g, 4°C) and monitored by phase microscopy for the expected change to an irregular morphology.
Isolation and purification of peptidoglycan.
Cells were harvested by centrifugation (9,000 × g, 4°C) for 12 min and frozen overnight at −20°C. Whole-cell pellets were then resuspended in 100 ml 25 mM sodium phosphate buffer (pH 6.0). Insoluble peptidoglycan and associated teichoic acids were extracted using the boiling 4% sodium dodecyl sulfate (SDS) procedure described by Holye and Beveridge (22), taking care to preserve natural levels of O acetylation (9). The SDS-insoluble cell wall material (for convenience referred to below as peptidoglycan) was recovered by ultracentrifugation at 160,000 × g for 55 min at 25°C, washed at least four times with 25 mM sodium phosphate buffer (pH 6.0), and lyophilized.
Determination of the extent of peptidoglycan O acetylation.
Isolated insoluble peptidoglycan was resuspended in 25 mM sodium phosphate buffer (pH 6.0) and fragmented by continuous sonication for 4 min using a Heat Systems XL2020 sonicator. Samples of insoluble peptidoglycan were treated with 500 mM (final concentration) NaOH for 30 min at the ambient temperature to release any ester-linked acetate and then subjected to ultracentrifugation (100,000 × g, room temperature) using a Beckman Airfuge (Beckman Coulter, Mississauga, ON, Canada). Released acetate was quantified both by high-performance liquid chromatography-based organic acid analysis using an Aminex HPX-87H Bio-Rad column as previously described (9) and using a Megazyme acetic acid assay kit (Megazyme International Ireland Ltd., Wicklow, Ireland). The extent of O acetylation was expressed as a percentage of the muramic acid content, which was determined by quantitative amino sugar analysis (10) of associated insoluble peptidoglycan pellets after hydrolysis in 4 M HCl at 96°C in vacuo for 18 h.
Susceptibility of peptidoglycans to lysozyme hydrolysis.
Peptidoglycan was isolated and purified as a substrate from cultures of E. faecalis grown to the mid-exponential, stationary, and VBNC phases. Suspensions of the peptidoglycan samples were prepared by brief ultrasonication as described above. The turbidometric assay of Hash (20) was used to assess the susceptibilities of the purified peptidoglycan preparations in 50 mM sodium phosphate buffer (pH 6.0) to 200 μg/ml (final concentration) of hen egg white lysozyme (HEWL), as previously described (16). De-O-acetylated peptidoglycan was prepared by treating peptidoglycan with 40 mM NaOH for 2 h at room temperature, followed by adjustment of the pH to 6.0 and reisolation by ultracentrifugation (16).
Zymography of SDS-stable autolysins produced by E. faecalis and E. hirae.
Peptidoglycans isolated from both E. faecalis and E. hirae, together with the corresponding de-O-acetylated forms, were used to assay separately the autolysin content associated with whole-cell lysates of the bacteria by zymography. In some experiments involving suspension of the peptidoglycan substrate in SDS-polyacrylamide gel electrophoresis separating gels at the typical alkaline pH (pH 8.6), the method of Bernadsky et al. (3) and Watt and Clarke (43) was used. In other experiments, in which it was necessary to preserve the natural levels of O acetylation in peptidoglycan, zymograms in which peptidoglycan was incorporated into the resolving gel of a discontinuous SDS-polyacrylamide gel electrophoresis system that was operated at a slightly acidic pH were prepared as previously described (40). Thus, [bis(2-hydroxyethyl)imino]tris(hydroxymethyl)methane (Bis-Tris) buffers at pH 6.8 and 6.3 were used for the separating and stacking gels, respectively, while the pH of both the N-2-acetamido-2-hydroxyethanesulfonic acid (ACES)-HCl anode buffer and the Bis-Tris cathode buffer was 6.8.
RESULTS AND DISCUSSION
Extent of peptidoglycan O acetylation in enterococcal species.
The peptidoglycans from different strains of four common species of pathogenic enterococci grown to the mid-exponential phase were isolated by the boiling SDS protocol, taking care to maintain the pH below 7 and thereby preserve natural levels of O acetylation. Following lyophilization, the preparations were subjected to analysis for ester-linked acetate. As shown in Table 1, bacteria representing each of these four species of Enterococcus were found to O acetylate their peptidoglycans. To our knowledge, this is the first report of O acetylation of peptidoglycan in any species of enterococci. The extent of this modification, relative to the muramic acid content, was found to range broadly from 19 to 72%. As observed with other species for which a number of strains have been analyzed, such as N. gonorrhoeae (35, 41) and Proteus mirabilis (9, 11), the extent of O acetylation in E. faecalis and E. hirae was strain dependent. Also similar to the other O-acetylated bacteria that have been studied, the levels increased as the cells entered the stationary phase. The increases ranged from at least 10% for E. faecium NRC 8012 (an increase from 46% to 57%) to almost 40% for E. hirae ATCC 10541 (an increase from 26% to 68%).
Susceptibility to muramidase.
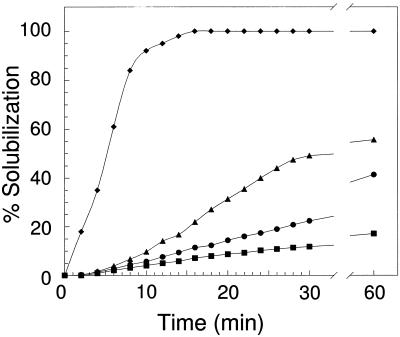
Consistent with the presence of ester-linked acetate, the enterococcal peptidoglycans were relatively resistant to hydrolysis by HEWL. As shown in Fig. 2, addition of HEWL to suspensions of insoluble peptidoglycan sacculi isolated from E. faecalis ATCC 19433 grown to the mid-exponential phase resulted in less than 20% solubilization within the first 15 min of the reaction, and the level reached approximately 50% after prolonged incubation. In contrast, prior removal of the O-acetyl groups from the peptidoglycan by mild-base hydrolysis resulted in complete solubilization within 15 min of enzyme addition under the same reaction conditions. It should be noted that the base hydrolysis treatment also removed any teichoic acid associated with the peptidoglycan, which, like acetate, is covalently attached at C-6 hydroxyl groups of muramoyl residues. Thus, both types of modification to the muramoyl residues likely contributed to the observed HEWL resistance. Nonetheless, peptidoglycans isolated from cells grown to the stationary phase and therefore having increased levels of O acetylation were even more resistant to HEWL-catalyzed hydrolysis, suggesting that this modification has an important role in conferring resistance. Similar observations were made with the other E. faecalis strains (data not shown).
FIG. 2.
Susceptibility of E. faecalis peptidoglycan to hen egg white lysozyme. Representative samples of peptidoglycan (approximately 0.5 mg/ml) in 50 mM sodium phosphate buffer (pH 6.0) were incubated at 25°C with 200 μg/ml (final concentration) of lysozyme, and the decrease in OD660 was monitored with time. The percentage of peptidoglycan solubilized was determined by comparison with the amount solubilized in an appropriate control to which lysozyme was not added. Peptidoglycans were isolated from E. faecalis ATCC 19433 grown to the mid-exponential phase (▴), the stationary phase (•), and the VBNC state (▪). ⧫, control (de-O-acetylated E. faecalis peptidoglycan prepared by incubation in 40 mM NaOH for 2 h).
These data are thus analogous to those obtained with both N. gonorrhoeae (6, 35, 36, 41) and P. mirabilis (9, 11, 16), in which resistance to HEWL was shown to be directly proportional to the levels of O acetylation. More importantly, however, the data help explain very early observations regarding large variations in the lytic response of enterococcal cells to HEWL (7, 42), which was later found to be dependent on the strain used and the growth phase from which the cells were obtained (17, 31). The fact that the previous studies were conducted using buffers with a basic pH further compromised the data due to the lability of the ester-linked acid in this environment. The observation that all enterococci are eventually lysed by mutanolysin, in contrast to the differential effects obtained with lysozyme, can also be accounted for by O acetylation because the action of mutanolysin (a muramidase produced by Streptomyces globisporus) is not affected by this modification to peptidoglycan. Finally, the fact that a single intravenous injection of mutanolysin resulted in complete resolution of acute arthritis and prevention of chronic joint disease induced by an athropathic dose of enterococcal peptidoglycan in a rat model (23) can also be accounted for by the presence of O acetylation. Although more active than HEWL, rat (and human) lysozyme does not efficiently hydrolyze O-acetylated peptidoglycan, thus permitting large fragments to persist in the mammalian host and thereby induce a variety of pathobiological effects (12, 16). The administration of mutanolysin, however, should lead to the immediate degradation of the circulating O-acetylated peptidoglycan sacculi into small fragments that may persist but are not arthropathic.
Autolysin profile of E. faecalis.
As we demonstrated that the most clinically relevant enterococcal species, E. faecalis, O acetylates its peptidoglycan, we were curious to know if this bacterium produces a complement of autolysins with various specificities for the modified cell wall polymer. To investigate this, we utilized the recently developed zymogram procedure involving acidic separating gels and running buffers (40). This system retains the natural levels of base-labile O acetylation on the peptidoglycan substrate throughout electrophoretic separation of protein samples, thereby permitting differentiation of any autolysins with specificity for the modified substrate.
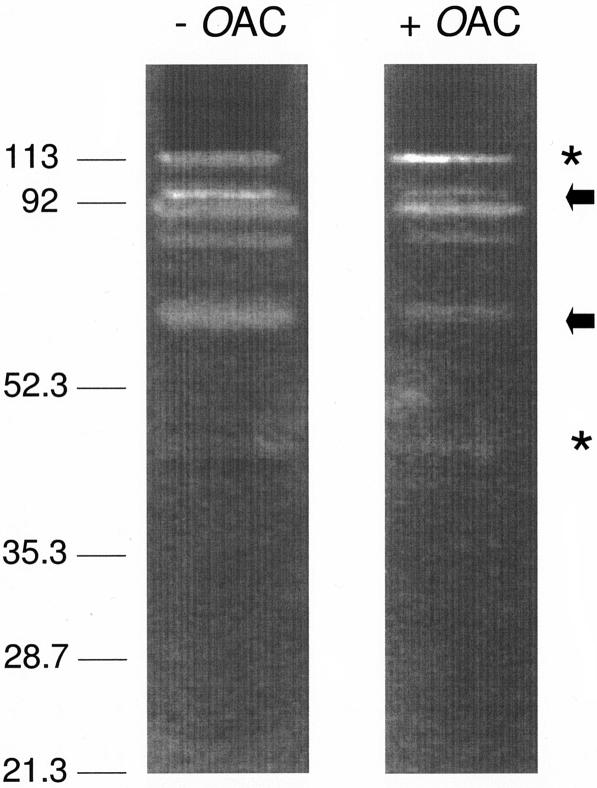
Identical samples of the total proteins present in E. faecalis ATCC 19433 grown to the late exponential phase were subjected to this electrophoretic system involving two separating gels, one containing O-acetylated E. faecalis peptidoglycan and the other containing de-O-acetylated material (prepared by mild-base hydrolysis). In both systems, at least five autolysin bands at apparent molecular masses of 105, 90.2, 88.0, 79.7, and 66.8 kDa were clearly visible in the separating gels counterstained with methylene blue (Fig. 3). Although this is the first report of E. faecalis autolysins, the presence of five lytic bands was somewhat surprising since only one major autolysin, a muramidase with an apparent molecular mass of 93.6 kDa, was previously known to be produced by E. hirae (formerly E. faecalis) (37). This E. hirae autolysin is processed into two smaller forms with molecular masses of 91.5 and 66.8 kDa (1, 8, 27). It is possible that the autolytic bands in Fig. 3 at apparent molecular masses of 90.2, 88.0, and 66.8 kDa correspond to the three forms of the E. hirae muramidase. Whereas the 105-kDa autolysin is probably a distinct enzyme that has not been characterized yet, it is possible that the 79.7-kDa autolysin represents another proteolytic form of the 90.2-kDa enzyme. In addition to the use of a different enterococcal species, the reasons for the discovery of two new putative autolysins could include the use of both an acidic electrophoretic system and different peptidoglycans for the zymography. In the current study, purified E. faecalis peptidoglycan sacculi were incorporated into the running gels of the zymograms, whereas Leclerc and Asselin (27) employed Micrococcus luteus cells in their zymogram analysis of E. hirae cell extracts. Indeed, these workers detected only one autolytic band at 67 kDa in zymograms of an identical cell extract when the cell walls of Corynebacterium sepedonicum were used as the substrate.
FIG. 3.
Zymogram analysis of E. faecalis ATCC 19433 autolysins under acidic conditions. Identical samples of the total proteins present in E. faecalis grown to the late exponential phase were subjected to the electrophoretic system involving two separating gels, one containing O-acetylated E. faecalis peptidoglycan (+OAc) and the other containing de-O-acetylated material (prepared by mild-base hydrolysis) (−OAc). The arrows and asterisks indicate the positions of autolysins with less and more activity with O-acetylated peptidoglycan, respectively.
While the acidic electrophoretic system used precluded the formation of tightly focused protein bands, as expected in basic systems, it was apparent that some of the intensities of the clearing zones were different, suggesting that there were variations in the specificities of the autolysins detected. Thus, the 90.2-kDa muramidase and its potential proteolytic forms, together with the 79.7-kDa autolysin, appeared to be more active with the de-O-acetylated peptidoglycan, whereas the newly discovered 105-kDa enzyme had greater specificity for the naturally O-acetylated material. In addition to these autolysins, a number of minor enzymes with lower molecular masses were barely detectable in the acidic zymograms. It is possible that some of these enzymes may be more active with the O-acetylated material, but further careful analysis is required for confirmation of this.
The finding that E. faecalis produces autolysins with different specificities for O-acetylated peptidoglycan is consistent with the hypothesis that this modification aids in the control of autolytic activity. With E. faecalis, it seems that the activity of the various forms of the so-called major muramidase (molecular masses, 90.2, 88.0, and 66.8 kDa) is controlled at the enzymatic level through O acetylation of the substrate, which, as shown in Table 1, increases with the age of the culture, thereby preventing autolysis as the cells remain in the stationary phase. To compensate for the lack of continued activity of this muramidase, which may still be required for routine maintenance of the peptidoglycan sacculus and/or insertion of various cell wall components, it is possible that the gene encoding the 105-kDa enzyme is then induced and expressed. Evidence for such expression of this enzyme in response to the state of the culture and, more specifically, the extent of peptidoglycan O acetylation is presented below.
O acetylation of VBNC E. faecalis.
The cell wall of E. faecalis grown to the VBNC state has recently been characterized by other workers in terms of the chemical composition, and comparisons were made with exponentially growing and stationary cells (39). This analysis revealed that (i) the extent of peptidoglycan cross-linking was greater in VBNC cells, (ii) the lipoteichoic acid levels doubled, and (iii) the amount of teichoic acid remained unchanged. However, the conditions employed for these studies (i.e., the method employed for isolation of the peptidoglycan and the chemical treatments used for characterization of the peptidoglycan) precluded detection of O acetylation. Since it is now known that the peptidoglycan of E. faecalis is O acetylated, there is still the question of whether the cell wall layer becomes more recalcitrant to hydrolysis by increasing its level of O acetylation when it enters the VBNC state. This issue is all the more intriguing in light of the fact that teichoic acids are covalently linked to the peptidoglycan of gram-positive cells through phosphoester linkages to the C-6 hydroxyl group of muramoyl residues (18) (viz., the same site as the site for O acetylation) (11). With no observed increase in teichoic acid levels in VBNC cells, the potential for increased O acetylation would exist.
In the current study, cells of three strains of E. faecalis were grown to the VBNC state for further analysis of both their peptidoglycans and autolysin profiles. Thus, following a minimum of 16 days of incubation at 4°C in raw well water, the morphology of the cells became more irregular, as expected, and the cells were slightly elongated (30). Confirmation that the cells had entered the VBNC state was obtained by unsuccessful attempts to culture them back on either BBEA or tryptic soy agar.
The cells were harvested, and their peptidoglycan sacculi were recovered, taking care to preserve the natural levels of any O acetylation. Analysis of this material revealed that the extent of O acetylation had increased by 10 to 16% from the stationary-phase levels. Thus, the VBNC cells had the highest levels of modification, which ranged from 44 to 72% depending on the strain (Table 1). These levels of O acetylation are among the highest known so far for bacteria characterized for this modification under natural conditions (9, 11, 12). In this regard, the level of O acetylation of Staphylococcus aureus peptidoglycan has been observed to increase to approximately 70% upon administration of the bacteriostatic antibiotic chloramphenicol (24). Presumably, in both conditions of bacteriostasis in which cell growth and division have been halted, the maturation of peptidoglycan O acetylation is allowed to continue.
Hydrolysis and autolysis of VBNC E. faecalis.
Not surprisingly given the high levels of O acetylation, the peptidoglycan associated with the VBNC E. faecalis was found to be recalcitrant to HEWL-catalyzed hydrolysis. As shown in Fig. 2, incubation of the O-acetylated peptidoglycan from strain ATCC 19433 (44%) with HEWL resulted in less than 12% solubilization over a 60-min period. With strain NRC 8023, which had 72% O acetylation in the VBNC state, the level of solubilization was less than 8% (data not shown). Hence, the extensive levels of modification protect the cell walls of the VBNC cells from exogenous hydrolytic agents and thereby permit the cells to survive in this otherwise vulnerable state. Such high levels of O acetylation also preclude any detrimental activity of the major endogenous muramidase produced by E. faecalis, which, interestingly, has been observed to be expressed at elevated levels in VBNC cells (39). As noted above, this enzyme appears to be inhibited by O acetylation of its substrate. Indeed, despite a reported 25 to 50% increase in the level of this muramidase, the rate of autolysis for VBNC cells was found to be only slightly higher than the rates of autolysis for either exponentially growing or stationary cells (39).
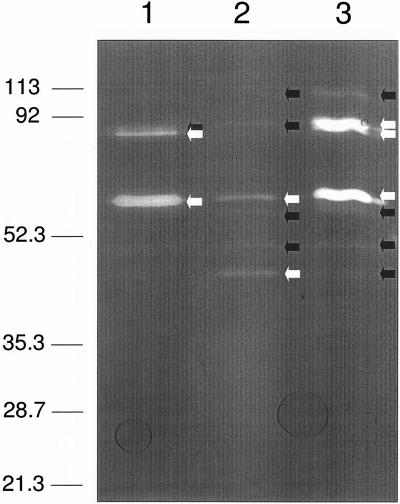
The increase in autolysin concentration associated with VBNC E. faecalis observed by Signoretto et al. (39) was confirmed in the current study. Figure 4 shows a zymogram for an experiment in which samples containing equal concentrations of E. faecalis cell sonicates obtained at different stages of growth were loaded into a separating gel containing E. faecalis peptidoglycan under basic conditions. Identical samples were loaded into a second zymogram gel, but this gel was stained with Coomassie brilliant blue after electrophoresis to confirm protein concentrations (data not shown). Under these conditions, in which the peptidoglycan substrate would have existed in its de-O-acetylated state, the three forms of the major muramidase were readily detected in the cells growing exponentially. When cells entered the stationary phase, the expression of this enzyme in all three states persisted, but at significantly reduced levels, with the smallest form predominating. In addition, four other lytic bands became evident. That this shift in autolysin production was not an artifact of unequal gel loading is clear from the obvious change in the proportions of the autolysins detected in the separate samples. One of the newly produced enzymes was the 105-kDa autolysin discussed above, which has specificity for both O-acetylated and unacetylated peptidoglycan. The three other enzymes, with apparent molecular masses of 62, 54, and 47.5 kDa, have not been detected previously. It is possible that one or more of these enzymes may represent latent forms of either the muramidase or the 105-kDa autolysin. Alternatively, they could represent unique autolysins that function specifically in stationary-phase cells.
FIG. 4.
Zymogram analysis of autolysins from E. faecalis ATCC 19433 grown to different culture states. Sonicates of equal amounts of cells from mid-exponential-phase (lane 1), stationary-phase (lane 2), and VBNC (lane 3) cultures were loaded into a zymogram gel containing peptidoglycan under basic conditions. The black and white arrows indicate autolytic bands with relatively low and high intensities, respectively.
Following conversion to the VBNC state, E. faecalis resumed expression of the major muramidase in all three of its forms while both maintaining production of the lower-molecular-mass autolysins and increasing production of the 105-kDa autolysin. In fact, production of the muramidase appeared to be at the highest level detected for all three cell states, which is consistent with the observations made by Signoretto et al. (39). Why E. faecalis resumes production of this muramidase at such high levels is not clear given our finding that the enzyme does not efficiently act on the highly O-acetylated peptidoglycan that exists in this VBNC cell state. This finding is especially puzzling in view of the cell's increased production of the 105-kDa autolysin, which hydrolyzes the O-acetylated wall polymer and thus has a housekeeping function. Moreover, although considered to be the major autolysin produced by E. faecalis, the muramidase is not essential for cell growth and plays no significant role in virulence (34). It has been proposed that this muramidase may aid in the persistence of VBNC E. faecalis in aquatic environments by participating at the cell surface in adhesion of the cells to surfaces, such as the chitin walls of copepods (38). No impairment of such adhesion was found with a Lyt mutant of E. faecalis, which produces the muramidase with apparently reduced hydrolytic activity (13); this finding resulted in dismissal of this proposal (38). However, the muramidase extracted from the Lyt mutant did not differ from the parent enzyme in terms of the ability to bind to cell wall substrates (13), leaving the possibility that the “dysfunctional” derivative of the muramidase may nonetheless be expressed and perform this binding function. Clearly, more studies are required to address this issue.
An alternative function for the increased production of the muramidase in VBNC E. faecalis could involve the role of the enzyme in predation. A variety of other bacteria are now known to release a complement of enzymes, including autolysins, when they are stressed, and these enzymes may both kill and degrade neighboring cells, thereby providing necessary nutrients. For example, Pseudomonas aeruginosa has been observed to release proteases, lipases, nucleases, and autolysins, including its major autolysin, in membrane vesicles when it is confronted with environmental stresses or administration of antibiotics (25, 28, 29). These enzyme packages have been demonstrated to be capable of attacking both other gram-negative bacteria and gram-positive bacteria. Hence, it is conceivable that VBNC E. faecalis in a nutrient-depleted environment releases its major autolysin, the 105-kDa muramidase, and its derivatives for the same purpose, thus preying on other bacteria while protecting itself from autolysis by increasing the level of peptidoglycan O acetylation.
Concluding remarks.
The presence of peptidoglycan O acetylation in the enterococci has escaped previous detection and study. This is true despite the significant attention that these bacteria, especially the vancomycin-resistant enterococci, have attracted. The fact that this modification of the peptidoglycan sacculus appears to play a role in the control of autolysins and thus the growth and division of the bacteria (5, 33) underscores the need for further investigation. Little is known about the pathway for peptidoglycan O acetylation, but O acetylation has been shown to be a maturation event, occurring outside the cell within the sacculus (reviewed in references 11 and 12). This suggests that the enzyme(s) involved in the process is relatively exposed to exogenous agents, such as administered antibiotics. Hence, this enzymatic system might prove to be an attractive new target for the development of a new class of antibiotics effective against the enterococci, as well as possibly many other bacteria.
Recently, an integral membrane protein in S. aureus N315 was found to function as a peptidoglycan O-acetyltransferase (OatA) (2). Using OatA (accession number BAB43658) as a probe, we identified a gene encoding a potential homolog in the E. faecalis genome (accession number AE016949) with 34.4% identity and 69.5% similarity. Thus, our future studies will involve cloning of this hypothetical gene and determination whether its translated product does in fact also function as a peptidoglycan O-acetyltransferase.
Acknowledgments
This work was supported by an operating grant to A.J.C. from the Canadian Institutes for Health Research (grant 62772) and by a postgraduate scholarship (PGSB) to J.T.W. from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- 1.Béliveau, C., C. Potvin, J. Trudel, A. Asseli, and G. Bellemare. 1991. Cloning, sequencing, and expression in Escherichia coli of a Streptococcus faecalis autolysin. J. Bacteriol. 173:5619-5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bera, A., S. Herbert, A. Jakob, W. Vollmer, and F. Gotz. 2005. Why are pathogenic staphylococci so lysozyme resistant? The peptidoglycan O-acetyltransferase OatA is the major determinant for lysozyme resistance of Staphylococcus aureus. Mol. Microbiol. 55:778-787. [DOI] [PubMed] [Google Scholar]
- 3.Bernadsky, G., T. J. Beveridge, and. A. J. Clarke. 1994. Analysis of the sodium dodecyl sulfate-stable autolysins of select gram-negative pathogens by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 176:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn, N. T., and A. J. Clarke. 2002. Families of lytic tranglycosylases. J. Mol. Evol. 52:78-84. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn, N. T., and A. J. Clarke. 2002. Characterization of soluble and membrane-bound family 3 lytic transglycosylases from Pseudomonas aeruginosa. Biochemistry 41:1001-1013. [DOI] [PubMed] [Google Scholar]
- 6.Blundell, J. K., G. J. Smith, and H. R. Perkins. 1980. The peptidoglycan of Neisseria gonorrhoeae: O-acetyl groups and lysozyme sensitivity. FEMS Microbiol. Lett. 9:259-261. [Google Scholar]
- 7.Chesbro, W. R. 1961. Lysozymes and the production of osmotic fragility in enterococci. Can. J. Microbiol. 7:952-955. [Google Scholar]
- 8.Chu, C. P., R. Kariyama, L. Daneo-Moore, and G. D. Shockman. 1992. Cloning and sequence analysis of the muramidase-2 gene from Enterococcus hirae. J. Bacteriol. 174:1619-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke, A. J. 1993. Extent of peptidoglycan O acetylation in the tribe Proteeae. J. Bacteriol. 175:4550-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, A. J. 1993. Compositional analysis of peptidoglycan by high-performance anion-exchange chromatography. Anal. Biochem. 212:344-350. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, A. J., and C. Dupont. 1992. O-Acetylated peptidoglycan: its occurrence, pathobiological significance and biosynthesis. Can. J. Microbiol. 38:85-91. [DOI] [PubMed] [Google Scholar]
- 12.Clarke, A. J., H. Strating, and N. T. Blackburn. 2000. Pathways for the O-acetylation of bacterial cell wall polymers, p. 187-223. In R. J. Doyle (ed.), Glycomicrobiology. Plenum Publishing Co. Ltd., New York, N.Y.
- 13.Cornett, J. B., B. E. Redman, and G. D. Shockman. 1978. Autolytic defective mutant of Streptococcus faecalis. J. Bacteriol. 133:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courvalin, P. 2005. Genetics of glycopeptide resistance in gram-positive pathogens. Int. J. Med. Microbiol. 294:479-486. [DOI] [PubMed] [Google Scholar]
- 15.Difco Laboratories. 1984. Difco manual, 10th ed. Difco Laboratories, Detroit, Mich.
- 16.Dupont, C., and A. J. Clarke. 1991. Dependence of lysozyme catalyzed solubilization of Proteus mirabilis peptidoglycan on the extent of O-acetylation. Eur. J. Biochem. 195:763-769. [DOI] [PubMed] [Google Scholar]
- 17.Glick, A. D., J. M. Ranhand, and R. M. Cole. 1972. Degradation of group A streptococcal cell walls by egg white lysozyme and human lysosomal enzymes. Infect. Immun. 6:403-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant, W. D., and A. J. Wicken. 1968. Muramic acid phosphate and the linkage of teichoic acid to peptidoglycan in Bacillus stearothermophilus cell walls. Biochem. Biophys. Res. Commun. 32:122-128. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, R. E. W. 2005. Mechanisms of action of newer antibiotics for gram-positive pathogens. Lancet Infect. Dis. 5:209-218. [DOI] [PubMed] [Google Scholar]
- 20.Hash, J. H. 1967. Measurement of bacteriolytic enzymes. J. Bacteriol. 93:1201-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Höltje, J.-V., D. Mirelmen, N. Sharon, and U. Schwarz. 1975. Novel type of murein transglycosylase in Escherichia coli. J. Bacteriol. 124:1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyle, B. D., and T. J. Beveridge. 1983. Metal binding by the peptidoglycan sacculus of Escherichia coli K-12. Can. J. Microbiol. 30:204-211. [DOI] [PubMed] [Google Scholar]
- 23.Janusz, M. J., C. Chetty, R. A. Eisenberg, W. J. Cromartie, and J. H. Schwab. 1984. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin. J. Exp. Med. 160:1360-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannsen, L., H. Labischinski, B. Reinicke, and P. Greisbrecht. 1983. Changes in the chemical structure of walls of Staphylococcus aureus grown in the presence of chloramphenicol. FEMS Microbiol. Lett. 16:313-316. [Google Scholar]
- 25.Kadurugamuwa, J. L., and T. J. Beveridge. 1996. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria, including pathogens: conceptually new antibiotics. J. Bacteriol. 178:2767-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kau, A. L., S. M. Martin, W. Lyon, E. Hayes, M. G. Caparon, and S. J. Hultgren. 2005. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect. Immun. 73:2461-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclerc, D., and A. Asselin. 1989. Detection of bacterial cell wall hydrolases after denaturing polyacrylamide gel electrophoresis. Can. J. Microbiol. 35:749-753. [DOI] [PubMed] [Google Scholar]
- 28.Li, Z., A. J. Clarke, and T. J. Beveridge. 1996. A major autolysin of Pseudomonas aeruginosa: subcellular distribution, potential role in cell growth and division, and secretion in surface membrane vesicles. J. Bacteriol. 178:2479-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Z., A. J. Clarke, and T. J. Beveridge. 1998. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 180:5478-5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lleo, M. M., M. C. Tafi, and P. Canepari. 1998. Nonculturable Enterococcus faecalis cells are metabolically active and capable of resuming active growth. Syst. Appl. Microbiol. 21:333-339. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf, R. H., and R. H. Deibel. 1972. Growth of Streptococcus faecium in the presence of lysozyme. Infect. Immun. 6:178-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, B. E. 1990. The life and times of Enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payie, K. G., H. Strating, and A. J. Clarke. 1996. The role of O-acetylation in the metabolism of peptidoglycan in Providencia stuartii. Microb. Drug Resist. 2:135-140. [DOI] [PubMed] [Google Scholar]
- 34.Qin, X., K. V. Singh, Y. Xu, G. M. Weinstock, and B. E. Murray. 1998. Effect of disruption of a gene encoding an autolysin of Enterococcus faecalis OG1RF. Antimicrob. Agents Chemother. 42:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenthal, R. S., J. K. Blundell, and H. R. Perkins. 1982. Strain-related differences in lysozyme sensitivity and extent of O-acetylation of gonococcal peptidoglycan. Infect. Immun. 37:826-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal, R. S., W. J. Folkening, D. R. Miller, and S. C. Swim. 1983. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect. Immun. 40:826-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shockman, G. D., J. S. Thompson, and M. J. Conover. 1967. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry 6:1054-1065. [DOI] [PubMed] [Google Scholar]
- 38.Signoretto, C., G. Burlacchini, C. Pruzzo, and P. Canepari. 2005. Persistence of Enterococcus faecalis in aquatic environments via surface interactions with copepods. Appl. Environ. Microbiol. 71:2756-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Signoretto, C., M. M. Lleo, M. C. Tafi, and P. Canepari. 2000. Cell wall chemical composition of Enterococcus faecalis in the viable but noncultruable state. Appl. Environ. Microbiol. 66:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strating, H., and A. J. Clarke. 2001. Differentiation of bacterial autolysins by zymogram analysis. Anal. Biochem. 291:149-154. [DOI] [PubMed] [Google Scholar]
- 41.Swim, S. G., M. A. Gfell, C. E. Wilde III, and R. S. Rosenthal. 1993. Strain distribution in extents of lysozyme resistance and O-acetylation of gonocccal peptidoglycan determined by high-performance liquid chromatography. Infect. Immun. 42:446-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toennies, G., L. Iszard, N. B. Rogers, and G. D. Shockman. 1961. Cell multiplication studied with an electronic particle counter. J. Bacteriol. 82:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watt, S., and A. J. Clarke. 1994. Initial characterization of two extracellular autolysins from Pseudomonas aeruginosa PAO1. J. Bacteriol. 176:4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weadge, T. J., J. M. Pfeffer, and A. J. Clarke. 2005. Identification of a new family of enzymes with potential O-acetylpeptidoglycan esterase activity in both gram-positive and gram-negative bacteria. BMC Microbiol. 5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]