Abstract
Members of the heat shock factor (HSF) family are evolutionarily conserved regulators that share a highly homologous DNA-binding domain. In mammals, HSF1 is the main factor controlling the stress-inducible expression of Hsp genes while the functions of HSF2 and HSF4 are less clear. Based on its developmental profile of expression, it was hypothesized that HSF2 may play an essential role in brain and heart development, spermatogenesis, and erythroid differentiation. To directly assess this hypothesis and better understand the underlying mechanisms that require HSF2, we generated Hsf2 knockout mice. Here, we report that Hsf2−/− mice are viable and fertile and exhibit normal life span and behavioral functions. We conclude that HSF2, most probably because its physiological roles are integrated into a redundant network of gene regulation and function, is dispensable for normal development, fertility, and postnatal psychomotor function.
The heat shock response is defined as the induced expression of Hsp genes in cells submitted not only to thermal stress but also to a plethora of other environmental conditions that can provoke protein misfolding and denaturation (30). First identified in Drosophila melanogaster, this pathway appears to be evolutionarily conserved from bacteria to humans. The regulatory mechanisms of the heat shock response have been extensively studied, demonstrating the role played by transcription factors (heat shock factors [HSFs]), which bind specific DNA sequences, termed the heat shock element (HSE). Screenings, based on homology at the level of the DNA-binding domain, have revealed that, in contrast to that found in lower organisms, mammals and chickens have evolved a family of HSFs (HSF1, HSF2, and HSF4 in mice and humans; HSF1, HSF2, and HSF3 in chickens) that are differentially and specifically expressed during embryonic development and organ differentiation (31, 32, 39, 40). Based on these observations, it has been hypothesized that, beyond the heat shock response mainly controlled by HSF1, each regulatory protein may impart specialized or unique properties in mammals (reviewed in reference 34).
Indeed, Jedlicka and colleagues first showed that, although Drosophila HSF protein is dispensable for cell survival, it exerts multiple requirements during organogenesis, larva development, and regulation of the heat shock response (16). Taking into account organism differences between flies and mammals, we made comparable observations with the Hsf1 knockout gene affecting at multiple levels stress response pathways, developmental processes, and cell survival. In particular, HSF1 deficiency produces abnormalities in extraembryonic development, postnatal growth, oogenesis, and reduced resistance to inflammatory challenge by bacterial endotoxin (4, 25, 46).
In contrast to HSF1, far less is known about the exact roles played by HSF2, though extensive analysis of the pattern of expression has suggested major developmental functions. Christians et al. have reported that HSF1 is abundant in oocytes and early embryos, whereas HSF2, initially absent in the newly formed zygote, starts to be expressed when the embryos contain four to eight cells (5). Of interest, the HSF2-like DNA-binding activity has been observed when embryos reached the blastocyst stage (3.5 days postcoitum [dpc]), which was coincident with stress inducibility. This has led to the hypothesis that HSF2 might participate or mediate developmental regulation of the heat shock pathway (26). A detailed analysis of HSF2 expression during postimplantation development has revealed that this factor is widely distributed in embryos until 15.5 dpc and, thereafter, restricted to the central nervous system, in particular, in mitotic neurons located within the ventricular layer (36). An additional report focusing on heart development has detected a strong upregulation of HSF2 between 11.5 and 12.5 dpc, a critical stage in the spatial organization of the tubular heart (9). Similarly, in rats, HSF2 expression peaks during the early organogenic phase while it decreases drastically from 9.5 to 15.5 dpc (27). Finally, HSF2 remains predominantly expressed in the brain and testes of the adult mouse and rat (1, 3, 11, 15, 44).
Unlike HSF1, HSF2 is typically unresponsive to heat shock in most cells and tissues. By using purified murine HSF1 and HSF2, in vitro experiments have shown differences in the interactions between these factors and the HSE (21), indicating that the DNA-binding site specific for either HSF1 or HSF2 could be defined (20). Extensive studies using human K562 erythroleukemia cells have implicated HSF2 in erythroid maturation following activation by the antioxidant and proteasome inhibitor hemin (42). Sistonen and coworkers also reported that hemin treatment in K562 cells triggers the formation of HSF2 homotrimers, which translocate to the nucleus and activate HSF2-HSE binding synergistically with HSF1 and induction of hsp70 transcription (41). However, these conclusions were challenged by other evidence that HSF1, and not HSF2, is primarily responsible for hemin-induced hsp70.1 transcription in K562 cells (47) and that the regulatory domain of HSF2 is actually unresponsive to hemin (48). Furthermore, a major role for HSF1 in the induction of Hsp expression by proteasome inhibition was suggested by the results of Pirkkala and coworkers using Hsf1-null cells, but whether HSF2 plays a minor role remains to be definitively addressed (33).
Homologous recombination and targeted mutagenesis remain the most powerful strategies to uncover functions exerted by a defined gene in intact organisms. Therefore, to directly assess the functional properties of HSF2 in vivo, we have generated Hsf2 knockout mice by deleting the first exon of the gene, which includes the translation start codon. Here, we report that Hsf2−/− mice are viable and appear to be normal, indicating that the requirements of mammalian HSF2 are subserved by other HSFs or pathways for development, fertility, and psychomotor function.
MATERIALS AND METHODS
Generation of the HSF2 targeting vector.
A 657-bp SphI-BglII section of the HSF2 cDNA fragment was used to screen a genomic library of the mouse embryonic stem (ES) cell DNA contained in a bacterial artificial chromosome vector (Genome Systems, Inc.). Two contiguous PstI fragments of 7.0 and 8.0 kb in which the 5′ 7.0-kb fragment contained a single 200-bp exon (−111 to +93) (39) carrying the translational start site of the HSF2 open reading frame were isolated (Fig. 1A). An internal ∼3.0-kb NotI-StuI genomic fragment spanning the 200-bp exon to be deleted was replaced with the 2.0-kb neomycin expression cassette (kindly provided by P. Soriano). The herpes simplex virus thymidine kinase expression cassette was ligated ∼0.8 kb upstream of the 5′ end of the neomycin cassette for negative selection (43). Finally, the 8.0-kb PstI fragment was ligated at the 3′ end of the neomycin cassette to complete the right arm of the targeting vector. The linearized vector was introduced into ES cells (129/Sv, KG1 passage 4), and double-resistant clones (G418/FIAU) were screened for homologous recombination by PCR (data not shown) and confirmed by Southern analysis with the indicated probe (Fig. 1B). Approximately 27% (13 of 48) of the clones screened contained the legitimate recombination event.
FIG. 1.
Targeted deletion of the murine HSF2 gene. Sizes of the different fragments are indicated in the scheme, which is not drawn to scale. (A) The wild-type genomic DNA (WT) used to prepare the targeting vector included two contiguous PstI fragments of 7 and 8 kb, as described in Materials and Methods. The 84-bp 5′ probe (black box with asterisk) used in Southern blot analysis was located at the 5′ end of the 7-kb fragment and therefore was excluded from the targeting vector. The NotI-StuI fragment containing the first exon (vertical grey box) was replaced by neomycin (NEO) in the targeting vector. The thymidine kinase expression cassette (open box) was attached to the Bgl2 site at the 5′ end of the left arm (0.8 kb) of the vector. The resulting deleted allele containing a smaller PstI fragment recognized by the 84-bp probe is illustrated. (B) Southern blot analysis of PstI-digested genomic DNA (15 μg) isolated from wild-type, heterozygous, and homozygous Hsf2 mice is shown. Arrows indicate the ∼7.0-kb wild-type band and the 3.0-kb recombinant band resulting from integration of the targeting vector at the HSF2 locus. (C) Northern blot analysis of RNA (20 μg) isolated from the brain, heart, kidney, liver, and lungs of wild-type and Hsf2-null animals is shown. (D) Western blot analysis was performed on whole-cell extracts from cells subject to ubiquitin-proteasome inhibition with lactacystin or MG132. Total cellular proteins were isolated from wild-type and Hsf2-null MEFs after a 6-h treatment. A low abundance of the HSF2 protein was present in unstressed conditions (lane 1, wild-type cells); increased amounts could be detected in wild-type cells exposed to either inhibitor (lanes 2 and 3). However, no immunoreactive protein of any size was observed in Hsf2-null cells exposed to treatment conditions (lanes 5 and 6). The gel was exposed for 12 h. A partially degraded product (∗) of HSF2 protein (arrow) is indicated (lane 2). Lane M, purified recombinant HSF2 protein as control. (E) Western blot analysis shows that the expression of HSF2 in testis has disappeared in Hsf2−/− organs (compare the WT lane with the expected 75-kDa band and Hsf2−/− lane).
Generation of Hsf2−/− mice and mouse embryonic fibroblasts (MEFs).
High- percentage chimeric males were derived from two independent ES cell clones (designated as clones 5 and 8), injected into C57BL/6 blastocysts, and further bred to both 129XI/SvJ and C57BL/6 to produce isogenic and hybrid strains, respectively. Embryos of F1 heterozygous crosses were harvested at E14.5 and eviscerated, and the remaining tissue was dissociated into individual cells with trypsin (25). The primary cells could be maintained in culture for up to seven passages, and all in vitro experiments were performed with early-passage primary cells.
Analysis of Hsf2−/− mice and cells. (i) HSF2 expression.
Total RNA was extracted from various tissues of Hsf2+/+ and Hsf2−/− animals and subjected to Northern blot analysis as previously described (25). The membrane was stained with 0.02% methylene blue in 0.5 M sodium acetate to assess the quality and quantity of samples loaded. An EcoRI-StuI fragment from Hsf2 cDNA was used as a probe. HSF2 protein expression was assessed by Western blotting with detergent-soluble extracts (20 μg) from wild-type and Hsf2-null MEFs subjected to a 6-h treatment with the ubiquitin-proteasome inhibitors MG132 (Peptides International) and lactacystin (stock solution in dimethyl sulfoxide, final concentration of 10 μM). For analysis of HSF2 expression in testis, whole tissue was homogenized in 5 ml of lysis buffer, stored on ice for 30 min, and centrifuged (20,000 × g for 15 min) (1). Equivalent protein (15 μg) was reduced and analyzed by electrophoresis on an 8% denaturing acrylamide gel and transferred to an Immobilon-P (Millipore) membrane. Resulting blots were probed either with rat monoclonal antibody (a gift from R. Morimoto) at a 1:200 dilution or with rabbit polyclonal anti-HSF2 (a gift from V. Zimarino) at a 1:5,000 dilution and then exposed to the corresponding horseradish peroxidase-conjugated secondary antibody. Blots were developed by using a chemiluminescence kit. Equal loading and transfer were monitored by staining the gel or the membrane with Coomassie blue.
(ii)Characterization of the heat shock response.
One hundred-millimeter-diameter plates of logarithmically growing embryonic cells were sealed, immersed in a water bath (43°C for 60 min), and allowed to recover at 37°C for the indicated times before extraction of total RNA. Total RNA was subjected to Northern analysis with the following cDNA probes: Hsp86, rat Hsp70i, human Hsc70, human Hsp27, and murine Hsp60 (25, 46). The membrane was stained with 0.02% methylene blue in 0.5 M sodium acetate to assess the quality and quantity of the samples loaded.
(iii) Characterization of proteasome inhibition response.
Whole-cell protein extracts derived from wild-type and null MEFs after treatment with the proteasome inhibitors MG132 (Peptides International) and lactacystin (stock solution in dimethyl sulfoxide, final concentration of 10 μM) were used in electrophoretic mobility shift assays (EMSAs) to determine the effect on HSE DNA-binding activities. EMSA with anti-HSF2 and HSF1 antibodies was performed as previously described (25). Aliquots (10 μg) of whole-cell extracts, prepared as previously described (2) from control and heat-shocked cells, were mixed in a binding reaction with a labeled double-stranded HSE oligonucleotide probe (5′AATTCGAAACCCCTGGAATATTCCCGACCTGGCAGC3′) and its complementary strand. The reaction was then subjected to nondenaturing polyacrylamide gel electrophoresis and autoradiography.
RNA was extracted from wild-type and null MEFs after treatment with the proteasome inhibitors MG132 and lactacystin (10 μM) and submitted to Northern analysis with the Hsp70 probe as described previously (25).
(iv) Morphological and immunohistochemical analyses.
Organs (brain, testis) were harvested from Hsf2+/+ and Hsf2−/− animals (n = 3, 2 to 3 months old), fixed in 10% formalin or 4% paraformaldehyde overnight at 4°C, and embedded in paraffin to prepare histological sections. When appropriate, testicular sections were stained with hematoxylin and eosin stain and brain sections were stained with Cresyl violet. Immunohistochemistry was performed on testicular sections with anti-Hsp70.2 (kindly provided by M. Eddy) (1:1,000), followed by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody (Vector Laboratories) (1:200) (37). Sections were developed with diaminobenzidine chromogen (Dako Corp., Carpineria, Calif.) and counterstained with hematoxylin.
(v) Assessment of behavioral performance.
In a blind study, 50 mature (7 ± 1.1 months) B6,129XI-Hsf2 mice were given a series of behavioral tests for cognitive, psychomotor, sensory, and reflexive capacity as described previously (12).
(a) Spatial navigation and contextual avoidance performance.
The Hsf2−/− and Hsf2+/+ mice were compared for their ability to learn and remember the location of a safe platform, hidden from view 1 cm below the surface in a pool of colored water. An acquisition phase (initial learning) consisted of eight sessions (two per day) in which the mouse had to swim to the hidden platform from a different starting position in the tank on each of five trials. After the learning phase, the mice were tested for retention of the platform location over a 66-h period (sessions 9 and 10) and subsequently tested for their ability to learn a new platform location (reversal; sessions 11 through 14). Contextual avoidance learning (14) was tested by administering four training sessions, spaced at 24-h intervals, in which the mice had to run to the correct arm of a small T-maze within 5 s in order to avoid a shock (0.25 mA) to the grid floor of the apparatus. The training trials (spaced at intervals of 1 min) continued on each day until a learning criterion was met (four of the last five trials with no errors). The correct side alternated between each session, such that the mice had to learn to run to the goal arm opposite that which had been correct on the previous day. Prior to the avoidance learning, shock aversion and intensity functions were compared in the Hsf2−/− and Hsf2+/+ mice by following a spatial preference procedure outlined previously (13) to determine if these groups of mice would respond differently to shock as a stimulus to motivate learning or conditioning of fear.
(b) Psychomotor and reflexive performance.
Spontaneous forward locomotion and rearing (standing on the hind paws) were sampled within 12-min periods for 2 h in a commercial photocell apparatus [model RXYZCM(16); Omnitech Electronics, Columbus, OH]. The unconditioned startle response to shock stimuli was measured with the mice restrained in acrylic tubes that rested on electromagnetic force transducers (SR Lab, San Diego Instruments). The floor of each restraint tube consisted of stainless steel bars wired to deliver a constant current, scrambled shocks of differing intensity (0.02 to 0.64 mA). Shock stimuli of 100-ms durations were presented every 30 s according to a semirandom sequence, until each intensity had been presented five times.
Maximum running capacity was measured by placing the mice on a cylinder that rotated with constant acceleration (Omnirotor Treadmill; Omnitech Electronics). The speed of rotation increased by 0.5 rpm/s until the mouse could no longer perform the running response and fell to a padded surface. The elapsed time at which the mouse fell was recorded as the measure of running performance. The mice were tested daily in sessions consisting of four trials until a performance stability criterion had been met. For the balance beam-walking test, the latency to fall was recorded after each mouse had been placed on the center of a rod suspended between two safe platforms (60 cm apart) located 45 cm above a padded surface. The level of difficulty was varied by testing the mice on one of four rods differing in shape and thickness (large square, large round, small square, or small round) during each of four consecutive sessions. The measure of performance was the overall average latency to fall over the four sessions.
In the wire grip test, each mouse was placed by its front paws on a horizontal wire approximately 27 cm above a padded surface and the latency to fall was recorded. For alley turning, the mouse was placed backward in a 3.5-cm-long alley and the latency to turn and face the open end was recorded. Negative geotaxis was scored by placing the mouse facing downward on a rough surface mounted at a 45° angle and measuring the latency to pivot 90° in either direction.
The significance level of genotype differences was determined using one- or two-way (with one repeated measure) analyses of variance on each dependent variable.
RESULTS
HSF2 is not essential for normal development.
We have generated Hsf2 knockout mice by homologous recombination with a targeting vector in 129/Sv ES cells (Fig. 1A), in which the neomycin cassette replaced a single exon of 200 bp encompassing the authentic translation start site of the HSF2 open reading frame and 5′ end of the DNA-binding domain (39). Approximately 27% (13 of 48) of the ES cell clones, which were screened by PCR and confirmed by Southern blot analysis, contained the disrupted Hsf2 allele (Fig. 1B). Two independent clones (clones 5 and 8) transmitted the Hsf2 mutation into the germ line of male chimeric mice that were mated to either 129XI/SvJ or C57BL/6J wild-type females. Intercrosses of the heterozygote progeny for both clones generated offspring at the expected Mendelian frequency (Table 1, shown for clone 8). As a result, clone 8 was arbitrarily selected for more in-depth analysis of lines of both isogenic 129XI and B6,129XI mixed genetic backgrounds. In addition, initial body weights and subsequent growth rates of homozygous Hsf2 mice were within 1 standard deviation of those for the wild-type and heterozygous animals (data not shown).
TABLE 1.
Genotype analysis of viable litters from heterozygote intercross
| Mouse strain | No. of live births | No. of litters | Genotype of offspringa (%)
|
||
|---|---|---|---|---|---|
| +/+ | +/− | −/− | |||
| 129XI | 114 | 16 | 35 (31) | 56 (49) | 23 (20) |
| C57BL/6J × 129XI (B6,129XI) | 118 | 15 | 34 (29) | 60 (51) | 24 (20) |
χ2 test for the actual genotype versus that of the predicted, P > 0.10.
The single-copy HSF2 gene, which shares 40% sequence identity with HSF1 (39) and encodes a 72-kDa polypeptide, is expressed as alternatively spliced variants (α and β isoforms) predominantly in the brain, heart, and testes of the mouse (1, 9, 15, 36). Using Northern blot analysis, we detected no HSF2 transcript of any size in the brain, heart, kidney, liver, or lung of Hsf2-null animals (Fig. 1C).
As the HSF2 protein is short-lived (t1/2 = 1 h), we assessed HSF2 expression in MEFs prepared from wild-type and Hsf2−/− mouse embryos that were treated with proteasome inhibitors, either MG132 or lactacystin (Fig. 1D) (10, 33). Western blot analyses showed that the concentration of labile HSF2 protein was increased in treated wild-type cells (Fig. 1D, lanes 1 through 3). In contrast, no immunologically detectable HSF2 protein was present in Hsf2-null cells either before or after treatment (Fig. 1D, lanes 4 through 6). Likewise, Western blot analysis of Hsf2−/− testis extract did not reveal any HSF2 protein, confirming that the introduced deletion produced a null allele (Fig. 1E). Equal loading after transfer of the samples was assessed by Coomassie blue staining of the membrane (data not shown). HSF2 deficiency did not lead to an increased expression of HSF1 (data not shown), providing evidence that HSF1 and HSF2 paralogs are independently regulated.
HSF2 deficiency does not affect either constitutive or stress-inducible expression of Hsp genes.
The proposed role of HSF2 as a transcription factor that regulates heat shock protein genes during development and cellular stress responses remains controversial (17, 41, 47). HSF-dependent transcription of Hsp genes requires stress-induced oligomerization and high-affinity DNA binding at alternating repeats of the conserved pentameric sequence 5′-nGAAn-3′, termed the HSE (34). On the basis of PCR amplification and selection, Kroeger and coworkers proposed differences between HSF1- and HSF2-HSE cooperative interactions with domain-specific (HSF) and single-base-pair (HSE) specificity (20, 21). Thus, the Hsf2 knockout offers the opportunity to clarify the physiologic role for HSF2 activity in cellular stress responses after heat shock and proteasome inhibition in vivo.
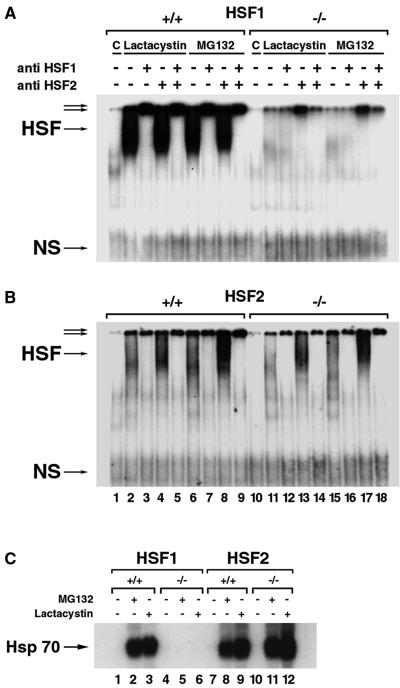
To test the role of HSF2 in the expression of Hsp, we carried out a series of RNA blotting experiments. Equivalent amounts of target Hsp transcripts were observed in wild-type and Hsf2−/− MEFs at normal temperature and, after heat shock (43°C for 60 min), followed by recovery (37°C for 1 to 4 h) (Fig. 2). We next assessed HSF1-HSE and HSF2-HSE binding in the presence of proteasome inhibition by EMSAs. In agreement with the role of HSF1 as the main factor activated by proteasome inhibition (33), HSE-DNA binding was abolished in the absence of HSF1 (Fig. 3A) but was fully maintained in Hsf2−/− cells (Fig. 3B). The induction of Hsp70 expression after treatment with proteasome inhibitor (e.g., lactacystin or MG132) was eliminated in Hsf1−/− cells containing basal HSF2 expression (Fig. 3C, lanes 5 and 6) but not in Hsf2−/− cells containing basal HSF1 expression (Fig. 3C, lanes 11 and 12). Inasmuch as MEF cells reflect the physiological role of HSF2, we conclude that the absence of HSF2 has no discernible effect on the classical heat shock response.
FIG. 2.
Characterization of the heat shock response in cultured cells. Northern analysis of RNA isolated from cells subjected to heat stress. Total RNA (20 μg) was isolated from the control (C), and heat shocked (43°C for 60 min). Wild-type, heterozygous, and Hsf2-null MEFs with no recovery period (0), 1-h recovery at 37°C (1), and 4-h recovery at 37°C (4) are shown. The same blot was stripped and reprobed for Hsp70, Hsc70, Hsp27, and Hsp60. Methylene blue staining of the rRNA was used to indicate the equivalent quality and quantity of the sample loaded.
FIG. 3.
EMSA of protein extracted from MEFs after control (C) conditions and treatment with 10 μM lactacystin and 10 μM MG132, respectively, for 6 h. Whole-cell protein extracts from wild-type and Hsf1 MEFs were derived from the control condition strain, 129XI (A) (25), and HSF2 MEFs were derived from the B6,129XI strain (B) (this study). The relative contribution of each transcription factor to the total binding activity was determined by supershifting with the indicated anti-HSF1 and/or anti-HSF2 antibodies. Protein samples from MEFs were subjected to the following treatments: no cellular protein (lanes 1 and 10), lactacystin in Hsf1+/+ cells (lanes 2 through 5) and Hsf1−/− cells (lanes 11 through 14), MG132 in Hsf1+/+ cells (lanes 6 through 9) and Hsf1−/− cells (lanes 15 through 18), anti-HSF1 antibody alone in the binding reaction (lanes 3, 7, 12, and 16), anti-HSF2 antibody alone in the binding reaction (lanes 4, 8, 13, and 17), or both antibodies in the binding reaction (lanes 5, 9, 14, and 18). (C) Northern blot analysis was performed on total RNA (20 μg) isolated from HSF2 wild-type and -null MEFs under control C conditions and after treatment with 10 μM lactacystin and 10 μM MG132, respectively, for 6 h and probed with an Hsp70 probe. An arrow indicates the position of Hsp70.
HSF2 deficiency and brain development.
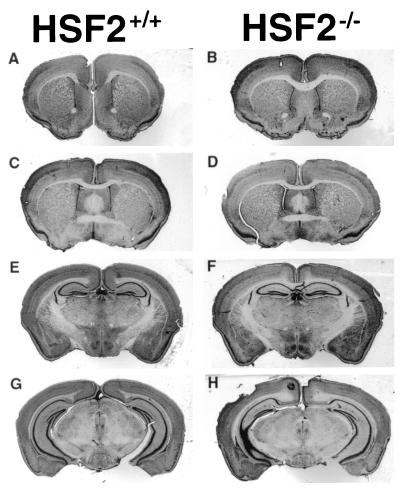
Since HSF2 expression and HSE-binding activities were reported during various stages in the development of the nervous system (3, 36), we anticipated that the disruption of HSF2 would cause postnatal abnormalities in brain structures such as the telencephalon by late midgestation. However, histological and morphological analyses revealed no differences between Hsf2+/+ and Hsf2−/− mice (B6,129XI strain). Noticeably, the cerebral ventricles were not enlarged in Hsf2−/− mice (Fig. 4, compare panels A, C, and E with panels B, D, and F, respectively). This observation contrasts with observations previously reported in other Hsf2−/− mice (17), suggesting that HSF2 is dispensable for brain development in mammals.
FIG. 4.
Coronal sections of adult brains (stained with Cresyl violet) showing the absence of differences between Hsf2+/+ (A, C, E, and G) and Hsf2−/− (B, D, F, and H). Sections were taken at the level of the lateral ventricles (for panels A and B, Bregma +1.10; for panels C and D, Bregma +0.14), at the level of the third ventricle (for panels E and F, Bregma −1.82), and at the level of the aqueduct (for panels G and H, Bregma −2.92).
HSF2 deficiency and behavioral performance.
Absence of morphological defect did not preclude that HSF2 deficiency may affect psychomotor function in adult animals, and to investigate this possibility, we subjected wild-type and Hsf2-null mice to a battery of behavioral tests of cognitive, sensory, and motor functions. Wild-type and Hsf2-null mice were indistinguishable in their ability to learn the spatial navigation task in the swim maze and the contextual avoidance task. In the swim maze task, both groups showed expected decreases in the path traversed to reach the platform during the acquisition phase, retention of the platform location, and efficient learning of the new platform position during the reversal phase (Fig. 5A ). The groups did not differ in shock aversiveness (Fig. 5B) and showed similar rates of learning the contextual avoidance problem when tested initially and following successive reversals of the correct arm in sessions 2, 3, and 4 (Fig. 5C).
FIG. 5.
Effect of Hsf2−/− on a behavioral test battery. (A) Hsf2−/− did not affect spatial navigation performance during any phase of testing (see panel divisions, left to right): (i) acquisition phase during which the mouse had to swim to a hidden platform from a different starting position for each of five trials during eight sessions; (ii) memory retention phase (Ret.) after a 66-h, no-test interval; and (iii) a reversal phase (Rev.) in which the platform position was changed and the mice were compared for their ability to learn the new location. Graphs from top to bottom: the average length of the path (in centimeters, ± standard error) taken by the mouse in swimming to the platform during each session, the average swim speed (in centimeters/second, ± standard error), and the average percentage (± standard error) of time spent within a 20-cm annulus in a probe trial administered after each session. (B) Shock aversion and intensity functions of Hsf2−/− and Hsf2+/+ mice were generated according to a spatial preference procedure outlined previously (13). Data reflect no difference in the percentage of time spent on the safe side of the apparatus, when escapable shocks of differing intensity were presented to the feet. (C) Hsf2−/− and Hsf2+/+ mice did not differ in their learning of contextual (discriminated) avoidance as a function of test sessions. This procedure (12, 14) involved four training sessions, spaced at 24-h intervals, in which the mice had to run to the correct arm of a small T-maze within 5 s in order to avoid shock to the grid floor of the apparatus. The training trials (spaced at intervals of 1 min) continued each day until a learning criterion was met. The correct side alternated between each session such that the mice had to learn to run to the goal arm opposite that which was correct on the previous day. The graph shows the number of trials required for learning (± standard error) in each session. (D) When tested over a 2-h period in a photocell apparatus, Hsf2+/+ and Hsf2−/− mice failed to differ in their initial levels of spontaneous locomotion (top graph) or rearing (standing on the hindpaws) (bottom graph) and showed no difference in the subsequent rate of habituation to the activity chamber. (E) When tested for startle reflex to a 100-ms foot shock, no effect in Hsf2−/− micewas evident in the magnitude of the startle response (force in g's) or in the reaction time (time to peak response) across a broad range of shock intensity (0.02 to 0.64 mA). (F) There was no effect of Hsf2−/− on (i) the maximum running speed, measured as the latency to fall from a motor-driven cylinder rotating with constant acceleration, (ii) the average latency to fall from four balance beams of varying width and shape, (iii) the latency to release grip when suspended from a horizontal wire, (iv) the latency to reverse direction in a blind alley, and (v) the latency to turn 180° in an upward direction when placed facing downward on a 45° inclined plane (geotaxis). Two-way analyses of variance (with one repeated measure) were performed on all data for experiments A through F, and none indicated effects or interactions involving genotype.
Wild-type and Hsf2-null mice also failed to differ in the exploration of or habituation to a novel environment (Fig. 5D), the startle response (Fig. 5E), sensorimotor coordination, balance, and reflexive postural adjustments (Fig. 5F). When data from each of the behavioral tests were subjected to two-way analyses of variance, no significant main effects or interactions involving genotype were obtained.
From these results, we can conclude that HSF2 deficiency has no major effect on nervous development and function in adults in the absence of any additional challenge.
HSF2-deficient mice are fertile.
Hsf2−/− females and males which were obtained in expected number (Table 1) were crossed with either wild-type or null animals in order to assess their fertility. No abnormality was detected in young adults regarding their reproductive development and sexual behavior. In the 129XI background, Hsf2−/− females (n = 7, 14 litters) produced 4.79 pups/litter compared to the 6 pups/litter that were produced when the females (4 females, 8 litters) were wild type (0.10 < P > 0.50). Fertility of 3-month-old males was assessed by the number of days between the mating, and the birth of a litter did not exhibit any significant difference between HSF2-deficient and wild-type animals (Table 2). Body and testis weights were measured at 3 months and did not exhibit any significant difference (Table 2). Nevertheless, we noticed that some degenerated seminiferous tubules can be observed in the testis of >3-month-old animals. We did not see any difference in fertility between 8-month-old Hsf2−/− and Hsf2+/+ males.
TABLE 2.
Body and testis weight and evaluation of male fertility in 3-month-old Hsf2+/+ and Hsf2−/− males
| Genotype | Mean ± SEM (n = 3)
|
||
|---|---|---|---|
| Body wt (g) | Testis wt (mg) | No. of days between mating and birth | |
| Hsf2+/+ | 26.4 ± 1.7 | 196 ± 13 | 23 ± 0.6 |
| Hsf2−/− | 25.2 ± 1.6 | 229 ± 43 | 24 ± 0.0 |
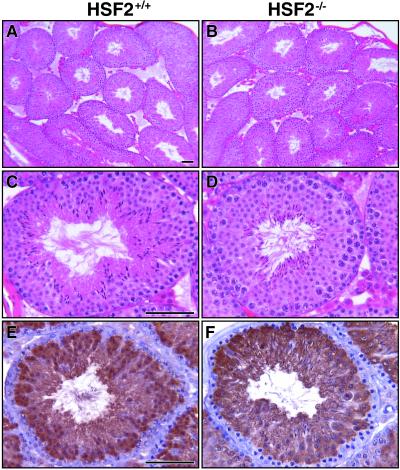
Though it was suggested that HSF2 might be a regulator for Hsp70.2, whose function is essential for spermatogenesis (6), we did not observe any significant reduction in Hsp70.2 expression in the Hsf2−/− testis. This expression was evaluated by reverse transcription-PCR, Western blot analysis (data not shown), and immunohistochemistry in 2- to 3-month-old animals (Fig. 6E and F). This result is consistent with the absence of severe abnormalities of the testis as observed in Hsp70.2−/− males (6) (Fig. 6A through D).
FIG. 6.
Histological and immunohistochemical analyses of Hsf2+/+ and Hsf2−/− testis collected from 3-month-old animals. Testis from Hsf2+/+ (A and C) and Hsf2−/− (B and D) mice presented similar well-organized seminiferous tubules, as observed in hematoxylin-and-eosin-stained sections at low (panels A and B) and high (panels C and D) magnification. (E and F) Immunohistochemical analyses of Hsp70.2 expression did not reveal any major difference between Hsf2+/+ and Hsf2−/− animals. Bars, 60 μm.
DISCUSSION
Since the discovery of multiple HSFs (HSF1 through HSF4) in higher vertebrates, considerable attention and controversy have arisen about the specialized role each factor might impart under physiological and stressful conditions (34). Gene targeting, which remains the most powerful approach to reveal gene function, has been successfully applied to HSF1 (25, 46) and, more recently, to HSF2 (reference 17 and this paper). Unexpectedly, we found that the Hsf2−/− mice created in our laboratory do not manifest any severe phenotypic abnormalities, indicating that HSF2 deficiency is dispensable for mouse embryogenesis, spermatogenesis, and fertility.
Unlike the Hsf2 knockout reported by Kallio and coworkers (17), for which exon 4-exon 5 was replaced by a β geo gene encoding a chimera of β-galactosidase and the G418 resistance gene (neomycin), we deleted the first exon of Hsf2 gene and replaced it with the neomycin transferase cassette (Fig. 1A). Using this approach, we observed that the F1 heterozygous intercrosses yield a similar and expected Mendelian distribution, indicating that HSF2, unlike HSF1, is dispensable for early embryogenesis (Table 1).
Although the heat shock response is mainly under the control of HSF1, as demonstrated by Hsf1 knockout (25, 46), the DNA-binding domain of HSF2 shares 70% homology with HSF1, raising the possibility that HSF2 might play a complementary function under stressful conditions. Notwithstanding, direct evidence that HSF2 regulates the heat shock response is lacking. Using HSF2-deficient cells to address this question, we did not observe any significant difference in the induced expression of Hsp genes after heat shock in Hsf2 wild-type and knockout cells (Fig. 2). Like heat shock, proteasome inhibition causes the accumulation of misfolded proteins, resulting in stressful conditions, and leads to HSF activation and the heat shock response. Previous studies, however, have produced contradictory results showing that either HSF2 (24), HSF1 (19, 33), or both were activated in cells treated with lactacystin or MG132 (18, 23, 35). Using HSF2-deficient cells, we confirmed that HSF1 is actually the main factor to be induced by proteasome inhibition whereas HSF2 appears to exert a secondary redundant role. Taken together, we conclude that HSF2 plays no direct role in the acute phase of the classical heat shock response in mammals.
Based on a previous description of the HSF2 profile of expression and HSF2 activation by developmental signals (reviewed in references 29, 30, and 34), four major systems were predicted to be affected by HSF2 deficiency: brain, testis, heart, and erythroid differentiation.
Because HSF2 is tightly regulated during the development of the central nervous system, HSF2 deficiency could be responsible for morphological and/or functional defects. Indeed, Kallio and coworkers have reported structural abnormalities in the adult Hsf2−/− brain, including enlargement of the lateral and third ventricles (17). In contrast, no anatomical or histological abnormalities were found in Hsf2−/− mice in the present study. In agreement with this observation, Hsf2−/− mice submitted to an extensive battery of behavioral tests for sensory, cognitive, and motor functions did not exhibit any significant difference in their response compared to that of Hsf2+/+ mice. Because Kallio and coworkers did not perform any behavioral tests, it remains to be established whether the anatomical differences may have direct functional consequences.
The testis is the second organ where HSF2 was expected to play an essential role based on its abundant expression during spermatogenesis from the early pachytene stage (1, 15). Consistent with the findings published by Kallio and coworkers (17), we found that HSF2 deficiency does not compromise the ability of null males to produce offspring. Nevertheless, these investigators have reported that Hsf2−/− males showed alteration in spermatogenesis. Since the progressive loss of active seminiferous tubules is observed in aging animals (45), it would be interesting to know more about the time course of testicular degeneration. Such analysis is presently being undertaken to determine whether HSF2 deficiency may accelerate age-related degeneration in testis.
Hsp70.2 expression is expressed specifically in the testes, and recombinant HSF2 binds to the Hsp70.2 promoter region in vitro, suggesting that this testis-specific Hsp is potentially under the regulatory control of HSF2 (38). Unexpectedly, HSF2 deficiency did not affect the expression of Hsp70.2, indicating either that this gene is not a direct target of HSF2 and/or that other known or unspecified factors predominantly control Hsp70.2 expression (7).
Finally, we observed that both cardiac and erythroid differentiation appear to be normal in Hsf2-deficient mice. It is noteworthy that normal morphology should not be equated with normal function or the ability to cope with functional or other pathophysiological challenges. For example, S100A1-deficient mice have normal cardiac morphology and function under baseline conditions but exhibit reduced responses to β-adrenergic stimulation (8). However, we have preliminarily observed that HSF2-deficient mice exhibit similar changes (e.g., cardiac hypertrophy) in response to cardiac β-adrenergic stimulation compared to that in wild-type mice (unpublished results). Likewise, we have observed no gross manifestations in HSF2-deficient mice consistent with defects of erythroid differentiation in either blood dyscrasias or abnormal oxygen delivery. However, more detailed studies in progress are needed to resolve these preliminary observations.
Perspectives on the Hsf2 knockout model.
The Hsf2 knockout reported in this paper differs to some extent from the Hsf2 knockout described recently by Kallio and coworkers (17). Multiple examples of discordant phenotypes after disruption of the same gene by knockout approaches have been previously reported (28). Genetic background plays an important role in penetrance of the phenotype, as previously described for the Hsf1 knockout (46), but other factors such as environmental conditions (diet, light, pathogens) exert influential effects. In addition, the Hsf2 gene contains a complex structure (22) and the different strategies used by the two laboratories may have inadvertently modulated the consequences of HSF2 deficiency. In our gene-targeting approach, the first exon of the Hsf2 gene was deleted and replaced by the neomycin transferase cassette, whereas Kallio and coworkers replaced part of exon 5 with the β geo gene, a chimera of β-galactosidase and the G418 resistance gene (neomycin) (17).
One important similarity, however, is that both knockouts produce homozygous offspring in the expected Mendelian distribution, which agrees with our conclusion that HSF2 is dispensable for early development. Because the Hsf2−/− mice created in our laboratory are healthy and fertile, we performed additional studies in a search of subtle postnatal phenotypes such as psychomotor abnormalities using sophisticated behavioral tests. In our opinion, such approaches are essential to resolve the functional consequences of anatomical differences and to assess other neurophysiological effects after pathophysiological challenges and stresses in the intact organism.
Of interest, the HSF2 deficiency differs substantially from what has been observed previously for HSF1 in which several developmental functions have been established (4, 46). To date, HSF1 appears to be a master regulator since its essential functions encompass development of the oocyte, placenta, and embryonic growth, all of which appear not to be complemented by other factors. On the other hand, the evolutionary necessity for multiple HSFs in higher vertebrates remains an open question. The redundant roles played by HSF1 and/or other HSF regulators might account for the absence of even subtle abnormalities (e.g., the central nervous system) in our Hsf2 knockout. Towards a better understanding of the specific functions exerted by the different HSFs, the models described herein should enable the development of combinatorial mutations to dissect the multifunctional relationships potentially shared by the mammalian HSFs.
Acknowledgments
We thank A. Davis for technical assistance during the course of this work, R. Morimoto and K. Sarge for the cDNAs of HSF2, V. Zimarino for the HSF2 antibodies, and M. Eddy for the Hsp70.2 antibody.
I.J.B. received support for this work from a National Institutes of Health Career Development grant (K14), an Established Investigator Award from the American Heart Association, and an NICHD grant (R01-HL60667-03).
REFERENCES
- 1.Alastalo, T. P., M. Lonnstrom, S. Leppa, K. Kaarniranta, M. Pelto-Huikko, L. Sistonen, and M. Parvinen. 1998. Stage-specific expression and cellular localization of the heat shock factor 2 isoforms in the rat seminiferous epithelium. Exp. Cell. Res. 240:16-27. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin, I. J., B. Kroger, and R. S. Williams. 1990. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc. Natl. Acad. Sci. USA 87:6263-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, I. R., and S. J. Rush. 1999. Cellular localization of the heat shock transcription factors HSF1 and HSF2 in the rat brain during postnatal development and following hyperthermia. Brain Res. 821:333-340. [DOI] [PubMed] [Google Scholar]
- 4.Christians, E., A. A. Davis, S. T. Thomas, and I. J. Benjamin. 2000. Maternal effect of Hsf1 on reproductive success. Nature 407:693-694. [DOI] [PubMed] [Google Scholar]
- 5.Christians, E., E. Michel, P. Adenot, V. Mezger, M. Rallu, M. Morange, and J.-P. Renard. 1997. Evidence for the involvement of mouse heat shock factor 1 in the atypical expression of the HSP70.1 heat shock gene during mouse zygotic genome activation. Mol. Cell. Biol. 17:778-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dix, D. J., J. W. Allen, B. W. Collins, C. Mori, N. Nakamura, P. Poorman-Allen, E. H. Goulding, and E. M. Reddy. 1996. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc. Natl. Acad. Sci. USA 93:3264-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dix, D. J., M. Rosario-Herrle, H. Gotoh, C. Mori, E. H. Goulding, C. V. Barrett, and E. M. Eddy. 1996. Developmentally regulated expression of Hsp70-2 and a Hsp70-2/lacZ transgene during spermatogenesis. Dev. Biol. 174:310-321. [DOI] [PubMed] [Google Scholar]
- 8.Du, X. J., T. J. Cole, N. Tenis, X. M. Gao, F. Kontgen, B. E. Kemp, and J. Heierhorst. 2002. Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol. Cell. Biol. 22:2821-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson, M., E. Jokinen, L. Sistonen, and S. Leppa. 2000. Heat shock factor 2 is activated during mouse heart development. Int. J. Dev. Biol. 44:471-477. [PubMed] [Google Scholar]
- 10.Fenteany, G., R. F. Standaert, W. S. Lane, S. Choi, E. J. Corey, and S. L. Schreiber. 1995. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268:726-731. [DOI] [PubMed] [Google Scholar]
- 11.Fiorenza, M. T., T. Farkas, M. Dissing, D. Kolding, and V. Zimarino. 1995. Complex expression of murine heat shock transcription factors. Nucleic Acids Res. 23:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster, M. J., A. Dubey, K. M. Dawson, W. A. Stutts, H. Lal, and R. S. Sohal. 1996. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 93:4765-4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster, M. J., and H. Lal. 1991. Neurobehavioral biomarkers of aging: influence of genotype and dietary restriction. Biomed. Environ. Sci. 4:144-165. [PubMed] [Google Scholar]
- 14.Forster, M. J., and H. Lal. 1992. Within-subject behavioral analysis of recent memory in aging mice. Behav. Pharmacol. 3:337-349. [PubMed] [Google Scholar]
- 15.Goodson, M. L., O. K. Park-Sarge, and K. D. Sarge. 1995. Tissue-dependent expression of heat shock factor 2 isoforms with distinct transcriptional activities. Mol. Cell. Biol. 15:5288-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jedlicka, P., M. A. Mortin, and C. Wu. 1997. Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J. 16:2452-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kallio, M., Y. Chang, M. Manuel, T. Alastalo, M. Rallu, Y. Gitton, L. Pirkkala, M. Loones, L. Paslaru, S. Larney, S. Hiard, M. Morange, L. Sistonen, and V. Mezger. 2002. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 21:2591-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawazoe, Y., A. Nakai, M. Tanabe, and K. Nagata. 1998. Proteasome inhibition leads to the activation of all members of the heat-shock-factor family. Eur. J. Biochem. 255:356-362. [DOI] [PubMed] [Google Scholar]
- 19.Kim, D., S. H. Kim, and G. C. Li. 1999. Proteasome inhibitors MG132 and lactacystin hyperphosphorylate HSF1 and induce hsp70 and hsp27 expression. Biochem. Biophys. Res. Commun. 254:264-268. [DOI] [PubMed] [Google Scholar]
- 20.Kroeger, P. E., and R. I. Morimoto. 1994. Selection of new HSF1 and HSF2 DNA-binding sites reveals difference in trimer cooperativity. Mol. Cell. Biol. 14:7592-7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroeger, P. E., K. D. Sarge, and R. I. Morimoto. 1993. Mouse heat shock transcription factors 1 and 2 prefer a trimeric binding site but interact differently with the HSP70 heat shock element. Mol. Cell. Biol. 13:3370-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuel, M., J. Sage, M. G. Mattei, M. Morange, and V. Mezger. 1999. Genomic structure and chromosomal localization of the mouse Hsf2 gene and promoter sequences. Gene 232:115-124. [DOI] [PubMed] [Google Scholar]
- 23.Mathew, A., S. K. Mathur, C. Jolly, S. G. Fox, S. Kim, and R. I. Morimoto. 2001. Stress-specific activation and repression of heat shock factors 1 and 2. Mol. Cell. Biol. 21:7163-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathew, A., S. K. Mathur, and R. I. Morimoto. 1998. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 18:5091-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillan, D. R., X. Xiao, L. Shao, K. Graves, and I. J. Benjamin. 1998. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J. Biol. Chem. 273:7523-7528. [DOI] [PubMed] [Google Scholar]
- 26.Mezger, V., M. Rallu, R. I. Morimoto, M. Morange, and J. P. Renard. 1994. Heat shock factor 2-like activity in mouse blastocysts. Dev. Biol. 166:819-822. [DOI] [PubMed] [Google Scholar]
- 27.Min, J. N., M. Y. Han, S. S. Lee, K. J. Kim, and Y. M. Park. 2000. Regulation of rat heat shock factor 2 expression during the early organogenic phase of embryogenesis. Biochim. Biophys. Acta 1494:256-262. [DOI] [PubMed] [Google Scholar]
- 28.Montagutelli, X. 2000. Effect of the genetic background on the phenotype of mouse mutations. J. Am. Soc. Nephrol. 11(Suppl. 16):S101-S105. [PubMed] [Google Scholar]
- 29.Morano, K. A., and D. J. Thiele. 1999. Heat shock factor function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 7:271-282. [PMC free article] [PubMed] [Google Scholar]
- 30.Morimoto, R. I. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12:3788-3796. [DOI] [PubMed] [Google Scholar]
- 31.Nakai, A., and R. I. Morimoto. 1993. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol. Cell. Biol. 13:1983-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakai, A., M. Tanabe, Y. Kawazoe, J. Inazawa, R. I. Morimoto, and K. Nagata. 1997. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol. Cell. Biol. 17:469-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pirkkala, L., T. P. Alastalo, X. Zuo, I. J. Benjamin, and L. Sistonen. 2000. Disruption of heat shock factor 1 reveals an essential role in the ubiquitin proteolytic pathway. Mol. Cell. Biol. 20:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirkkala, L., P. Nykanen, and L. Sistonen. 2001. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 15:1118-1131. [DOI] [PubMed] [Google Scholar]
- 35.Pritts, T. A., E. S. Hungness, D. D. Hershko, B. W. Robb, X. Sun, G. J. Luo, J. E. Fischer, H. R. Wong, and P. O. Hasselgren. 2002. Proteasome inhibitors induce heat shock response and increase IL-6 expression in human intestinal epithelial cells. Am. J. Physiol. Endocrinol. Metab. 282:R1016-R1026. [DOI] [PubMed] [Google Scholar]
- 36.Rallu, M., M. Loones, Y. Lallemand, R. Morimoto, M. Morange, and V. Mezger. 1997. Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc. Natl. Acad. Sci. USA 94:2392-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosario, M. O., S. L. Perkins, D. A. O'Brien, R. L. Allen, and E. M. Eddy. 1992. Identification of the gene for the developmentally expressed 70 kDa heat-shock protein (P70) of mouse spermatogenic cells. Dev. Biol. 150:1-11. [DOI] [PubMed] [Google Scholar]
- 38.Sarge, K. D., O. K. Park-Sarge, J. D. Kirby, K. E. Mayo, and R. I. Morimoto. 1994. Expression of heat shock factor 2 in mouse testis: potential role as a regulator of heat-shock protein gene expression during spermatogenesis. Biol. Reprod. 50:1334-1343. [DOI] [PubMed] [Google Scholar]
- 39.Sarge, K. D., V. Zimarino, K. Holm, C. Wu, and R. I. Morimoto. 1991. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 5:1902-1911. [DOI] [PubMed] [Google Scholar]
- 40.Schuetz, T. J., G. J. Gallo, L. Sheldon, P. Tempst, and R. E. Kingston. 1991. Isolation of a cDNA for HSF2: evidence for two heat shock factor genes in humans. Proc. Natl. Acad. Sci. USA 88:6911-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sistonen, L., K. D. Sarge, and R. I. Morimoto. 1994. Human heat shock factors 1 and 2 are differentially activated and can synergistically induce hsp70 gene transcription. Mol. Cell. Biol. 14:2087-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sistonen, L., K. D. Sarge, B. Phillips, K. Abravaya, and R. I. Morimoto. 1992. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol. Cell. Biol. 12:4104-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriano, P., C. Montgomery, R. Geske, and A. Bradley. 1991. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64:693-702. [DOI] [PubMed] [Google Scholar]
- 44.Stacchiotti, A., R. Rezzani, L. Rodella, L. Tiberio, L. Schiaffonati, and R. Bianchi. 1999. Cell-specific expression of heat shock transcription factors 1 and 2 in unstressed rat spinal cord. Neurosci. Lett. 268:73-76. [DOI] [PubMed] [Google Scholar]
- 45.Tanemura, K., M. Kurohmaru, K. Kuramoto, and Y. Hayashi. 1993. Age-related morphological changes in the testis of the BDF1 mouse. J. Vet. Med. Sci. 55:703-710. [DOI] [PubMed] [Google Scholar]
- 46.Xiao, X., X. Zuo, A. A. Davis, D. R. McMillan, B. B. Curry, J. A. Richardson, and I. J. Benjamin. 1999. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 18:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshima, T., T. Yura, and H. Yanagi. 1998. Heat shock factor 1 mediates hemin-induced hsp70 gene transcription in K562 erythroleukemia cells. J. Biol. Chem. 273:25466-25471. [DOI] [PubMed] [Google Scholar]
- 48.Zhu, Z., and N. F. Mivechi. 1999. Regulatory domain of human heat shock transcription factor-2 is not regulated by hemin or heat shock. J. Cell. Biochem. 73:56-69. [DOI] [PubMed] [Google Scholar]