Abstract
A steroid-hormone-dependent growth suppression was observed in Escherichia coli efflux-deficient backgrounds containing mutations in the major RND- and MFS-type tripartite multidrug efflux systems, AcrAB-TolC and EmrAB-TolC, respectively. In addition to their previously known natural steroid spectrum, which includes bile acids, both systems were shown to transport the hormones estradiol and progesterone, whereas hydrocortisone served as a substrate of only AcrAB-TolC. Furthermore, at least two other RND-type pumps, YhiV and AcrD, were capable of transporting such hormones when overexpressed on plasmid vectors (with some demonstrable specificity observed with AcrD). When this activity was examined in a wild-type background, cell-associated estradiol levels remained largely unaffected by competition with exogenous bile acids and hydrocortisone, in contrast to progesterone, which produced a significant modulation in estradiol uptake.
In Escherichia coli, there are several multiple-drug efflux (MDE) pump proteins from the resistance nodulation division (RND), major facilitator superfamily (MFS), small multidrug resistance (SMR), and multidrug and toxic compound extrusion (MATE) families that provide resistance to bile acids (13). These amphipathic steroid molecules are found exclusively in the gastrointestinal (GI) tract and are critical for the absorption of dietary lipophilic ingesta in the lumen of the small intestine because of their capacity to spontaneously form micelles above certain critical concentrations. It is estimated that bile acids are present at high concentrations in this region (approximately 4 to 20 mM in the duodenum [17, 21]) and that their reabsorption in the terminal ileum is incomplete, leaving 200 to 600 mg per day in humans that escapes to the colon, where complex microbial populations exist (2). Thus, from the perspective of the bacterium, they are powerfully noxious emulsifying agents that have been implicated as the “natural substrates” for some MDE pumps, especially in a commensal organism like E. coli (17, 25).
Accordingly, bile acid steroids at high concentrations may have potentially significant effects on gram-negative bacterial drug resistance by competing for MDE pump function or inducing the expression of MDE proteins (15-17). Perhaps the largest intrinsic resistance to drugs and bile acids in E. coli is provided by two tripartite systems which are expressed constitutively: AcrAB-TolC of the RND and EmrAB-TolC of the MFS (11). Interestingly, every known multidrug RND-type efflux pump encoded in the E. coli genome (AcrB, AcrD, AcrF, YhiV, and MdtBC), encompassing five separate systems, transports bile acids with no apparent specificity for conjugated or deconjugated species (1, 5, 6, 12). This observation is actually unremarkable considering the structural variety of typical MDE pump substrates but can be explained by a recent structural study which identified several separate substrate binding sites within the AcrB protein (24).
Like bile acids, certain steroid hormones are subject to enterohepatic circulation and are secreted in bile once conjugated to either glucuronide or sulfate by the liver at levels approximating 6 to 13 mg per day (2). As with bile acids, reports of bacterial interactions with steroid hormones are limited to the hydrolysis of such conjugates (deconjugation) and transformations of the steroid ring structure by several genera of gram-positive commensal bacteria (2). The significance of these activities to the bacterium remains unclear but may involve sequestering substrates for assimilatory or metabolic pathways (22) or providing a mechanism to modulate membrane permeability (4). Thus far, these activities have been attributed to a gram-negative organism in only a few cases, which, interestingly, implicated E. coli in the hydrolysis of steroid glucuronides (8) and the 7α-dehydroxylation of bile acids (23).
The physiological role of MDE pumps remains an enigma, considering the genetic dedication and presumed redundancy in substrate specificities that exist within a given genome (14). It is therefore conceivable that other endogenous agents derived from the host organism may serve as substrates for these systems. In an effort to address MDE function in the commensal-host environment, we examined whether free (deconjugated) steroid hormones could affect E. coli fitness in an efflux-deficient genetic background and discovered that, indeed, these steroids can serve as strong and specific substrates for at least two major efflux systems.
(Part of this research was presented at the 105th General Meeting of the American Society for Microbiology, 2005.)
Effects of steroids on E. coli growth fitness.
Recently, a gonococcal efflux pump homolog of the tripartite systems studied here was shown to enhance the survival of Neisseria gonorrhoeae in a female mouse model of genital tract infection. Specifically, efflux-deficient gonococcal mutants were more rapidly cleared from mice secreting gonadal hormones and were more sensitive to progesterone than comparable wild-type strains when assessed in vitro with MIC determinations and growth inhibition studies (10). Therefore, we assessed the growth of E. coli MDE mutants with various steroid hormones (estradiol, progesterone, hydrocortisone, cholesterol, ergosterol, and stigmasterol) in a hyperresistant E. coli strain, AG102 (marR1) (7), and its isogenic derivatives AG102MB (acrB::kan) (6) and HNCE4 (acrB::kan emrAB::cat), containing deficiencies in either one or two major, constitutively expressed bile acid efflux systems, AcrAB-TolC and EmrAB-TolC. Strain HNCE4 was constructed from strain AG102MB using the λ Red recombinase system encoded by pKD46 and the PCR-amplified cat gene generated from pKD3 with integration site-specific hybrid primers as described previously (3).
Overnight cultures of E. coli AG102, AG102MB, and HNCE4 were grown with appropriate antibiotic selection (25 μg/ml kanamycin and chloramphenicol for AG102MB and HNCE4, respectively), normalized to turbidity at a wavelength of 550 nm, and used to inoculate (1%, vol/vol) nonselective Luria-Bertani (LB) broth containing 256 μg/ml dimethyl sulfoxide (DMSO)-dissolved steroid and, in tandem, LB broth supplemented with DMSO alone. With each hormone tested, AG102 growth was unaffected by steroid treatment of any kind; however, both mutant strains exhibited some effect on growth, resulting in a decrease in culture density of at least 1 log10 and at most 3 log10 CFU/ml during log phase (data not shown). In addition, growth suppression was generally higher with the double-knockout mutant, HNCE4, than with the single-knockout mutant, AG102MB, in at least three cases: with estradiol, progesterone, and cholesterol. For example, an inoculum of AG102 on the order of 106 CFU/ml into prewarmed LB broth (with vigorous shaking) produced a late-log-phase cell density of approximately 9 × 1012 (DMSO treatment) and 2.2 × 1012 (estradiol treatment) CFU/ml after 6 h of growth. As consistently observed, the AG102MB and HNCE4 cell densities were approximately 1.62 × 1012 and 1.4 × 1013 CFU/ml (DMSO treatment), respectively, and 1.18 × 1010 and 4 × 1010 (estradiol treatment) CFU/ml, respectively. For hydrocortisone, the levels of suppression were generally similar for AG102MB and HNCE4 and approximately 2 log10 CFU/ml lower than that for the respective DMSO-treated cells. Interestingly, the nonmammalian hormones stigmasterol and ergosterol are potentially the most lipophilic of the sterols tested in this study yet had the smallest suppressive effect (approximately 1 log10 CFU/ml) on the growth of the mutant strains (data not shown).
The mechanism of growth suppression in the absence of these pumps, however, is unclear at best but may involve secondary effects of steroid hormone interaction with regulatory proteins similar to what has been described recently with specific classes of bile acids (15-17). Alternatively, Silver and Levine demonstrated some time ago that steroidal diamines may affect sugar transport in addition to affecting cell permeability (19). Nevertheless, it must be noted that simple steroid lipophilicity, and hence any potential ramification of steroid-membrane association on growth, cannot account for our observations, which, accordingly, should be much more pronounced with highly hydrophobic molecules, such as cholesterol (log Kow, 8.74), ergosterol (log Kow, 8.86), and stigmasterol (log Kow, 9.43), than with moderately hydrophobic ones, such as estradiol (log Kow, 3.94), progesterone (log Kow, 3.67), and hydrocortisone (log Kow, 1.62) (Kow is the estimated octanol-water partition coefficient for a neutral molecule calculated by using the Syracuse Research Corporation KowWin Software).
Steroid uptake with MDE mutants.
The observed hormone-dependent growth suppression in AG102MB and HNCE4 suggested that the AcrAB-TolC and EmrAB-TolC systems may be involved in the efflux of steroids. Therefore, uptake studies were undertaken with various tritium-labeled steroids to determine their levels in a set of four isogenic E. coli genomic mutants constructed, using a methodology similar to that used for constructing HNCE4, to also include a mutant with a single knockout of the EmrAB-TolC system (CE1 emrAB::cat) in the AG102 background. [1,2-3H(N)]cholesterol, [2,4-3H]cholic acid, [6,7-3H(N)]estradiol, [1,2-3H]hydrocortisone, and [1,2-3H(N)]progesterone were obtained commercially in ethanol at a concentration of 1 mCi/ml and a specific activity of 50 Ci/mmol except for cholesterol and cholic acid, which were obtained at specific activities of 40 and 20 Ci/mmol, respectively.
An uptake assay was performed essentially as described previously (4). Briefly, cultures were grown to mid-log phase (approximately 4 to 5 h), harvested by centrifugation, resuspended in 0.5 volume of LB broth, and normalized by turbidity analysis. The cultures were further concentrated 10-fold in LB broth and dispensed into 200-μl aliquots on ice. After a 7.5-min preincubation in a 37°C water bath, tritium-labeled steroid (2.5 μCi) was added and incubated with the cell suspension for 3.5 min. The reaction was immediately quenched with a 1-ml ice-cold 100 mM lithium chloride-100 mM potassium phosphate buffer (pH 7.0). The cells were pelleted immediately, and after a second ice-cold buffer wash, the resulting cell pellet was digested in 1 ml formamide at 65°C for 1 hour. The cell-associated radioactivity was quantified in 10 ml of Ultima Gold scintillation cocktail using a Packard 1600TR TRI-CARB liquid scintillation analyzer.
Interestingly, the uptake of cholic acid, progesterone, estradiol, and hydrocortisone increased in AG102MB cells approximately 2-, 6-, 10-, and 15-fold, respectively, over levels in wild-type AG102 cells, suggesting that the primary RND-type tripartite efflux pump (AcrAB-TolC) recognizes and expels these steroid molecules (Fig. 1). In addition, the uptake of progesterone, cholic acid, and estradiol in HNCE4 cells increased approximately 2-, 3-, and 4-fold, respectively, over that of AG102MB cells (11-, 7-, and 38-fold over levels in AG102 cells, respectively), further suggesting that the primary MFS-type tripartite system (EmrAB-TolC) also recognizes at least two steroid hormones in addition to the deconjugated bile acid cholic acid but not hydrocortisone. With CE1 cells, uptake profiles were similar (expectedly so) to those of wild-type AG102 cells presumably because of the overriding influence of the AcrAB-TolC system that has previously been observed for drug resistance (for example, compare references 13 and 20). In comparison, uptake levels of cholesterol in AG102, AG102MB, and CE1 cells were indistinguishable but were also approximately twofold lower than in HNCE4 cells (Fig. 1).
FIG. 1.
Uptake of various tritium-labeled steroid molecules by E. coli AG102 and its efflux-deficient derivatives. Error bars represent standard deviations from three replicates with the same bacterial suspension.
Secondary active energy dependence of cell-associated steroid levels.
The AcrAB-TolC and EmrAB-TolC systems are composed of secondary active pump proteins (AcrB and EmrB, respectively) and, thus, require the proton motive force (PMF) for efflux. To test the dependence of this energy source on efflux, the four E. coli strains were exposed to the ionophore and PMF uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP), at 0.1 mM, prior to the addition of tritium-labeled hormone in the uptake assay. CCCP increased both estradiol and progesterone accumulation in each strain to levels that were generally similar in all four strains, indicating that the hormone levels associated with E. coli cells are subject to a PMF-dependent process (data not shown). Most notably, CCCP increased estradiol and progesterone accumulation even in the double-knockout strain HNCE4 to 5.24 ± 0.65 and 10.33 ± 0.58 pmol, respectively. These levels were approximately twofold higher than those of the respective non-CCCP-treated HNCE4 cells, 2.88 ± 0.19 and 6.62 ± 0.16 pmol, respectively, which suggests the presence of other steroid efflux processes. Indeed, we found that two other RND-type pumps are involved in steroid efflux (see below); however, their basal-level expression and respective native contributions to such efflux remains questionable since they were overexpressed on plasmid vectors in our study.
Mutant complementation with RND-type pumps from the E. coli genome.
AcrAB-TolC maintained an overwhelming influence on steroid levels in the AG102 background (Fig. 1, compare the AG102MB and CE1 profiles), yet there are several, perhaps redundant, RND-type bile acid efflux systems in the E. coli genome that are subject to heavy regulation and are essentially silent under typical laboratory conditions (11). In some cases, as has previously been shown (5, 6), these pumps can function heterologously with the AcrB adaptor coupling, AcrA, and can be functionally overexpressed on pSportI plasmid vectors (amp lacI) in an AG102-derived background. Two such pumps, AcrD (encoded on pAcrD) and YhiV (pYhiV), in addition to AcrB (pAcrB), were tested for the capacity to complement the acrB mutant portion of the HNCE4 steroid efflux-deficient phenotype (for expression, 0.2 mM isopropyl-β-d-thiogalactopyranoside was added approximately 2 h before cell harvesting for the uptake assay). As expected, plasmid-encoded AcrB directly complemented its deficiency in HNCE4 by reducing the levels of cell-associated cholic acid, estradiol, hydrocortisone, and progesterone approximately 2.5-fold more than in cells harboring the pSportI plasmid (Fig. 2). Surprisingly, YhiV produced the largest effect by reducing steroid levels over eightfold for estradiol and four- to fivefold for cholic acid, hydrocortisone, and progesterone. In contrast, AcrD provided modest reductions, 1.8-fold and 1.4-fold, respectively, for cholic acid and progesterone, whereas it failed to reduce cell-associated estradiol and hydrocortisone below control (pSportI) levels (Fig. 2). These findings with AcrD are not surprising since this pump provides resistance to strongly hydrophilic molecules like aminoglycosides (18) and has been shown to produce only modest effects on bile acid resistance when similarly overexpressed (6). However, it remains intriguing that some level of specificity indeed exists for steroid hormones unlike what has been observed thus far for bile acid steroids.
FIG. 2.
Uptake of tritium-labeled steroid molecules by HNCE4 cells complemented with pSportI plasmid-encoded RND pumps from the E. coli genome. Of the six RND-type pumps encoded within the E. coli genome, AcrD and YhiV were previously shown to function heterologously with AcrA to produce a drug resistance phenotype (5, 6).
Steroid competition.
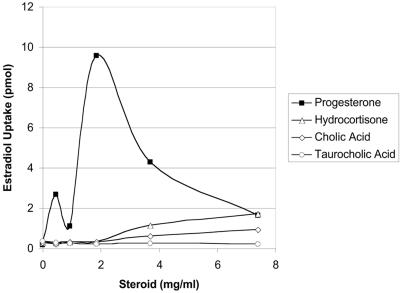
Commensal and potentially pathogenic organisms are undoubtedly exposed to multiple types of host-derived steroid molecules within their respective niches in the GI, vaginal, or urinary tract. A study with fully efflux-proficient wild-type AG102 cells was conducted to address estradiol efflux when the cells were exposed to various unlabeled steroids (Fig. 3). Thus, estradiol uptake was monitored at increasing concentrations of either conjugated (taurocholic) or deconjugated (cholic) bile acid within GI tract physiological ranges of <10 mg/ml (approximately 18 to 20 mM) supplied exogenously in the assay medium during the preincubation period of the uptake assay (4). Exogenous hydrocortisone, like exogenous taurocholic and cholic acids, failed to significantly modulate estradiol uptake, unlike exogenous progesterone, which provided the largest modulation and produced a biphasic profile. It is tempting to suggest that this profile results from the separate inhibition of estradiol efflux by each major efflux system; however, further detailed study of the issue is required before a conclusion of this type can be reached. Most importantly with regard to the microbial ecology of the GI tract, these data demonstrate that cell-associated estradiol levels remain low despite challenges with physiological levels of endogenous GI tract bile acids. Furthermore, AcrAB-TolC is apparently responsive to low concentrations of steroid hormones (125 nM tritium-supplied estradiol), which are expected in the various host-derived niches.
FIG. 3.
Effect of exogenous, unlabeled steroids on the uptake of tritium-labeled estradiol by fully efflux-proficient wild-type AG102 cells.
Conclusions.
Apart from steroid deconjugation and transformation, an additional interaction involving the efflux of mammalian steroid hormones by tripartite MDE pumps in bacteria is reported here for the first time. These pumps may be essential for survival under conditions where steroids are present, such as in the GI, vaginal, and urinary tracts. In such environments, MDE pump function may be complex and undoubtedly dynamic considering the number of potential, endogenous substrates involved as well as any transient, ingested substrates taken as drug therapy. Uptake studies performed with genomic mutants (Fig. 1) and plasmid-expressed RND pumps (Fig. 2) indicate that MDE pumps recognize specific steroid hormones. Such specificity may have evolved from a commensal association or, alternatively or in addition, a specific pathogenicity. Moreover, estradiol levels were shown to remain low even though exogenous substrates like conjugated and deconjugated bile acids were present at concentrations several orders of magnitude higher than that of tritium-labeled estradiol (Fig. 3). Thus, our findings may extend previous observations of bile acid steroids outperforming other drug substrates in AcrB-mediated phospholipid extrusion, suggesting that they are the strongest natural substrates of the AcrAB-TolC system (25).
Whether steroid hormones may influence the nature of intrinsic resistance to typical antimicrobial agents in commensal or pathogenic bacteria is a subject for future study. We have, in the course of this study, conducted some preliminary two-dimensional MIC determinations with increasing concentrations of estradiol and progesterone which failed to modulate MICs of a typical pump substrate like ethidium bromide (data not shown). In a related scenario, steroids can modulate mammalian P-glycoprotein (Pgp) efflux activity, which mediates resistance to chemotherapy agents (9). Interestingly, it is the non-Pgp steroid substrates, such as testosterone and progesterone, that inhibit its efflux activity (9). It is therefore important to address the impact of natural and synthetic steroid drugs on the intrinsic resistances of gram-negative bacteria, particularly the effects that newly designed steroids may have on the evolution and function of multiple-drug efflux resistance mechanisms.
Acknowledgments
We thank Carl Cerniglia, Fatemeh Rafii, and R. Doug Wagner for critically reviewing the manuscript and providing several constructive comments during the course of the study. We also acknowledge a helpful suggestion from F. A. Martín to assess steroid efflux by other E. coli RND pumps, based on a preliminary account of this study presented at the 105th General Meeting of the American Society for Microbiology in Atlanta, GA.
This study was funded by the U.S. Food and Drug Administration and the National Center for Toxicological Research under protocol E0718001.
The views presented in this article do not necessarily reflect those of the Food and Drug Administration.
REFERENCES
- 1.Baranova, N., and H. Nikaido. 2002. The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 184:4168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, S. F., and P. B. Hylemon. 1997. Biotransformation of bile acids, cholesterol, and steroid hormones, p. 470-510. In R. I. Mackie and B. A. White (ed.), Gastrointestinal microbiology, vol. I. Gastrointestinal ecosystems and fermentations. International Thomson Publishing, New York, N.Y. [Google Scholar]
- 3.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkins, C. A., and L. B. Mullis. 2004. Bile-mediated aminoglycoside sensitivity in Lactobacillus species likely results from increased membrane permeability attributable to cholic acid. Appl. Environ. Microbiol. 70:7200-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkins, C. A., and H. Nikaido. 2003. Chimeric analysis of AcrA function reveals the importance of its C-terminal domain in its interaction with the AcrB multidrug efflux pump. J. Bacteriol. 185:5349-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkins, C. A., and H. Nikaido. 2002. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J. Bacteriol. 184:6490-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George, A. M., and S. B. Levy. 1983. Amplifiable resistance to tetracycline, chloramphenicol, and other antibiotics in Escherichia coli: involvement of a nonplasmid-determined efflux of tetracycline. J. Bacteriol. 155:531-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graves, V., E. Suraya, and O. Nishikiza. 1977. Hydrolysis of steroid glucuronides with β-glucuronidase from bovine liver, Helix pomatia and Escherichia coli. Clin. Chem. 23:532-535. [PubMed] [Google Scholar]
- 9.Hamilton, K. O., M. A. Yazdanian, and K. L. Audus. 2001. Modulation of P-glycoprotein activity in Calu-3 cells using steroids and beta-ligands. Int. J. Pharm. 228:171-179. [DOI] [PubMed] [Google Scholar]
- 10.Jerse, A. E., N. D. Sharma, A. N. Simms, E. T. Crow, L. A. Snyder, and W. M. Shafer. 2003. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 71:5576-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li, X. Z., and H. Nikaido. 2004. Efflux-mediated drug resistance in bacteria. Drugs 64:159-204. [DOI] [PubMed] [Google Scholar]
- 12.Nagakubo, S., K. Nishino, T. Hirata, and A. Yamaguchi. 2002. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 184:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulsen, I. T., J. Chen, K. E. Nelson, and M. H. Saier, Jr. 2001. Comparative genomics of microbial drug efflux systems. J. Mol. Microbiol. Biotechnol. 3:145-150. [PubMed] [Google Scholar]
- 15.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150:775-783. [DOI] [PubMed] [Google Scholar]
- 16.Prouty, A. M., I. E. Brodsky, J. Manos, R. Belas, S. Falkow, and J. S. Gunn. 2004. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol. Med. Microbiol. 41:177-185. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg, E. Y., D. Bertenthal, M. L. Nilles, K. P. Bertrand, and H. Nikaido. 2003. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48:1609-1619. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg, E. Y., D. Ma, and H. Nikaido. 2000. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver, S., and E. Levine. 1968. Action of steroidal diamines on active transport and permeability properties of Escherichia coli. J. Bacteriol. 96:338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Eldere, J., J. Robben, G. De Pauw, R. Merckx, and H. Eyssen. 1988. Isolation and identification of intestinal steroid-desulfating bacteria from rats and humans. Appl. Environ. Microbiol. 54:2112-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto, T., H. Higashi, A. Kanatani, X. S. Lin, H. Nagai, H. Oyama, K. Kurazono, and D. Tsuru. 1991. Cloning and sequencing of the 7 α-hydroxysteroid dehydrogenase gene from Escherichia coli HB101 and characterization of the expressed enzyme. J. Bacteriol. 173:2173-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, E. W., G. McDermott, H. I. Zgurskaya, H. Nikaido, and D. E. Koshland, Jr. 2003. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 300:976-980. [DOI] [PubMed] [Google Scholar]
- 25.Zgurskaya, H. I., and H. Nikaido. 1999. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:7190-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]