Abstract
Unlike Saccharomyces cerevisiae RNA polymerase III, human RNA polymerase III has not been entirely characterized. Orthologues of the yeast RNA polymerase III subunits C128 and C37 remain unidentified, and for many of the other subunits, the available information is limited to database sequences with various degrees of similarity to the yeast subunits. We have purified an RNA polymerase III complex and identified its components. We found that two RNA polymerase III subunits, referred to as RPC8 and RPC9, displayed sequence similarity to the RNA polymerase II RPB7 and RPB4 subunits, respectively. RPC8 and RPC9 associated with each other, paralleling the association of the RNA polymerase II subunits, and were thus paralogues of RPB7 and RPB4. Furthermore, the complex contained a prominent 80-kDa polypeptide, which we called RPC5 and which corresponded to the human orthologue of the yeast C37 subunit despite limited sequence similarity. RPC5 associated with RPC53, the human orthologue of S. cerevisiae C53, paralleling the association of the S. cerevisiae C37 and C53 subunits, and was required for transcription from the type 2 VAI and type 3 human U6 promoters. Our results provide a characterization of human RNA polymerase III and show that the RPC5 subunit is essential for transcription.
Eukaryotic cells contain three nuclear RNA polymerases, RNA polymerases I, II, and III, which are highly related to each other. RNA polymerase III synthesizes RNA components of the protein synthesis, pre-mRNA splicing, and tRNA processing apparatuses. Saccharomyces cerevisiae RNA polymerase III has been well defined, and all of its subunits have been characterized (see references 6 and 20 for reviews). Its two largest subunits are related to the β′ and β subunits of Escherichia coli RNA polymerase and form the catalytic core. They have paralogues in all multisubunit RNA polymerases characterized to date, including RNA polymerases I and II. The enzyme also contains two subunits shared with RNA polymerase I that correspond to the E. coli α subunits. Eight additional subunits are unique to RNA polymerase III, and five small subunits are shared with RNA polymerases I and II.
Much less is known about human RNA polymerase III. The enzyme has been purified both by conventional chromatography (49, 60) and from cell lines expressing tagged Homo sapiens RPC4 (HsRPC4)/HsRPC53/BN51 (57, 58), and cDNAs corresponding to five of its subunits [HsRPC4/HsRPC53 (26, 27); HsRPC1/HsRPC155 (50); and HsRPC3/RPC62, HsRPC6/RPC39, and HsRPC7/RPC32 (58)] have been characterized. For the other subunits, the available information is their apparent molecular weight on sodium dodecyl sulfate (SDS)-polyacrylamide gels (58, 60) and sequences present in various databases that display a high degree of similarity to the yeast RNA polymerase subunit sequences (49; reviewed in reference 25). Putative human orthologues of S. cerevisiae C128, the second largest RNA polymerase III subunit, and C37 have not been identified.
RNA polymerase III promoters can be divided into three types, called type 1, 2, and 3 promoters. The type 1 promoters are present in the ribosomal 5S genes, and the type 2 promoters are present in tRNA genes as well as in a number of other genes, including the adenovirus 2 VAI gene. Both types of promoters are gene-internal and recruit TFIIIC, either directly, in the case of type 2 promoters, or through prior binding of TFIIIA, in the case of type 1 promoters. The binding of TFIIIC then allows recruitment of the TFIIIB factor, which consists of TBP, the TFIIB-related factor Brf1, and the SANT domain protein Bdp1, followed by RNA polymerase III (reviewed in reference 20; see reference 63 for a universal nomenclature of TFIIIB subunits).
The type 3 promoters, exemplified by the human U6 promoter, have a gene-external promoter whose core elements comprise a proximal sequence element that recruits the five-subunit complex SNAPc (also called PSE transcription factor [PTF] or PSE binding protein [PBP]) and a TATA box that recruits a specialized version of TFIIIB in which the Brf1 polypeptide is replaced by Brf2 (20, 47, 53). Additional factors such as Bfr2-associated polypeptides, the autoantigen La, topoisomerase 1, PC4, and NF1 polypeptides have also been implicated in human RNA polymerase III transcription (21, 22, 34, 35, 53, 56, 59). However, the reconstitution of a completely defined RNA polymerase III transcription system from human cells has been hampered by the lack of a defined RNA polymerase III preparation.
As a first step towards the reconstitution of an entirely defined RNA polymerase III transcription system from HeLa cells, we have purified a human RNA polymerase III complex and determined its composition. This analysis provides the identification of human orthologues for all but the smallest of the yeast RNA polymerase III subunits. In particular, we characterized the second largest subunit of human RNA polymerase II and identified two human RNA polymerase III subunits as paralogues of the RPB7 and RPB4 subunits of RNA polymerase II. We also show that an 80-kDa protein with limited sequence similarity to S. cerevisiae C37 is the human orthologue of S. cerevisiae C37 and is required for transcription from type 2 and 3 promoters.
MATERIALS AND METHODS
Constructs.
The pBabeBN51Flag/His construct is a derivative of pBabepuro (41) containing a cDNA fragment encoding C-terminal Flag and His-tagged full-length RPC4/HsRPC53 (GenBank accession number AY092086). cDNA fragments encoding N-terminally hemagglutinin (HA)-tagged RPC4/HsRPC53 or RPC4/HsRPC53 amino acids 1 to 253 or RPC4/HsRPC53 amino acids 254 to 398 were cloned into a derivative of the vector pCITE (Novagen).
The RPC5 and RPC8 coding sequences were subcloned from cDNA clones KAT11904 and KAT02533 (kind gifts of H. Hata and S. Sugano, Department of Virology, University of Tokyo, Tokyo, Japan), which contained full-length open reading frames. The sequence of the cDNA clone KAT11904 was confirmed (GenBank accession number AY092085), and the RPC5 coding region was amplified and inserted into a pSBet vector (46) modified to fuse a Flag and a His tag to the N and C termini of the protein, respectively. cDNA fragments encoding RPC5, RPC5 amino acids 1 to 163, or RPC5 amino acids 164 to 854 were also cloned into a derivative of the vector pCITE (Novagen). The sequence of the cDNA clone KAT02533 was confirmed (GenBank accession number AY092087), and the RPC8 coding region was amplified and cloned into pCITE and a derivative of pCITE in which an HA tag is fused to the N terminus of the protein. The RPC9 coding sequence was amplified from a human cDNA library and inserted into the same two vectors.
To assemble the sequence encoding RPC2 (GenBank accession number AY092084), we used the sequence AK001161 (encoding the protein sequence BAA91527.1, identified by mass spectrometry), which encodes the last 342 amino acids of RPC2 to search the expressed sequence tag (EST) database. With successive rounds of Blast (1) searches, we assembled a putative sequence encoding a protein with high similarity to the S. cerevisiae C128 polypeptide. We then sequenced the clones CS0DI084YD19 (GenBank accession number AL577130), which contained nucleotides 1 to 2136 of our sequence, and 3452733 (GenBank accession number BE541441), which contained nucleotides 2684 to 4102. The missing internal sequences were amplified by PCR from a commercial HeLa cell cDNA library.
Generation of cell lines expressing tagged RPC4/HsRPC53.
We transfected 3 μg of the plasmid pBabeBN51Flag/His together with 25 μg of salmon sperm DNA into HeLa S cells by electroporation. The transfected cells were selected by addition of 1 μg of puromycin per ml to the medium, and clonal cell lines were derived by serial dilution and screened for tagged RPC4/HsRPC53 expression levels. One clone expressing high levels of RPC4 (PH9-8) was selected for further studies.
Purification of tagged RNA polymerase III complex.
Whole-cell extracts (36) (12 mg of protein/ml) prepared from 48 liters of HeLa cells or from the clonal cell line PH9-8 were fractionated by ammonium sulfate precipitation. The proteins precipitated between 18 and 40% ammonium sulfate were dissolved in TBS120 buffer (50 mM Tris-HCl [pH 8.0], 120 mM NaCl, 5% glycerol) to a final salt concentration of 150 mM and loaded onto anti-Flag immunoaffinity beads (Sigma). The anti-Flag beads were rotated overnight at 4°C and washed with 30 column volumes of TBS300 and 20 column volumes of TBS150. The bound proteins were eluted with 5 column volumes of a Flag peptide gradient ranging from 100 to 300 μg of peptide per ml in TBS150 buffer.
The fractions active for U6 transcription were pooled, adjusted to 300 mM NaCl by dilution with buffer A (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, adjusted to pH 8.0), and incubated with Ni2+-nitrilotriacetic acid (NTA) agarose beads (Qiagen) overnight at 4°C. The beads were washed with buffer B (50 mM NaH2PO4, 20 mM imidazole, pH 8.0) containing 300 or 600 mM NaCl as described in the figure legends. The bound proteins were eluted with 5 column volumes of buffer B containing 250 to 300 mM imidazole. The Ni2+-NTA fractions were dialyzed against buffer D100 (50 mM HEPES [pH 7.9], 0.2 mM EDTA, 20% glycerol, 0.1% Tween 20, 100 mM KCl, 3 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride).
Protein identification by mass spectrometry.
The purified RNA polymerase III complex was fractionated on an SDS-4 to 20% polyacrylamide gel and visualized by Coomassie blue staining. All the visible bands were excised for mass spectrometry identification. The samples were subjected to both matrix-assisted laser desorption ionization (MALDI) and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. MALDI spectra were acquired with a Perseptive Biosystems DE-PRO, the data were analyzed by m/z (ProteoMetrics), and protein identification was performed with the Profound search engine (65). The spectra resulting from LC-MS/MS were analyzed with the Sequest (14) software or the SONARS (ProteoMetrics) software or interpreted manually.
Conventional purification of RNA polymerase III complex.
Endogenous RNA polymerase III complex was purified as described previously (49).
In vitro transcriptions.
In vitro transcription reactions were performed as described previously (31, 47). Immunodepletions were performed with a 2:1 volume ratio of beads covalently coupled to antibodies to extract overnight at 4°C.
RESULTS
Nomenclature of RNA polymerase III subunits.
The first column of Table 1 shows the commonly used nomenclature for the subunits of S. cerevisiae RNA polymerase III (6, 20). The fourth column lists our suggested names, in which the letters A, B, and C indicate whether the subunits belong only to RNA polymerase III (C) or are common to RNA polymerases I and III (AC) or I, II, and III (ABC), as in the original nomenclature of Sentenac and colleagues (6, 48). The numbers 1, 2, 3, etc., order separately the S. cerevisiae subunits unique to RNA polymerase III, those common to RNA polymerases I and III, and those common to all three RNA polymerases by decreasing apparent molecular weight. The nomenclature could be expanded to include the unique RNA polymerase I and II subunits (which would be listed as RPA1, RPA2, etc., and RPB1, RPB2, etc.) and in some cases is already in use in the GenBank database. In this article, we use this proposed nomenclature for the S. cerevisiae subunits as the guide to refer to the human RNA polymerase III subunits, as indicated in Table 1, and consequently the numbering of the human subunits does not always correspond to their decreasing apparent molecular weights.
TABLE 1.
S. cerevisiae and H. sapiens RNA polymerase (pol) III subunitsa
| S. cerevisiae RNA pol III subunits | Size (kDa) | Accession no. | Suggested nomenclature | Corresponding S. cerevisiae RNA pol II subunits | H. sapiens RNA pol III subunits | Size (kDa) | Accession no. | % Amino acid identities between H. sapiens and S. cerevisiae pol III subunitsb |
|---|---|---|---|---|---|---|---|---|
| C160 (β′-like) | 162.1 | P04051 | ScRPC1 | RPB1 | HsRPC1/RPC155 | 155.6 | AAB86536 | 50 (1,356/1,391) |
| C128 (β-like) | 129.3 | AAB59324 | ScRPC2 | RPB2 | HsRPC2 | 127.6 | AY092084 | 63 (1,115/1,133) |
| C82 | 73.6 | CAA45072 | ScRPC3 | HsRPC3/RPC62 | 60.5 | NP_006459/XP_034604 | 22 (163/534) | |
| C53 | 46.6 | P25441 | ScRPC4 | HsRPC4/RPC53 | 44.4 | AY092086 | 28 (134/398) | |
| C37 | 32.1 | NP_012950 | ScRPC5 | HsRPC5 | 79.8 | AY092085 | 26 (160/708) | |
| C34 | 36.1 | P32910 | ScRPC6 | HsRPC6/RPC39 | 35.6 | NP_006457/XP_009639 | 26 (216/316) | |
| C31 | 27.7 | P17890 | ScRPC7 | HsRPC7/RPC32 | 25.9 | AAB63676/XP_036456 | 35 (44/223) | |
| C25 | 24.3 | P35718 | ScRPC8 | RPB7 | HsRPC8 | 22.9 | AY092087 | 42 (201/204) |
| C17 | 18.6 | P47076 | ScRPC9 | RPB4 | HsRPC9/CGRP-RC | 16.8 | AAC25992 | 30 (122/148) |
| C11 | 12.5 | AAD12060 | ScRPC10 | RPB9 | HsRPC10/RPC11 | 12.8 | NP_057394 | 52 (108/108) |
| AC40 (α-like) | 37.6 | P07703 | ScRPAC1 | RPB3 | HsRPAC1/RPA5,RPA39 | 38.6 | NP_004866 | 47 (287/342) |
| AC19 (α-like) | 16.1 | P28000 | ScRPAC2 | RPB11 | HsRPAC2/RPA9,RPA16 | 15.2 | NP_057056 | 45 (119/133) |
| ABC27 | 25.1 | P20434 | ScRPABC1 | RPB5 | HsRPABC1/RPB5,RPB25 | 24.6 | P19388 | 42 (207/210) |
| ABC23 (ω-like) | 17.9 | AAA34989 | ScRPABC2 | RPB6 | HsRPABC2/RPB6,RPB14.4 | 14.5 | P41584 | 72 (83/127) |
| ABC14.5 | 16.5 | CAA37383 | ScRPABC3 | RPB8 | HsRPABC3/RPB8,RPB17 | 17.1 | P52434 | 35 (147/150) |
| ABC10α | 7.7 | AAA64417 | ScRPABC4 | RPB12 | HsRPABC4/RPB7.0 | 7.0 | P53803 | 52 (42/58) |
| ABC10β | 8.2 | P22139 | ScRPABC5 | RPB10 | HsRPABC5/RPB10,RPB7.6 | 7.6 | P52436 | 73 (67/67) |
Subunits in bold do not have paralogues in RNA polymerases I and II; those in bold and underlined form a complex separable from the rest of the enzyme (58, 61, 62). Subunits corresponding to the E. coli β′, β, α, and ω subunits are indicated. ABC27 and RPB5, ABC23 and RPB6, ABC14.5 and RPB8, ABC10α and RPB12, and ABC10β and RPB10 in each case designate the same protein. For HsRPC62, HsRPC39, and HsRPC32, the sequence under the first accession number (58) differs in several positions from both the sequences deposited by NCBI (second accession number) and genomic sequences.
The first number in parentheses indicates the length of the region of similarity, and the second number indicates the total length of the human protein.
Purification of an RNA polymerase III complex.
We generated stable cell lines expressing the RPC4/HsRPC53 subunit of RNA polymerase III (26, 27, 49) fused to both Flag and His tags at its C terminus. Purification of the RNA polymerase III complex was achieved by ammonium sulfate precipitation followed by anti-Flag immunoaffinity and nickel agarose chromatography, as summarized in Fig. 1A. Figure 1B shows the polypeptide pattern obtained after fractionation of the resulting material by SDS-polyacrylamide gel electrophoresis (PAGE). A few background bands were visible in the material purified from control cells with only untagged RPC4/HsRPC53 (Fig. 1A, lane 1), but these bands disappeared when the Ni2+-NTA column was washed with 600 mM KCl (Fig. 1A, lane 3). In contrast, the polypeptide bands visible in material purified from tagged RPC4/HsRPC53-containing cells were not reduced by a high-salt wash (Fig. 1A, compare lanes 2 and 4).
FIG. 1.
Composition of the tagged RNA polymerase III complex. (A) Purification scheme of the tagged RNA polymerase III complex. (B) Polypeptide composition of the purified tagged RNA polymerase III. Whole-cell extracts (WCE) from either HeLa cells (lanes 1 and 3) or HeLa cells expressing tagged RPC4/HsRPC53 (lanes 2 and 4) were used as starting material for the purification scheme summarized in A. The proteins were separated on an SDS-4 to 20% polyacrylamide gel and stained with Coomassie blue. In lanes 1 and 2, the nickel-agarose beads were washed with a buffer containing 300 mM KCl before elution with 250 mM imidazole. In lanes 3 and 4, the beads were washed with 600 mM KCl. The positions of the molecular size markers are shown on the left (in kilodaltons). The identities of each polypeptide as determined by mass spectrometry analysis are indicated on the right. Solid circles indicate cytoskeletal and other known proteins that were not characterized further. Triangles indicate previously characterized RNA polymerase III subunits. Asterisks indicate polypeptides whose function has not been analyzed before in the context of RNA polymerase III transcription.
We identified each visible band by mass spectrometry and grouped them into three categories. The bands labeled with a solid circle in Fig. 1B correspond to cytoskeletal and other known abundant proteins and were not characterized further. The bands labeled with a triangle correspond to previously characterized RNA polymerase III subunits [RPC1/RPC155 (50), RPC3/RPC62, RPC6/RPC39, and RPC7/RPC32 (58), RPC4/HsRPC53 (26, 27, 50), and RPABC1, RPABC2, RPABC3, and RPABC5 (8, 38, 40, 51)]. The bands labeled with asterisks correspond to either known [RPC9/HsCGRP (Homo sapiens calcitonin gene-related peptide) receptor component (18, 33), RPC10 (7), and RPAC1/RPA39 (12)] or novel (RPC2, RPC5, RPC8, and RPAC2) polypeptides whose function has not been analyzed in the context of human RNA polymerase III transcription but that show different degrees of similarity to S. cerevisiae RNA polymerase III subunits.
Structure of RPC2, the second largest subunit of RNA polymerase III.
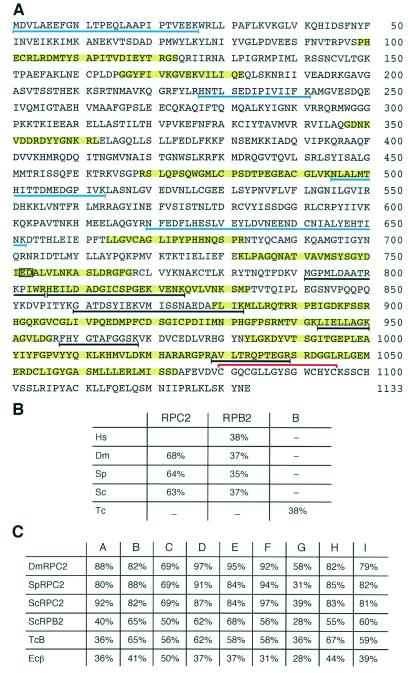
Mass spectrometry analysis of the polypeptide labeled RPC2 gave matches in two protein sequences (NP_060552.1 and BAA91527.1) that correspond to the C-terminal part of the full-length protein sequence. These were used as a starting point to assemble, through database searches and PCR amplification from a cDNA library, a cDNA encoding the 1,133-amino-acid protein depicted in Fig. 2A (GenBank accession number AY092084). The thick black underlines show the first peptide matches identified by mass spectrometry. These clustered towards the C terminus of the protein because only the C terminus of RPC2 was represented in the database. The blue underlines show the additional matches obtained upon reanalysis of the original mass spectrometry data with the full-length sequence available.
FIG. 2.
Structure of HsRPC2. (A) Predicted protein sequence of HsRPC2 (GenBank accession number AY092084). The thick black underlines show the first peptide matches identified by mass spectrometry. These cluster towards the C terminus of the protein because only the C terminus of RPC2 was represented in the database. The blue underlines show the additional matches obtained upon reanalysis of the original mass spectrometry data with the full-length sequence. The homology blocks A to I (52) are shaded in yellow. The invariant E and D residues corresponding to those in close proximity to metal B in the structure of S. cerevisiae RNA polymerase II (11) are boxed. The zinc-binding domain is bracketed in red. (B) The percent identities between H. sapiens (Hs) RPC2 and the second largest subunits of D. melanogaster (Dm), S. pombe (Sc), and S. cerevisiae (Sc) RNA polymerase III (RPC2) and II (RPB2), as well as T. celer (Tc) polypeptide B, are indicated. The sequences were aligned with Blast. (C) The percent identities between HsRPC2 and the indicated second-largest subunits, including the E. coli β subunit (Ec β) in the conserved A to I blocks are shown.
The protein HsRPC2 had a calculated molecular mass of 128 kDa and an isoelectric point of 8.6. As shown in Fig. 2B, it was more similar to the second-largest RNA polymerase III subunits of Drosophila melanogaster, Schizosaccharomyces pombe, and S. cerevisiae (68, 64, and 63% identical, respectively) than to the human second-largest RNA polymerase II subunit (38% identical), consistent with its identification as the second-largest subunit of human RNA polymerase III. The protein was also related to RNA polymerase B subunits from archaea (for example, 38% identical to that of Thermococcus celer) and to the β subunit of E. coli RNA polymerase.
The blocks highlighted in yellow in Fig. 2A correspond to the A to I sequence homology blocks originally noted in an alignment of the E. coli β subunit with the second-largest subunit of S. cerevisiae RNA polymerase II (52) and then confirmed by structural studies (11). Figure 2C shows the percent identities within these blocks between HsRPC2 and the second-largest subunits of several RNA polymerases. All blocks were highly conserved in the human RPC2 subunit. Region F contained the invariant E and D residues (positions 752 and 753, boxed in Fig. 2A), corresponding to the E836 and D837 residues of the S. cerevisiae orthologue found in the active site in close proximity to a metal ion (11). Like all other eukaryotic RNA polymerase second-largest subunits analyzed to date, and unlike the E. coli β subunit (48), HsRPC2 also contained a zinc-binding domain near its C terminus (Fig. 2A, red bracket), which is part of the clamp in the crystal structure of yeast RNA polymerase II (11).
RPC8, RPC9, RPC10, RPAC1, and RPAC2: human orthologues of S. cerevisiae RPC25, RPC17, RPC11, RPAC40, and RPAC19, respectively.
The bands labeled RPC8, RPC9, RPC10, RPAC1, and RPAC2 in Fig. 1B all gave matches in protein GenBank entries with strong similarity to yeast RNA polymerase III subunits. Thus, RPC8 was 42% identical to S. cerevisiae C25, RPC9 was 30% identical to C17, as noted before (18, 33), RPC10 was 52% identical to C11, as noted before (7), RPAC1 was 47% identical to AC40, and RPAC2 was 45% identical to AC19. These findings are indicated in Table 1, as well as the calculated molecular weights of these proteins and the corresponding GenBank accession numbers. The observation that these polypeptides with high similarity to yeast RNA polymerase III subunits are indeed present in the highly purified RNA polymerase III complex confirms their identity as subunits of human RNA polymerase III.
RPC8 and RPC9 are paralogues of the RNA polymerase II subunits RPB7 and RPB4.
A Blast search (1) with the human RPC8 sequence as the query revealed that the N-terminal region of the protein is similar to the N-terminal region of the RNA polymerase II RPB7 subunit from a number of species (not shown), as noted before for the S. cerevisiae C25 orthologue (44). Figure 3A shows a Clustal W Fast (54) alignment of the HsRPC8 subunit with the RNA polymerase II RPB7 subunits from S. pombe, S. cerevisiae, and H. sapiens. This alignment shows 22 identical (13%; stars in Fig. 3A) and a total of 62 identical and similar (36%; stars and colons in Fig. 3A) amino acids out of the N-terminal 172 amino acids of the human sequence in all four polypeptides. This region encompasses the sequences that are predicted to assume an OB-fold motif (4) in the S. cerevisiae RPB7 subunit (43) and that were shown to do so in the crystal structure of an archaeal homologue (55). The OB-fold motif is present in the E. coli ribosomal protein S1 (4) as well as in many other proteins capable of binding single-stranded nucleic acids (42).
FIG. 3.
HsRPC8 and RPC9 are paralogues of the RNA polymerase II RPB7 and RPB4 subunits, respectively. (A) Clustal W Fast alignment of H. sapiens (Hs) RPC8 and the S. pombe (Sp), S. cerevisiae (Sc), and H. sapiens (Hs) RPB7 subunits performed with default parameters. Stars indicate identical amino acids, colons indicate similar amino acids (A, V, F, P, M, I, L, and W; D and E; R, K, and H; and S, T, Y, H, C, N, G, and Q are considered similar), and periods indicate semiconserved substitutions. (B) Clustal W Fast alignment of H. sapiens (Hs) RPC9 and the S. pombe (Sp), S. cerevisiae (Sc), and H. sapiens (Hs) RPB4 subunits performed with default parameters. The symbols are the same as in A. (C) HsRPC8 and HsRPC9 associate with each other. Untagged and HA-tagged HsRPC8 and HsRPC9 were translated in vitro with [35S]methionine and used for coimmunoprecipitation experiments. Lanes 1, 2, 5, and 6 show 1 μl of each of the in vitro translation reaction mixes loaded directly on the gel (input). In lanes 3, 4, 7, and 8, the proteins indicated above the lanes were used for immunoprecipitation (IP) with anti-HA antibody beads. The immunoprecipitates were washed with a buffer containing 300 mM KCl. The circles and triangles indicate untagged and tagged proteins, respectively.
Strikingly, a Blast search with HsRPC9 as the query then identified a conserved domain also found in the RNA polymerase II RPB4 subunits of S. pombe and S. cerevisiae (not shown). The Clustal W Fast alignment of this sequence with the RNA polymerase II RPB4 subunits from the same species as above is shown in Fig. 3B. In this case, the alignment showed 11 identical amino acids (9%) and a total of 40 (31%) identical and similar amino acids among the N-terminal 128 amino acids of HsRPC9 in all four polypeptides.
In RNA polymerase II, RPB4 forms a heterodimer with RPB7 that reversibly associates with the core enzyme (15). We therefore tested whether the RNA polymerase III RPC8 and RPC9 subunits might similarly associate with one another in the absence of the other RNA polymerase III subunits. We generated both HA-tagged and untagged RPC8 and RPC9 by translation in vitro and tested them in coimmunoprecipitation experiments. As shown in Fig. 3C, these proteins were well expressed (lanes 1, 2, 5, and 6). Untagged RPC8 and RPC9 were not immunoprecipitated by anti-HA antibodies, as expected (Fig. 3C, lanes 3 and 7). However, when untagged RPC8 was mixed with HA-tagged RPC9, both proteins were recovered in the immunoprecipitate (Fig. 3C, lane 4). The same was true when untagged RPC9 was mixed with HA-tagged RPC8 (Fig. 3C, lane 8). Thus, like RPB7 and RPB4, HsRPC8 and HsRPC9 associate with each other. Together with the sequence similarities linking RPC8 to RPB7 and RPC9 to RPB4, this observation strongly suggests that the HsRPC8 and HsRPC9 subunits are in fact paralogues of the RNA polymerase II RPB7 and RPB4 subunits, respectively. We have therefore indicated them as such in Table 1.
Identification of RPC5, a human orthologue of S. cerevisiae C37.
One of the prominent bands in the purified RNA polymerase III complex (labeled RPC5 in Fig. 1B) corresponded to the protein encoded by the cDNA clone KAT11904 (GenBank accession number AK026645), shown in Fig. 4A. The protein had a calculated molecular mass of 79.8 kDa and an isoelectric point of 5.9. The peptides identified by mass spectrometry are underlined with thick lines, and a glutamate-rich region is boxed.
FIG. 4.
Structure of RPC5. (A) Predicted RPC5 protein sequence (GenBank accession number AY092085). The thick underlines show peptide matches identified by mass spectrometry, the thin underlines indicate synthetic peptides used to generate antibodies (CS1534, amino acids 1 to 9; CS1542, amino acids 207 to 222), and the shaded box shows the glutamic acid-rich domain. (B) Regions of similarity between HsRPC5 and a Mus musculus (Mm) homologue of Drosophila sex-lethal interactor (BAB23761), the Drosophila sex-lethal interactor (Dm SIN, AAF51670 [13]), putative proteins from A. thaliana (At) (NP_199764), C. elegans (Ce) (AAK29861), S. pombe (Sp) (T41323), and S. cerevisiae (Sc) C37 (NP_012950). The percentages show amino acid identities between the colored regions of the various proteins and the regions in HsRPC5 delimited by thin lines of corresponding color as determined by the Blast program. The numbers indicate amino acid numbers. (C) Blast alignment of H. sapiens RPC5 and S. cerevisiae C37.
As shown in Fig. 4B, the sequence was highly similar over its entire length to the mouse homologue of Drosophila sex-lethal interactor (Mm BAB23761; 85% identical; shown in orange) and over its N-terminal 425 amino acids to Drosophila sex-lethal interactor itself (Dm SIN, shown in green). Drosophila SIN, whose function is unknown, is identified as a protein interacting in a two-hybrid assay with the RNA binding domain 1 of D. melanogaster sex-lethal (13), a regulator of alternative RNA splicing and translation (2, 3, 28). The N-terminal 444, 431, 228, and 163 amino acids were also similar to Caenorhabditis elegans, Arabidopsis thaliana, and S. pombe open reading frames and, very interestingly, to the S. cerevisiae RNA polymerase III subunit C37, respectively. The Blast (1) alignment of the human and S. cerevisiae proteins is shown in Fig. 4C. The two proteins showed 26% identities over a region limited to the N-terminal 163 amino acids of the 80-kDa human protein. As described below, our data suggest that the 80-kDa protein is a human orthologue of S. cerevisiae C37, and we therefore refer to it as HsRPC5.
RPC5 is a subunit of human RNA polymerase III.
We have previously developed a protocol, shown in Fig. 5A, to partially purify an untagged RNA polymerase III complex (49). We used a phosphocellulose (P11) fraction from the second step of this purification as well as a doubly tagged recombinant RPC5 for immunoblotting with an anti-RPC5 antibody. As shown in Fig. 5B, the antibody reacted with a polypeptide in the P11 fraction (lane 1) that migrated slightly faster than the doubly tagged recombinant RPC5 (lane 3), and the interaction was abolished by blocking of the antibody with the peptide against which it was raised (lanes 2 and 4). This indicates that the antibody is specific and that the cDNA very likely encodes a full-length protein.
FIG. 5.
RPC5 copurifies and is associated with RPC1/RPC155 in cells expressing only untagged RNA polymerase III. (A) Conventional purification scheme of an untagged RNA polymerase III complex (49). WCE, whole-cell extract; CV, column volume. (B) Doubly tagged RPC5 expressed in E. coli migrates slightly slower than endogenous RPC5 from HeLa cells. An active P11 phosphocellulose fraction (8 μl, 427 μg/ml) (lanes 1 and 2) or 2 μl of doubly tagged, purified recombinant RPC5 (11.2 μg/ml) (lanes 3 and 4) were fractionated by SDS-PAGE, and the proteins were transferred to nitrocellulose. The membrane was then cut and probed with an anti-RPC5 antibody (CS1542) for lanes 1 and 3 or with the same antibody preincubated with the peptide against which it was raised for lanes 2 and 4. The positions of RPC5 and the molecular size markers (in kilodaltons) are indicated. (C) Endogenous RPC5 is associated with other RNA polymerase subunits in vivo. A HeLa cell nuclear extract was used as the starting material for nondenaturing immunoprecipitations (IP) with the antibodies indicated above the lanes, and the immunoprecipitates were fractionated by SDS-PAGE, transferred to a membrane, and probed by immunoblot with the antibodies indicated at the right of the three panels. preimm., preimmune serum. (D) Copurification of RPC5 and RPC1/RPC155 during Mono Q chromatography. Mono Q fractions 1 to 20 (lanes 1 to 20, respectively) and Mono Q flowthrough (FT, lane 21) were analyzed by immunoblot with the antibodies indicated at the right of the two panels.
We used this antibody as well as an anti-RPC1/RPC155 antibody for nondenaturing immunoprecipitations from HeLa cells. As shown in the upper panel of Fig. 5C, both RPC4/HsRPC53 and RPC1/RPC155 could be detected in material immunoprecipitated with anti-RPC5 (lane 2) but not with preimmune antibodies (lane 1). Similarly, RPC5 was specifically present in material immunoprecipitated with anti-RPC1/RPC155 antibodies (Fig. 5C, lower panel, lane 2). Moreover, we analyzed each Mono Q fraction from the penultimate step in the purification of untagged RNA polymerase III (see Fig. 5A). Like RPC4/HsRPC53 and U6 transcription activity (49), RPC5 coeluted exactly with RPC1/RPC155 (Fig. 5D, lane 11). Thus, RPC5 is associated with other RNA polymerase III subunits not only in cell lines expressing a tagged RPC4/HsRPC53 subunit, as shown in Fig. 1B, but also in cells expressing only endogenous RPC4/HsRPC53, suggesting that it is indeed an RNA polymerase III subunit.
RPC5 is required for transcription from both the VAI and U6 promoters but not from the U1 and adenovirus 2 major late promoters.
To explore the role of RPC5 in transcription, we immunodepleted extracts of endogenous RPC5 and tested the depleted extracts for transcription from two types of RNA polymerase II promoters, the U1 snRNA promoter and the adenovirus 2 major late mRNA promoter. In both cases, transcription was unaffected by the depletion and by addition of either recombinant RPC5 or purified tagged RNA polymerase III complex (data not shown).
We next tested the immunodepleted extracts for transcription from the RNA polymerase III type 2 VAI promoter and type 3 U6 snRNA promoter. In preliminary experiments, we had noticed that immunodepletion with the anti-RPC5 antibodies rendered the concentration of certain RNA polymerase III transcription factors other than RPC5 limiting. This is probably because the depletions were performed in relatively low salt concentrations (80 to 100 mM KCl), and under these conditions, RNA polymerase III is known to associate with several of its transcription factors (58). Therefore, before depletion with the anti-RPC5 antibody CS1534, the extracts were supplemented with recombinant TATA box binding protein (TBP) and Brf1 for the VAI transcriptions and TBP, Brf2, and SNAPc for the U6 transcriptions. Depletion with another anti-RPC5 antibody, CS1542, did not render SNAPc limiting, and thus, in this case, SNAPc was not added.
In sharp contrast to what was observed with RNA polymerase II promoters, depletion with the anti-RPC5 antibodies strongly reduced transcription from the U6 snRNA and VAI promoters, as shown in Fig. 6A and C (compare lanes 2 to lanes 1). In an extract depleted with the anti-RPC5 antibody CS1534, transcription from both the U6 and the VAI promoters was restored by addition of purified RNA polymerase III complex but not recombinant RPC5 (Fig. 6A, lanes 3 to 7). This suggested that the antibody removed not only RPC5 but also additional RNA polymerase III subunits. Indeed, as shown in Fig. 6B, the levels of both RPC5 and RPC1/RPC155 were strongly reduced in extracts depleted with the CS1534 antibodies (lanes 2 and 4) compared to extracts depleted with preimmune antibodies (lanes 1 and 3). These results indicate that either RPC5 itself or RPC5-associated polypeptides are required for RNA polymerase III transcription.
FIG. 6.
RPC5 is required for RNA polymerase III transcription. (A) Addition of purified tagged RNA polymerase III (pol III) complex restores transcription in CS1534-treated extracts. HeLa whole-cell extracts were supplemented with 30 ng of recombinant TBP and either 80 ng of recombinant Brf1 (VAI transcription, upper panel) or 30 ng of recombinant TBP, 50 ng of recombinant Brf2, and 400 ng of recombinant SNAPc (third panel, U6 transcription), as indicated on top of the figure. The extracts were then incubated with either preimmune (preimm., lane 1) or anti-RPC5 CS1534 (lane 2 to 7) antibody beads. The treated extracts were complemented with 50 (lane 3) and 100 (lane 4) ng of recombinant RPC5 or with 30 (lane 5), 150 (lane 6), and 300 (lane 7) ng of purified tagged RNA polymerase III complex and tested for VAI or U6 transcription. The bands labeled IC correspond to the internal control signals. (B) Both RPC5 and RPC1/RPC155 are removed from the extracts by CS1534 treatment. The same extracts complemented with TBP and Brf1 or TBP, Brf2 and SNAPc used for VAI (lanes 1 and 2) or U6 (lane 3 and 4) transcription in panel A were analyzed by immunoblotting with the antibodies indicated on the right. (C) RPC5 itself, not only associated proteins, is essential for RNA polymerase III transcription. HeLa whole-cell extracts were complemented with TBP and Brf1 or Brf2 as in A. The extracts were then incubated with either preimmune (lane 1) or anti-RPC5 CS1542 (lanes 2 to 8) antibody beads. The treated extracts were complemented with 10 (lane 3), 50 (lane 4), and 100 (lane 5) ng of recombinant RPC5 or 30 (lane 6), 150 (lane 7), and 300 (lane 8) ng of purified tagged RNA polymerase III complex. (D) RPC1/RPC155 is present in the CS1542-depleted extracts. The same extracts complemented with TBP and Brf1 or TBP and Brf2 and used for VAI (lanes 1 and 3) or U6 (lanes 2 and 4) transcription in panel C were analyzed by immunoblotting with the antibodies indicated on the right.
Surprisingly, in extracts depleted with the CS1542 antibody, both VAI and U6 transcription could be reconstituted not only by addition of purified tagged RNA polymerase III complex, as expected, but also by addition of recombinant RPC5, as shown in Fig. 6C, lanes 3 to 8. In this case, depletion with the antibody removed RPC5 but not RPC1/RPC155 (Fig. 6D, compare lanes 3 and 4 to lanes 1 and 2). Thus, it appears that the anti-RPC5 antibody CS1542 disrupts the association of RPC5 with the rest of RNA polymerase III. Importantly, the ability to restore transcription by addition of just recombinant RPC5 indicates that the RPC5 polypeptide itself is absolutely required for efficient transcription from both the type 2 VAI and type 3 U6 RNA polymerase III promoters.
Interaction between S. cerevisiae C53 and C37 is conserved in the corresponding RPC4/HsRPC53 and RPC5 human subunits.
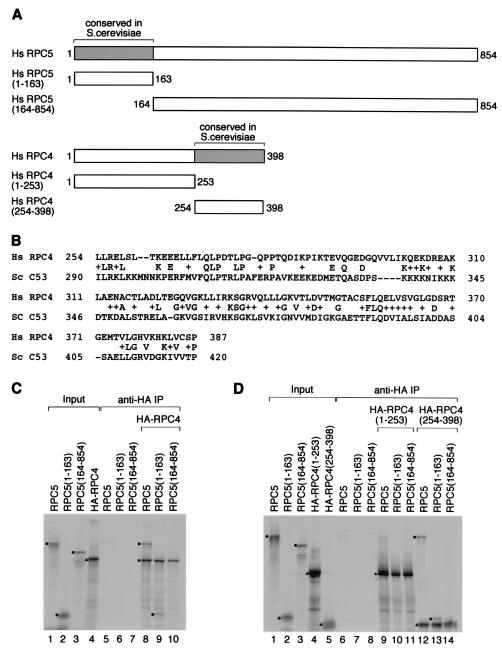
Most RNA polymerase subunits show a high degree of conservation between S. cerevisiae and human cells. Two notable exceptions are RPC4/HsRPC53 and RPC5. As mentioned above and illustrated in Fig. 7A, human RPC5 showed similarity to S. cerevisiae C37 in its N-terminal 163 amino acids (see alignment in Fig. 4C); RPC4/HsRPC53 showed similarity to S. cerevisiae C53 in its C-terminal part (Fig. 7A), where it was 28% identical to the C-terminal part of the S. cerevisiae protein, as shown in Fig. 7B. The C37 and C53 subunits interact with each other in a yeast two-hybrid assay (19). We tested whether this property was conserved in the human subunits by coimmunoprecipitation. We generated full-length RPC4/HsRPC53 and RPC5 by translation in vitro as well as truncated forms consisting in each case of either the sequences conserved or not conserved in the S. cerevisiae subunits, as illustrated in Fig. 7A. All the RPC4/HsRPC53 constructs carried an N-terminal HA tag.
FIG. 7.
RPC5 associates with HsRPC4/RPC53. (A) RPC5 and RPC4 derivatives used for immunoprecipitations. The regions conserved in the S. cerevisiae counterparts are indicated. The numbers refer to amino acids. (B) Blast alignment of H. sapiens RPC4/RPC53 and S. cerevisiae C53. (C) RPC5 amino acids 1 to 163 are sufficient for association with HsRPC4/RPC53. Full-length and truncated RPC5 proteins as well as HA-tagged full-length HsRPC4/RPC53 were translated in vitro with [35S]methionine and used for coimmunoprecipitation experiments. Lanes 1 to 4 show 1 μl of each of the in vitro translation reaction mixes loaded directly on the gel (input). In lanes 5 to 7, the proteins indicated above the lanes were used for immunoprecipitation (IP) with anti-HA antibody beads. In lanes 8 to 10, the proteins indicated above the lanes were mixed with HA-tagged HsRPC4/RPC53 before immunoprecipitation. The immunoprecipitates were washed with a buffer containing 300 mM KCl. The circles and triangles indicate untagged and tagged proteins, respectively. (D) HsRPC4/RPC53 amino acids 254 to 398 are sufficient for interaction with RPC5 amino acids 1 to 163. The proteins indicated above the lanes were either loaded directly (lanes 1 to 5) or used for coimmunoprecipitation experiments as in C. The circles and triangles are as in C.
As shown in Fig. 7C, full-length RPC5, truncated RPC5 containing the first 163 amino acids [RPC5(1-163)] or amino acids 164 to 854 [RPC5(164-854)], and HA-RPC4/HsRPC53 were well expressed in a reticulocyte lysate (lanes 1 to 4), and none of the untagged RPC5 proteins were immunoprecipitated by anti-HA antibodies (lanes 5 to 7). However, when the various RPC5 proteins were mixed with HA-RPC4/HsRPC53, full-length RPC5 as well as RPC5(1-163) but not RPC5(164-854) was coimmunoprecipitated with HA-RPC4/HsRPC53 (Fig. 7C, lanes 8 to 10). Thus, the part of RPC5 that shows sequence conservation with yeast C37 associates with RPC4/HsRPC53.
Next, we mapped the region of RPC4/HsRPC53 that interacts with RPC5. As shown in Fig. 7D, none of the RPC5 derivatives were coimmunoprecipitated with HA-RPC4/HsRPC53(1-253) (lanes 9 to 11). However, both full-length RPC5 and RPC5(1-163) but not RPC(164-854) were coimmunoprecipitated with HA-RPC4/HsRPC53(254-398) (Fig. 7D, lanes 12 to 14). Thus, the conserved N-terminal part of RPC5 associates with the conserved C-terminal part of RPC4/HsRPC53, indicating that despite the divergence between the S. cerevisiae and human proteins, their ability to associate with each other has been conserved and can be mapped to the conserved domains.
DISCUSSION
Unlike S. cerevisiae RNA polymerase III, the composition of human RNA polymerase III has never been determined. In this work, we have extensively purified human RNA polymerase III so that the identity of every component in the complex could be determined. This analysis has identified human orthologues for all but the smallest yeast RNA polymerase III subunits, including orthologues whose corresponding cDNA sequences were either absent from the database or present but not recognized as putative RNA polymerase III subunits, and thus represents a key step toward a defined RNA polymerase III transcription system from HeLa cells.
Composition of human RNA polymerase III.
The composition of human RNA polymerase III as determined by our mass spectrometry analysis is summarized in Table 1. We detected the human orthologues of four of the five S. cerevisiae subunits common to all three RNA polymerases, HsRPABC1, HsRPABC2, HsRPABC3, and HsRPABC5. HsRPABC4 was not detected, most probably because of its very small size (7 kDa). HsRPABC1 was cloned before as a subunit of RNA polymerase I (8), and HsRPABC2 to HsRPABC5 but not HsRPABC1 were shown to complement S. cerevisiae strains lacking the endogenous corresponding subunit (38, 40, 51). We also detected two polypeptides highly similar to the yeast subunits (AC40 and AC19) common to RNA polymerases I and III. HsRPAC1 was cloned before as a subunit of RNA polymerase I (hRPA40 [12]), whereas HsRPAC2 had not been characterized.
A number of the subunits unique to RNA polymerase III have been characterized before. Thus, RPC1/RPC155 (50), RPC3/RPC62, RPC6/RPC39, and RPC7/RPC32 (58), and RPC4/RPC53 (26, 27, 50) have all been shown to be part of RNA polymerase III by biochemical methods. Our analysis completes the characterization of the unique subunits with the identification of the HsRPC2, HsRPC5, HsRPC8, HsRPC9 [HsCGRP receptor component (18, 33)], and HsRPC10 (7) polypeptides. Of these, the ones that possess paralogues in other RNA polymerases are conserved with their S. cerevisiae counterpart, with identities ranging from 30 to 63% (Table 1).
The HsRPC9 subunit has been characterized before as an intracellular peripheral membrane protein that facilitates CGRP signaling and associates with the CGRP receptor CRLR (calcitonin receptor-like receptor) (5, 17, 33), but its similarity to the S. cerevisiae C17 subunit prompted the suggestion that it might correspond to a human RNA polymerase III subunit (18). Its presence in the highly purified RNA polymerase III complex confirms that this is the case. Thus, HsRPC9 may constitute an example of a factor with different functions in the nucleus and the cytoplasm.
The characterization of human RPC8 and RPC9 brought an unexpected result. Blast searches revealed that RPC8 is related to RPB7, as noted earlier for the S. cerevisiae HsRPC8 orthologue C25 (44). In addition, however, RPC9 contains a conserved domain also found in RPB4 subunits. Moreover, like the RNA polymerase II RPB7 and RPB4 subunits from S. cerevisiae (15), A. thaliana (32), and H. sapiens (29), the human RNA polymerase III RPC8 and RPC9 subunits associate with each other, as symbolized in Fig. 8 with the red arrow. This strongly suggests that RPC8 and RPC9 are paralogues of RPB7 and RPB4, respectively, as symbolized by the blue arrows in Fig. 8, and that the corresponding S. cerevisiae RNA polymerase III subunits C25 and C17 can similarly associate with each other.
FIG. 8.
Subunit-subunit associations. HsRPC4 and HsRPC5 associate with each other, and so do HsRPC8 and HsRPC9, as well as the RNA polymerase II (pol II) subunits RPB7 and RPB4, as symbolized by red arrows. HsRPC8 and HsRPC9 are paralogues of the RNA polymerase II subunits RPB7 and RPB4, as symbolized by blue arrows.
RPB7 but not RPB4 is essential for S. cerevisiae cell viability (39, 64). The dimer has been compared to prokaryotic σ factors (43) because, like σ factors, it is dispensable for RNA chain elongation and associates reversibly with the rest of the enzyme (15). Moreover, the RPB4 and RPB7 subunits are less abundant than the other RNA polymerase II subunits in S. cerevisiae cells, and RPB4 is essential, like many σ factors, for cellular responses to stress (9). Thus, in vivo, the requirement for the RPB4 subunit may be promoter specific. The OB-fold motif of RPB7 is similar to that present in the E. coli ribosomal protein S1 (4) and is found in many other proteins with single-stranded nucleic acid-binding activity (42). Indeed, the S. cerevisiae RPB7-RPB4 dimer binds strongly to both single-stranded DNA and RNA, and in vitro, it is required for transcription initiation at a step subsequent to recruitment of RNA polymerase II to the promoter (43). Structural considerations suggest that the RPB4-RPB7 dimer is located downstream of the catalytic site, in the cleft (10).
Together, these observations have prompted the suggestion that the RPB4-RPB7 complex may bind either to nascent RNA or to single-stranded DNA in the transcription bubble and thus stabilize the open promoter complex prior to initiation as well as, perhaps, the early transcribing complex prior to promoter escape (43, 55). The S. cerevisiae RNA polymerase III paralogues of RBP7 and RPB4, C25 and C17, respectively, are both essential for viability in S. cerevisiae (18, 44). Moreover, yeast two-hybrid and coimmunoprecipitation experiments indicate that C17 interacts with the transcription initiation factor Brf1 and with the RNA polymerase III C31 subunit (18), which is itself part of a trimeric complex required specifically for transcription initiation (58, 61, 62). Thus, the RNA polymerase III paralogues may also be involved in transcription initiation, but in this case, perhaps simply because many of the RNA polymerase III genes encode components essential for basic cell metabolism, both subunits are essential for S. cerevisiae cell viability.
In contrast to most subunits with paralogues in other RNA polymerases, RPC5 is quite divergent between S. cerevisiae and human cells, with only a short region of homology at the N terminus of the human protein. Nevertheless, HsRPC5 is clearly a subunit of human RNA polymerase III, as it copurifies and is associated with other RNA polymerase III subunits (Fig. 5). In addition, it is clearly an orthologue of S. cerevisiae C37, as it shows some sequence similarity with the S. cerevisiae protein (Fig. 4C) and it associates with HsRPC4/HsRPC53 (Fig. 7), paralleling the association of yeast C37 and C53. The observation that this association, which is symbolized by the red arrow in Fig. 8, is through the HsRPC4/HsRPC53 and HsRPC5 regions that are conserved in their S. cerevisiae counterparts suggests that amino acids 290 to 420 of yeast C53 (see Fig. 7B) and amino acids 65 to 207 of yeast C37 (see Fig. 4C) are sufficient for interaction of the S. cerevisiae proteins.
HsRPC5 is 32% identical to Drosophila sex-lethal interactor protein and 85% identical to its mouse homologue, strongly suggesting that the Drosophila and mouse proteins are in fact RPC5 RNA polymerase III subunits. The observation that Drosophila SIN interacts with RNA-binding domain 1 of sex-lethal (13) suggests that HsRPC5 may interact with other RNA-binding proteins, perhaps RNA polymerase III transcript processing factors, similar to the interaction of RNA polymerase II with pre-mRNA processing factors (37).
In addition to RNA polymerase III subunits, the active complex that we purified contained some abundant proteins such as spectrin, myosin, clathrin, and β-actin. These may represent contaminants or proteins that play roles not tested in our in vitro transcription assay, such as targeting the RNA polymerase III machinery to the correct nuclear compartment. On the other hand, actin was implicated in transcription a number of years ago (16, 45), and more recently, β-actin has been found in nuclear complexes involved in transcription, such as the SWI/SNF-like chromatin remodeling complex BAF (Brg-associated factor), where it is required for maximal ATPase activity of Brg1 as well as for association of the complex with the nuclear matrix (66). Such observations suggest that some of these abundant proteins may play a direct role in RNA polymerase III transcription.
RPC5 is required for RNA polymerase III transcription.
Of the five subunits that do not have paralogues in RNA polymerase II, three of them, RPC3/RPC62, RPC6/RPC39, and RPC7/RPC32, form a complex that is separable from the rest of the enzyme and is required for transcription initiation (58, 61). RPC6 from both S. cerevisiae (C34) and human cells (RPC39) associates with Brf1, and HsRPC6 associates with TBP and the TFIIIC90 subunit of TFIIIC2 (23, 30, 58, 61). HsRPC3/RPC62 associates with TFIIIC90 as well as with the TFIIIC63 subunit of TFIIIC2 (24). Thus, this subcomplex is probably involved in promoter recognition.
HsRPC5 is required for transcription (Fig. 6C) and associates with HsRPC4 (Fig. 7), paralleling the association of the S. cerevisiae C37 and C53 counterparts (61), and yeast C53 itself associates with one of the TFIIIC subunits (19). This suggests that HsRPC5, like HsRPC4, may be in proximity to the promoter and thus that all of the RNA polymerase III subunits without paralogues in RNA polymerase II may play roles in promoter recognition. Their divergence from the S. cerevisiae subunits parallels the differences between S. cerevisiae and human RNA polymerase III transcription initiation factors.
Acknowledgments
We thank S. Sepehri, who started this work; H. Hata and S. Sugano for sending us cDNA clones KAT11904 and KAT02533; D. Bogenhagen, P. Hearing, A. Krainer, and A. Stenlund for discussions and suggestions; B. Ma and X. Zhao for reagents; S. Lopez for technical assistance with mass spectrometry; W. Herr and L. Schramm for comments on the manuscript; and J. Duffy and P. Renna for artwork and photography.
This work was funded in part by NIH grant GM38810. N.H. and Y.S. are supported by the Howard Hughes Medical Institute.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped Blast and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashaw, G. J., and B. S. Baker. 1997. The regulation of the Drosophila msl-2 gene reveals a function for Sex-lethal in translational control. Cell 89:789-798. [DOI] [PubMed] [Google Scholar]
- 3.Bell, L. R., E. M. Maine, P. Schedl, and T. W. Cline. 1988. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell 55:1037-1046. [DOI] [PubMed] [Google Scholar]
- 4.Bycroft, M., T. J. Hubbard, M. Proctor, S. M. Freund, and A. G. Murzin. 1997. The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold. Cell 88:235-242. [DOI] [PubMed] [Google Scholar]
- 5.Chang, C. P., R. V. Pearse, 2nd, S. O'Connell, and M. G. Rosenfeld. 1993. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 11:1187-1195. [DOI] [PubMed] [Google Scholar]
- 6.Chedin, S., M. L. Ferri, G. Peyroche, J. C. Andrau, S. Jourdain, O. Lefebvre, M. Werner, C. Carles, and A. Sentenac. 1998. The yeast RNA polymerase III transcription machinery: a paradigm for eukaryotic gene activation. Cold Spring Harb. Symp. Quant. Biol. 63:381-389. [DOI] [PubMed] [Google Scholar]
- 7.Chedin, S., M. Riva, P. Schultz, A. Sentenac, and C. Carles. 1998. The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev. 12:3857-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheong, J. H., M. Yi, Y. Lin, and S. Murakami. 1995. Human RPB5, a subunit shared by eukaryotic nuclear RNA polymerases, binds human hepatitis B virus X protein and may play a role in X transactivation. EMBO J. 14:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choder, M., and R. A. Young. 1993. A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol. Cell. Biol. 13:6984-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cramer, P., D. A. Bushnell, J. Fu, A. L. Gnatt, B. Maier-Davis, N. E. Thompson, R. R. Burgess, A. M. Edwards, P. R. David, and R. D. Kornberg. 2000. Architecture of RNA polymerase II and implications for the transcription mechanism. Science 288:640-649. [DOI] [PubMed] [Google Scholar]
- 11.Cramer, P., D. A. Bushnell, and R. D. Kornberg. 2001. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292:1863-1876.11313498 [Google Scholar]
- 12.Dammann, R., and G. P. Pfeifer. 1998. Cloning and characterization of the human RNA polymerase I subunit hRPA40. Biochim. Biophys. Acta 1396:153-157. [DOI] [PubMed] [Google Scholar]
- 13.Dong, Z., and L. R. Bell. 1999. SIN, a novel Drosophila protein that associates with the RNA binding protein sex-lethal. Gene 237:421-428. [DOI] [PubMed] [Google Scholar]
- 14.Ducret, A., I. Van Oostveen, J. K. Eng, J. R. Yates 3rd, and R. Aebersold. 1998. High throughput protein characterization by automated reverse-phase chromatography/electrospray tandem mass spectrometry. Protein Sci. 7:706-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards, A. M., C. M. Kane, R. A. Young, and R. D. Kornberg. 1991. Two dissociable subunits of yeast RNA polymerase II stimulate the initiation of transcription at a promoter in vitro. J. Biol. Chem. 266:71-75. [PubMed] [Google Scholar]
- 16.Egly, J. M., N. G. Miyamoto, V. Moncollin, and P. Chambon. 1984. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 3:2363-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans, B. N., M. I. Rosenblatt, L. O. Mnayer, K. R. Oliver, and I. M. Dickerson. 2000. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 275:31438-31443. [DOI] [PubMed] [Google Scholar]
- 18.Ferri, M. L., G. Peyroche, M. Siaut, O. Lefebvre, C. Carles, C. Conesa, and A. Sentenac. 2000. A novel subunit of yeast RNA polymerase III interacts with the TFIIB-related domain of TFIIIB70. Mol. Cell. Biol. 20:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flores, A., J. F. Briand, O. Gadal, J. C. Andrau, L. Rubbi, V. Van Mullem, C. Boschiero, M. Goussot, C. Marck, C. Carles, P. Thuriaux, A. Sentenac, and M. Werner. 1999. A protein-protein interaction map of yeast RNA polymerase III. Proc. Natl. Acad. Sci. USA 96:7815-7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiduschek, E. P., and G. A. Kassavetis. 2001. The RNA polymerase III transcription apparatus. J. Mol. Biol. 310:1-26. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb, E., and J. A. Steitz. 1989. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 8:851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb, E., and J. A. Steitz. 1989. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 8:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsieh, Y. J., T. K. Kundu, Z. Wang, R. Kovelman, and R. G. Roeder. 1999. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Mol. Cell. Biol. 19:7697-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh, Y. J., Z. Wang, R. Kovelman, and R. G. Roeder. 1999. Cloning and characterization of two evolutionarily conserved subunits (TFIIIC102 and TFIIIC63) of human TFIIIC and their involvement in functional interactions with TFIIIB and RNA polymerase III. Mol. Cell. Biol. 19:4944-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, Y., and R. J. Maraia. 2001. Comparison of the RNA polymerase III transcription machinery in Schizosaccharomyces pombe, Saccharomyces cerevisiae and human. Nucleic Acids Res. 29:2675-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ittmann, M., J. Ali, A. Greco, and C. Basilico. 1993. The gene complementing a temperature-sensitive cell cycle mutant of BHK cells is the human homologue of the yeast RPC53 gene, which encodes a subunit of RNA polymerase C (III). Cell Growth Differ. 4:503-511. [PubMed] [Google Scholar]
- 27.Jackson, A. J., M. Ittmann, and B. F. Pugh. 1995. The BN51 protein is a polymerase (Pol)-specific subunit of RNA Pol III which reveals a link between Pol III transcription and pre-rRNA processing. Mol. Cell. Biol. 15:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley, R. L., J. Wang, L. Bell, and M. I. Kuroda. 1997. Sex lethal controls dosage compensation in Drosophila by a non-splicing mechanism. Nature 387:195-199. [DOI] [PubMed] [Google Scholar]
- 29.Khazak, V., J. Estojak, H. Cho, J. Majors, G. Sonoda, J. R. Testa, and E. A. Golemis. 1998. Analysis of the interaction of the novel RNA polymerase II (Pol II) subunit hsRPB4 with its partner hsRPB7 and with Pol II. Mol. Cell. Biol. 18:1935-1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoo, B., B. Brophy, and S. P. Jackson. 1994. Conserved functional domains of the RNA polymerase III general transcription factor BRF. Genes Dev. 8:2879-2890. [DOI] [PubMed] [Google Scholar]
- 31.Kuhlman, T. C., H. Cho, D. Reinberg, and N. Hernandez. 1999. The general transcription factors IIA, IIB, IIF, and IIE are required for RNA polymerase II transcription from the human U1 snRNA promoter. Mol. Cell. Biol. 19:2130-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin, R. M., and T. J. Guilfoyle. 1998. Two small subunits in Arabidopsis RNA polymerase II are related to yeast RPB4 and RPB7 and interact with one another. J. Biol. Chem. 273:5631-5637. [DOI] [PubMed] [Google Scholar]
- 33.Luebke, A. E., G. P. Dahl, B. A. Roos, and I. M. Dickerson. 1996. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc. Natl. Acad. Sci. USA 93:3455-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maraia, R. J. 1996. Transcription termination factor La is also an initiation factor for RNA polymerase III. Proc. Natl. Acad. Sci. USA 93:3383-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maraia, R. J., D. J. Kenan, and J. D. Keene. 1994. Eukaryotic transcription termination factor La mediates transcript release and facilitates reinitiation by RNA polymerase III. Mol. Cell. Biol. 14:2147-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroney, P. A., G. J. Hannon, and T. W. Nielsen. 1990. Transcription and cap trimethylation of a nematode spliced leader RNA in a cell-free system. Proc. Natl. Acad. Sci. USA 87:709-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCracken, S., E. Rosonina, N. Fong, M. Sikes, A. Beyer, K. O'Hare, S. Shuman, and D. Bentley. 1998. Role of RNA polymerase II carboxy-terminal domain in coordinating transcription with RNA processing. Cold Spring Harb. Symp. Quant. Biol. 63:301-309. [DOI] [PubMed] [Google Scholar]
- 38.McKune, K., P. A. Moore, M. W. Hull, and N. A. Woychik. 1995. Six human RNA polymerase subunits functionally substitute for their yeast counterparts. Mol. Cell. Biol. 15:6895-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKune, K., K. L. Richards, A. M. Edwards, R. A. Young, and N. A. Woychik. 1993. RPB7, one of two dissociable subunits of yeast RNA polymerase II, is essential for cell viability. Yeast 9:295-299. [DOI] [PubMed] [Google Scholar]
- 40.McKune, K., and N. A. Woychik. 1994. Functional substitution of an essential yeast RNA polymerase subunit by a highly conserved mammalian counterpart. Mol. Cell. Biol. 14:4155-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murzin, A. G. 1993. OB (oligonucleotide/oligosaccharide binding)-fold: common structural and functional solution for non-homologous sequences. EMBO J. 12:861-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlicky, S. M., P. T. Tran, M. H. Sayre, and A. M. Edwards. 2001. Dissociable Rpb4-Rpb7 subassembly of rna polymerase II binds to single-strand nucleic acid and mediates a postrecruitment step in transcription initiation. J. Biol. Chem. 276:10097-10102. [DOI] [PubMed] [Google Scholar]
- 44.Sadhale, P. P., and N. A. Woychik. 1994. C25, an essential RNA polymerase III subunit related to the RNA polymerase II subunit RPB7. Mol. Cell. Biol. 14:6164-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheer, U., H. Hinssen, W. W. Franke, and B. M. Jockusch. 1984. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell 39:111-122. [DOI] [PubMed] [Google Scholar]
- 46.Schenk, P. M., S. Baumann, R. Mattes, and H.-H. Steinbiss. 1995. Improved high level expression system for eucaryotic genes in E. coli using T7 RNA polymerase and rare Arg tRNAs. BioTechniques. 19:196-200. [PubMed] [Google Scholar]
- 47.Schramm, L., P. S. Pendergrast, Y. Sun, and N. Hernandez. 2000. Different human TFIIIB activities direct RNA polymerase III transcription from TATA-containing and TATA-less promoters. Genes Dev. 14:2650-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sentenac, A., M. Riva, P. Thuriaux, J.-M. Buhler, I. Treich, C. Carles, M. Werner, A. Ruet, C. Mann, N. Chiannilkulchai, S. Stettler, and S. Mariotte. 1992. Yeast RNA polymerase subunits and genes, p. 27-54. In S. L. McKnight, and K. R. Yamamoto (ed.), Transcriptional regulation. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 49.Sepehri Chong, S., P. Hu, and N. Hernandez. 2001. Reconstitution of transcription from the human U6 small nuclear RNA promoter with eight recombinant polypeptides and a partially purified RNA polymerase III complex. J. Biol. Chem. 276:20727-20734. [DOI] [PubMed] [Google Scholar]
- 50.Sepehri, S., and N. Hernandez. 1997. The largest subunit of human RNA polymerase III is closely related to the largest subunit of yeast and trypanosome RNA polymerase III. Genome Res. 7:1006-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shpakovski, G. V., J. Acker, M. Wintzerith, J. F. Lacroix, P. Thuriaux, and M. Vigneron. 1995. Four subunits that are shared by the three classes of RNA polymerase are functionally interchangeable between Homo sapiens and Saccharomyces cerevisiae. Mol. Cell. Biol. 15:4702-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sweetser, D., M. Nonet, and R. A. Young. 1987. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc. Natl. Acad. Sci. USA 84:1192-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teichmann, M., Z. Wang, and R. G. Roeder. 2000. A stable complex of a novel transcription factor IIB-related factor, human TFIIIB50, and associated proteins mediate selective transcription by RNA polymerase III of genes with upstream promoter elements. Proc. Natl. Acad. Sci. USA 97:14200-14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, J. D., D. G. Higgins, and T. J. Gobson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todone, F., P. Brick, F. Werner, R. O. Weinzierl, and S. Onesti. 2001. Structure of an archaeal homolog of the eukaryotic RNA polymerase II RPB4/RPB7 complex. Mol. Cell 8:1137-1143. [DOI] [PubMed] [Google Scholar]
- 56.Wang, Z., L. Bai, Y. J. Hsieh, and R. G. Roeder. 2000. Nuclear factor 1 (NF1) affects accurate termination and multiple-round transcription by human RNA polymerase III. EMBO J. 19:6823-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, Z., T. Luo, and R. G. Roeder. 1997. Identification of an autonomously initiating RNA polymerase III holoenzyme containing a novel factor that is selectively inactivated during protein synthesis inhibition. Genes Dev. 11:2371-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, Z., and R. Roeder. 1997. Three human RNA polymerase III-specific subunits form a subcomplex with a selective function in specific transcription initiation. Genes Dev. 11:1315-1326. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Z., and R. G. Roeder. 1998. DNA topoisomerase I and PC4 can interact with human TFIIIC to promote both accurate termination and transcription reinitiation by RNA polymerase III. Mol. Cell 1:749-757. [DOI] [PubMed] [Google Scholar]
- 60.Wang, Z., and R. G. Roeder. 1996. TFIIIC1 acts through a downstream region to stabilize TFIIIC2 binding to RNA polymerase III promoters. Mol. Cell. Biol. 16:6841-6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werner, M., N. Chaussivert, I. M. Willis, and A. Sentenac. 1993. Interaction between a complex of RNA polymerase III subunits and the 70-kDa component of transcription factor IIIB. J. Biol. Chem. 268:20721-20724. [PubMed] [Google Scholar]
- 62.Werner, M., S. Hermann-Le Denmat, I. Treich, A. Sentenac, and P. Thuriaux. 1992. Effect of mutations in a zinc-binding domain of yeast RNA polymerase C (III) on enzyme function and subunit association. Mol. Cell. Biol. 12:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willis, I. M. 2002. A universal nomenclature for subunits of the RNA polymerase III transcription initiation factor TFIIIB. Genes Dev. 16:1337-1338. [DOI] [PubMed] [Google Scholar]
- 64.Woychik, N. A., and R. A. Young. 1989. RNA polymerase II subunit RPB4 is essential for high- and low-temperature yeast cell growth. Mol. Cell. Biol. 9:2854-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, W., and B. T. Chait. 2000. ProFound: an expert system for protein identification using mass spectrometric peptide mapping information. Anal. Chem. 72:2482-2489. [DOI] [PubMed] [Google Scholar]
- 66.Zhao, K., W. Wang, O. J. Rando, Y. Xue, K. Swiderek, A. Kuo, and G. R. Crabtree. 1998. Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95:625-636. [DOI] [PubMed] [Google Scholar]