Abstract
It has been proposed that the bitopic membrane protein SecG undergoes topology inversion during translocation of (pre)proteins via SecYEG. Here we show that SecG covalently cross-linked to SecY cannot invert its topology while remaining fully functional in protein translocation. Our results strongly disfavor topology inversion of SecG during protein translocation.
The translocation of proteins across the cytoplasmic membrane of bacteria occurs via an integral membrane protein complex composed of the three proteins SecY, SecE, and SecG (3). The functions of SecY and SecE are well established: SecY forms the protein-conducting channel (2, 9), and SecE is required for the stability of SecY (12, 21). SecG stimulates the activity of SecYE but is not required for viability or in vitro protein translocation (8, 16, 17). Although early studies on a secG deletion strain suggested that SecG is required for growth at low temperature (16), later studies showed that cold sensitivity is only observed in strains that also carry a mutation in GlpR, the regulator of glycerol phosphate metabolism (6). The molecular basis for this synergistic effect remains to be established, but it explains why all the secG deletion strains that have been constructed in a glpR+ background are not cold sensitive (1, 5, 7, 11; N. Nouwen, unpublished data).
The membrane topology of SecG has been determined with PhoA fusions (18) and cysteine labeling studies (15). SecG consists of two transmembrane segments that are connected by a mildly hydrophobic cytoplasmic loop, while both termini are located in the periplasm. During protein translocation SecG has been proposed to completely invert its membrane topology (14, 18). This is a highly unusual phenomenon for a stably membrane-integrated protein. The topology inversion theory is, however, mostly based on indirect accessibility studies with proteases and chemical reagents. We reasoned that we could critically test the theory with a topologically fixed version of SecG. Disulfide cross-linking seemed the method of choice to fix the SecG topology, since it can efficiently and reversibly generate covalent bonds between proteins.
In a cysteine-directed cross-linking study we have previously identified a cytoplasmic residue in SecY (Thr179) that can be chemically cross-linked to SecG (23). Mutagenesis of Lys26 of SecG to arginine abolished this cross-link (data not shown) (20). To investigate whether SecY(T179C) could form a disulfide bond with SecG(K26C) we expressed both mutants together with SecE in the Escherichia coli SF100-derived secG deletion strain NN104 [F− ΔlacX74 galK thi rpsL strA ΔphoA(PvuII) ΔompT ΔsecG] (22). When inner membrane vesicles (IMVs) overexpressing SecY(T179C)EG(K26C) complexes were oxidized by a 30-min incubation with 1 mM Na2S4O6 at 37°C, a SecY-SecG cross-link product was formed with more than 75% efficiency (Fig. 1A to C, lane 6). The oxidation could be reversed by the addition of dithiothreitol (data not shown).
FIG. 1.
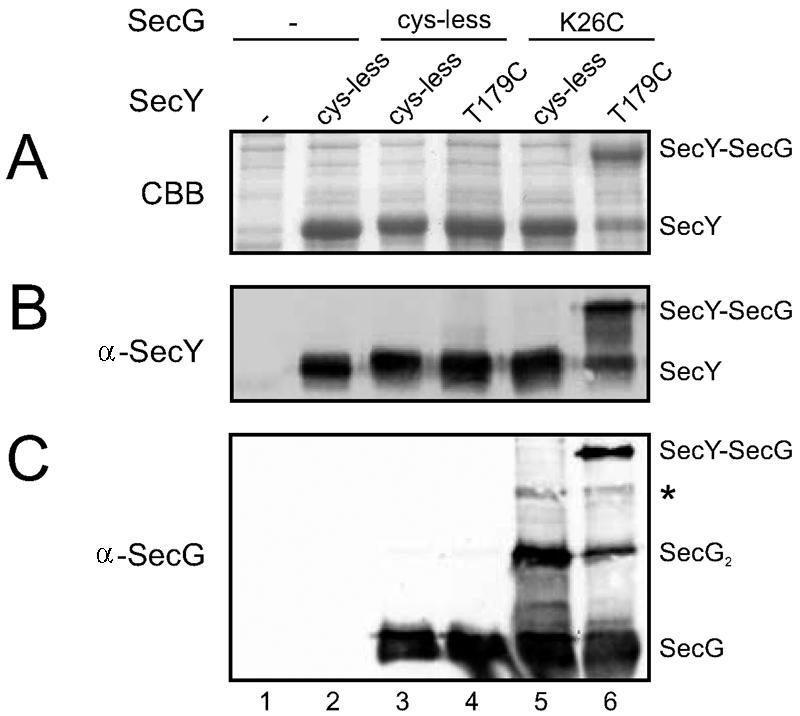
Highly efficient disulfide cross-linking of SecY(T179C) to SecG(K26C). NN104-derived IMVs overexpressing different combinations of SecY (SecE) and SecG or the empty expression vector (lanes 1) were oxidized with Na2S4O6 and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie brilliant blue staining (A), anti-SecY (B), or anti-SecG immunodetection (17) (C) according to standard procedures. Bands corresponding to SecY, SecG, dimeric SecG (SecG2), and the SecY-SecG cross-link product are labeled correspondingly, and the weak band indicated with an asterisk represents SecG cross-linked to the N-terminal proteolytic fragment of SecY (23). For quantitation of the SecY-SecG cross-linking efficiency, the Coomassie brilliant blue-stained gel was imaged and analyzed. Note that no quantitative information can be gained from the Western blots due to the reduced blotting efficiency of the cross-linked adducts.
It seems unlikely that SecG which is cross-linked to the shortest cytoplasmic loop of SecY can invert its topology, as this would require a major topological inversion of several SecY helices as well. To corroborate this, we subjected the oxidized SecY(T179C)EG(K26C) complexes to the proteolytic topology inversion assay. When wild-type IMVs are incubated with an externally added protease under nontranslocating conditions, SecG is cleaved in its cytoplasmic loop, resulting in a 9-kDa C-terminal fragment that can be detected with antibodies directed against the extreme C terminus of SecG (18). Similarly, cleavage of the 50-kDa SecY-SecG cross-link product is accompanied by the appearance of the 9-kDa C-terminal fragment (Fig. 2A, panels 1 and 2), indicating that cross-linked SecG has the same topology as non-cross-linked SecG.
FIG. 2.
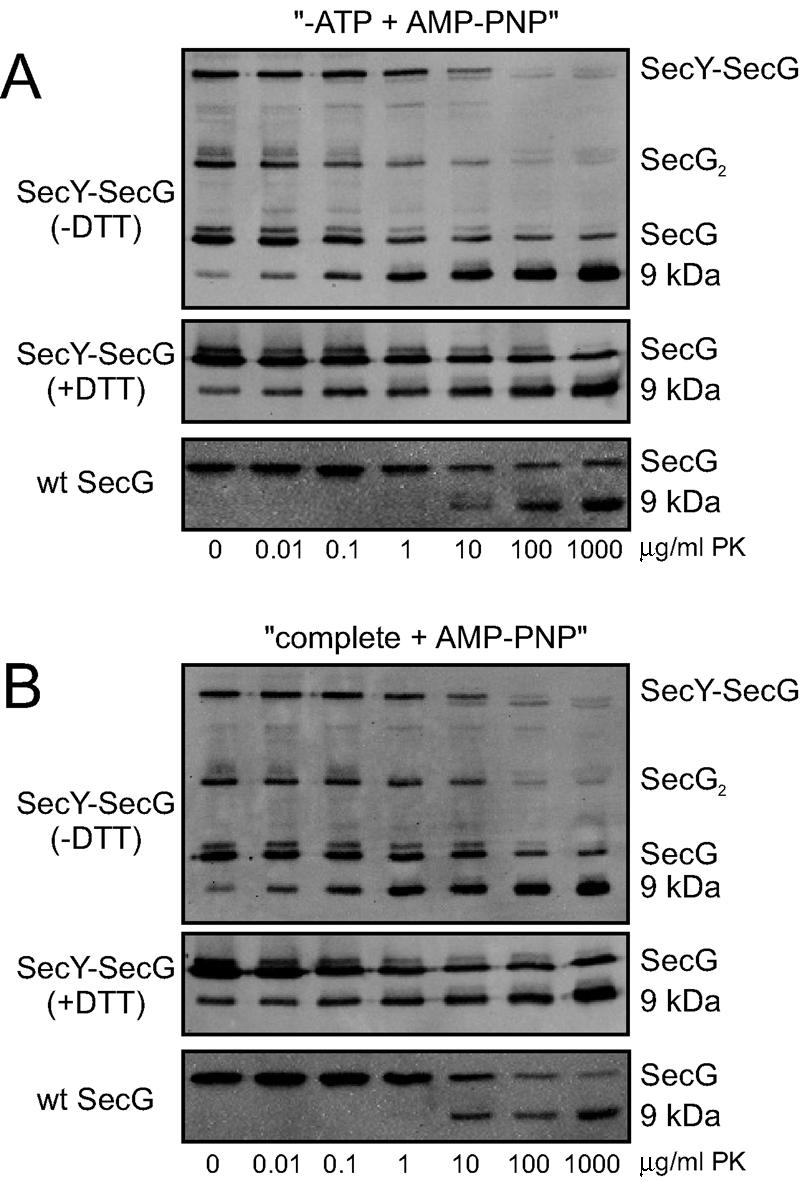
SecG cross-linked to SecY does not invert its membrane topology. Oxidized IMVs overexpressing SecY(T179C)EG(K26C) (panels 1 and 2) or wild-type SF100 IMVs (panels 3) were subjected to the SecG topology inversion assay (A: −ATP plus AMP-PNP; B: complete plus AMP-PNP; see text for details) as described before (18) under nonreducing conditions. Samples were analyzed by nonreducing (panels 1) or reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (panels 2 and 3) followed by immunodetection with antibodies raised against the extreme C terminus of SecG (17). Bands corresponding to the SecY-SecG cross-link product, SecG, dimeric SecG (SecG2), and the 9-kDa C-terminal fragment of SecG, are indicated. The applied concentrations of proteinase K (PK) are indicated at the bottom of each panel. Note that a small amount of the 9-kDa fragment is always observed upon overexpression of SecG (18).
In the proposed topology inversion model, the inverted SecG is characterized by the complete disappearance of the C-terminal epitope when translocation of a preprotein is blocked by the nonhydrolyzable ATP analog adenylyl imidodiphosphate (AMP-PNP) after initiation with ATP (18). When IMVs containing the cross-linked SecYEG complexes were subjected to these conditions, the 9-kDa C-terminal fragment was still quantitatively generated (Fig. 2B, panels 1 and 2), indicating that cross-linked SecG does not invert its topology. From these data we conclude that SecG cross-linked to SecY has a fixed topology resembling that of wild-type SecG under nontranslocating conditions.
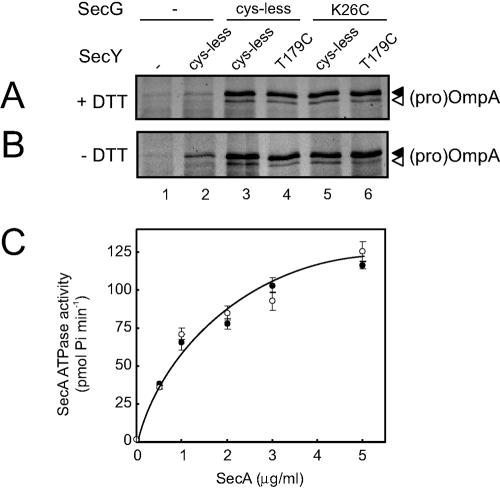
Since the topology inversion assay requires a functional SecYEG complex, it could be argued that cross-linked SecG does not invert its topology because it is inactive. Therefore we determined the activity of SecYEG complexes containing cross-linked SecG with two different activity assays. After oxidation as described above and reharvesting of the membranes to remove the oxidator, we assayed IMVs containing cross-linked SecY(T179C)EG(K26C) complexes under reducing (Fig. 3A) and nonreducing conditions (Fig. 3B) for the in vitro translocation of fluorescently labeled pro-OmpA as described before (23). Translocation reactions were incubated for 7 min at 37°C and contained limiting amounts of IMVs to ensure that it reflects the activity of the cross-linked SecYEG complexes. The stimulatory effect of SecG can be clearly observed by comparing IMVs containing overexpressed SecYE (Fig. 3, lanes 2) to those with overexpressed SecYEG (lanes 3). Neither the single cysteine mutations in SecY (lanes 4) (20, 23) and SecG (lanes 5) nor the combination of both mutations (lanes 6) influences the translocation activity of SecYEG. Importantly, the cross-linked SecYEG complexes (Fig. 3B, lane 6) show translocation activity similar to that of non-cross-linked SecYEG (Fig. 3A and B, lanes 3 to 5).
FIG. 3.
Cross-linked SecY(T179C)EG(K26C) is as active as wild-type SecYEG. Oxidized IMVs overexpressing the indicated SecYEG complexes (see legend to Fig. 1) were analyzed for in vitro translocation of fluorescein maleimide-labeled pro-OmpA(C302S) under nonreducing conditions (A) or in the presence of 5 mM dithiothreitol (DTT) (B) as described (23). Cysteineless SecYEG has previously been shown to be as active as wild-type SecYEG (10). (C) Oxidized IMVs overexpressing SecYEG (open symbols) or SecY(T179C)EG(K26C) (solid symbols) were analyzed for pro-OmpA-stimulated SecA ATPase activity under nonreducing conditions as described (4) with the indicated amounts of SecA.
We also analyzed the effect of the SecY-SecG cross-link on the pro-OmpA-stimulated SecA ATPase activity as a function of the SecA concentration by assaying oxidized IMVs overexpressing SecY(T179C)EG(K26C) or cysteineless SecYEG under nonreducing conditions. As can be seen in Fig. 3C, both cross-linked (open symbols) and non-cross-linked SecYEG complexes (solid symbols) exhibit identical SecA dependency of the translocation ATPase activity. From these two activity assays we conclude that SecYEG complexes containing cross-linked SecG are fully functional and that the static behavior in the topology inversion assay is not caused by inactivation of the complex. Importantly, this implies that the proposed topology inversion of SecG is not required for the functionality of the SecYEG complex.
The results presented here disfavor the topology inversion theory. However, in previous studies (13, 19), the topology inversion assay has also yielded results that differ substantially from those originally described by Nishiyama et al. (14, 18). In both of these studies (13, 19), under conditions in which the C-terminal epitope of SecG was expected to disappear completely, SecG was hardly cleaved by the protease and a small amount of the 9-kDa fragment was generated. Importantly, these results disfavor the topology inversion theory. Our attempts to reproduce the topology inversion with endogenous levels of wild-type SecG in IMVs derived from various E. coli strains (K002, K003, DH5α, SF100, and NN100) also failed and resulted in the generation of the 9-kDa fragment, very similar to the results described above for cross-linked SecG (Fig. 2A and B, panels 3). Based on these three independent observations, in particular the finding that topologically fixed SecG is fully functional, we conclude that SecG maintains its original topology during protein translocation via SecYEG. We propose that the reported changes in accessibility for proteases and cysteine-modifying reagents reflect a SecA-induced conformational change of SecG within the SecYEG complex rather than an inversion of the SecG topology.
Acknowledgments
This work was supported by the Council for Chemical Sciences of the Netherlands Organization for Scientific Research (CW-NWO) and a fellowship from the Royal Academy of Sciences of the Netherlands (KNAW) to N.N.
We thank Hajime Tokuda (University of Tokyo) for the generous gift of SecG antibodies and Jeanine de Keyzer for critical reading of the manuscript.
REFERENCES
- 1.Bost, S., and D. Belin. 1995. A new genetic selection identifies essential residues in SecG, a component of the Escherichia coli protein export machinery. EMBO J. 14:4412-4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon, K. S., E. Or, W. M. Clemons, Jr., Y. Shibata, and T. A. Rapoport. 2005. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J. Cell Biol. 169:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Keyzer, J., C. van der Does, and A. J. M. Driessen. 2003. The bacterial translocase: a dynamic protein channel complex. Cell, Mol. Life Sci. 60:2034-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Keyzer, J., E. O. van der Sluis, R. E. Spelbrink, N. Nijstad, B. de Kruijff, N. Nouwen, C. van der Does, and A. J. M. Driessen. 2005. Covalently dimerized SecA is functional in protein translocation. J. Biol. Chem. 280:35255-35260. [DOI] [PubMed] [Google Scholar]
- 5.Duong, F., and W. Wickner. 1997. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 16:2756-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flower, A. M. 2001. SecG function and phospholipid metabolism in Escherichia coli. J. Bacteriol. 183:2006-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flower, A. M., L. L. Hines, and P. L. Pfennig. 2000. SecG is an auxiliary component of the protein export apparatus of Escherichia coli. Mol. Gen. Genet. 263:131-136. [DOI] [PubMed] [Google Scholar]
- 8.Hanada, M., K. Nishiyama, S. Mizushima, and H. Tokuda. 1994. Reconstitution of an efficient protein translocation machinery comprising SecA and the three membrane proteins, SecY, SecE, and SecG (p12). J. Biol. Chem. 269:23625-23631. [PubMed] [Google Scholar]
- 9.Joly, J. C., and W. T. Wickner. 1993. The SecA and SecY subunits of translocase are the nearest neighbors of a translocating preprotein, shielding it from phospholipids. EMBO J. 12:255-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann, A., E. H. Manting, A. K. J. Veenendaal, A. J. M. Driessen, and C. van der Does. 1999. Cysteine-directed cross-linking demonstrates that helix 3 of SecE is close to helix 2 of SecY and helix 3 of a neighboring SecE. Biochemistry 38:9115-9125. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, G., H. Mori, and K. Ito. 1998. Roles of SecG in ATP- and SecA-dependent protein translocation. Proc. Natl. Acad. Sci. USA 95:13567-13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuyama, S., J. Akimaru, and S. Mizushima. 1990. SecE-dependent overproduction of SecY in Escherichia coli. Evidence for interaction between two components of the secretory machinery. FEBS Lett. 269:96-100. [DOI] [PubMed] [Google Scholar]
- 13.Mori, H., H. Sugiyama, M. Yamanaka, K. Sato, M. Tagaya, and S. Mizushima. 1998. Amino-terminal region of SecA is involved in the function of SecG for protein translocation into Escherichia coli membrane vesicles. J. Biochem. (Tokyo) 124:122-129. [DOI] [PubMed] [Google Scholar]
- 14.Nagamori, S., K. Nishiyama, and H. Tokuda. 2002. Membrane topology inversion of SecG detected by labeling with a membrane-impermeable sulfhydryl reagent that causes a close association of SecG with SecA. J. Biochem. (Tokyo) 132:629-634. [DOI] [PubMed] [Google Scholar]
- 15.Nagamori, S., K. Nishiyama, and H. Tokuda. 2000. Two SecG molecules present in a single protein translocation machinery are functional even after crosslinking. J. Biochem. 128:129-137. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama, K., M. Hanada, and H. Tokuda. 1994. Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J. 13:3272-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiyama, K., S. Mizushima, and H. Tokuda. 1993. A novel membrane protein involved in protein translocation across the cytoplasmic membrane of Escherichia coli. EMBO J. 12:3409-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama, K., T. Suzuki, and H. Tokuda. 1996. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell 85:71-81. [DOI] [PubMed] [Google Scholar]
- 19.Sato, K., H. Mori, M. Yoshida, M. Tagaya, and S. Mizushima. 1997. In vitro analysis of the stop-transfer process during translocation across the cytoplasmic membrane of Escherichia coli. J. Biol. Chem. 272:20082-20087. [DOI] [PubMed] [Google Scholar]
- 20.Satoh, Y., G. Matsumoto, H. Mori, and K. Ito. 2003. Nearest neighbor analysis of the SecYEG complex. 1. Identification of a SecY-SecG interface. Biochemistry 42:7434-7441. [DOI] [PubMed] [Google Scholar]
- 21.Taura, T., T. Baba, Y. Akiyama, and K. Ito. 1993. Determinants of the quantity of the stable SecY complex in the Escherichia coli cell. J. Bacteriol. 175:7771-7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Laan, M., N. Nouwen, and A. J. M. Driessen. 2004. SecYEG proteoliposomes catalyze the Deltaphi-dependent membrane insertion of FtsQ. J. Biol. Chem. 279:1659-1664. [DOI] [PubMed] [Google Scholar]
- 23.van der Sluis, E. O., N. Nouwen, and A. J. M. Driessen. 2002. SecY-SecY and SecY-SecG contacts revealed by site-specific crosslinking. FEBS Lett. 527:159. [DOI] [PubMed] [Google Scholar]