Abstract
3-Oxoadipate:succinyl-coenzyme A (CoA) transferase and 3-oxoadipyl-CoA thiolase carry out the ultimate steps in the conversion of benzoate and 3-chlorobenzoate to tricarboxylic acid cycle intermediates in bacteria utilizing the 3-oxoadipate pathway. This report describes the characterization of DNA fragments with the overall length of 5.9 kb from Pseudomonas sp. strain B13 that encode these enzymes. DNA sequence analysis revealed five open reading frames (ORFs) plus an incomplete one. ORF1, of unknown function, has a length of 414 bp. ORF2 (catI) encodes a polypeptide of 282 amino acids and starts at nucleotide 813. ORF3 (catJ) encodes a polypeptide of 260 amino acids and begins at nucleotide 1661. CatI and CatJ are the subunits of the 3-oxoadipate:succinyl-CoA transferase, whose activity was demonstrated when both genes were ligated into expression vector pET11a. ORF4, termed catF, codes for a protein of 401 amino acid residues with a predicted mass of 41,678 Da with 3-oxoadipyl-CoA thiolase activity. The last three ORFs seem to form an operon since they are oriented in the same direction and showed an overlapping of 1 bp between catI and catJ and of 4 bp between catJ and catF. Conserved functional groups important for the catalytic activity of CoA transferases and thiolases were identified in CatI, CatJ, and CatF. ORF5 (catD) encodes the 3-oxoadipate enol-lactone hydrolase. An incomplete ORF6 of 1,183 bp downstream of ORF5 and oriented in the opposite direction was found. The protein sequence deduced from ORF6 showed a putative AMP-binding domain signature.
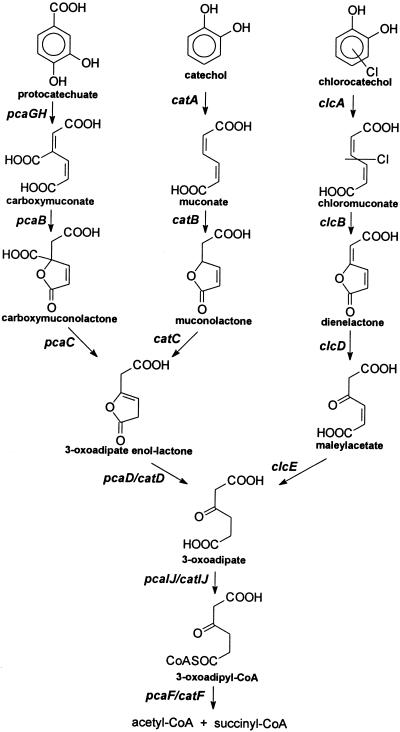
The majority of chloroaromatic compounds are degraded via chlorocatechols as the central metabolites and further through the modified ortho cleavage pathway with chlorine elimination. Convergence of the pathway for aromatics with the one degrading lower-chlorinated catechols, with 3-oxoadipate as the common metabolite, is seen.
The following gene clusters encoding the modified ortho cleavage pathway have been studied: clcABDE, encoding the 3-chlorobenzoate-degradative enzymes of Pseudomonas putida AC866 (23), tfdCDEF, encoding the 2,4-dichlorophenoxyacetate-degradative enzymes of Ralstonia eutropha JMP134 (53), tcbCDEF, encoding the 1,2,4-trichlorobenzene-degradative enzymes of Pseudomonas sp. strain P51 (50), and the part of clcDE encoding the 3-chlorobenzoate-degradative enzymes of Pseudomonas sp. strain B13 (37) (Fig. 1). However, the molecular basis for the connection to the Krebs cycle is an aspect of chloroaromatic degradation that has received no attention, while the genes encoding the lower 3-oxoadipate pathway have been the subject of intensive investigations (22).
FIG. 1.
Schematic presentation of the protocatechuate and catechol branches of the 3-oxoadipate pathway plus the modified ortho pathway. Gene designations are in italics.
We report here the identification and characterization of the genes, designated catIJ, encoding the two subunits of 3-oxoadipate:succinyl-coenzyme A (CoA) transferase from Pseudomonas sp. strain B13. In addition, gene catF, encoding the 3-oxoadipyl-CoA thiolase, was found next to the transferase genes. When the sequence of CatF was compared with sequences of bacterial and eukaryotic thiolases, a high degree of amino acid identity was seen. The CoA transferase of strain B13 showed highest identity with enzymes from the strictly anaerobic bacteria Acidaminococcus fermentans, Pseudomonas aeruginosa PAO1, and Mesorhizobium loti, but lower similarity to analogous enzymes involved in the degradation of aromatic compounds. Biochemical aspects of both enzymes are reported in the accompanying publication (26).
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The plasmids and bacterial strains used in this study are listed in Table 1. Pseudomonas sp. strain B13 (DSMZ6978) was grown at 30°C in mineral medium containing 3-chlorobenzoate (10 mM) as the substrate (13). Escherichia coli strains harboring plasmids were grown in Luria-Bertani (LB) medium (33) with ampicillin (100 μg/ml). For expression experiments E. coli BL21(DE3)pLysS with pET11a was cultivated at 37°C in LB medium with ampicillin (50 μg/ml) and chloroamphenicol (30 μg/ml) to an absorbance of 0.7 at 546 nm. Induction was achieved by adding 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG), followed by further incubation for 1.5 h.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype, phenotype, or description | Reference or source |
|---|---|---|
| Strains | ||
| Pseudomonas sp. strain B13 | Wild type; benzoate+ 3-chlorobenzoate+ | 13 |
| Escherichia coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK−) phoA supE44 λ−thi-1 gyrA96 relA1 | Gibco BRL |
| XL10-Gold Kan | Tetr Δ(mcrA) 183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Tn5 (Kanr) Amy] | Stratagene |
| SoloPack Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac Hte [F′ proAB lacIqZΔM15 Tn10 (Tetr) Amy Camr] | Stratagene |
| BL21(DE3)pLysS | F−ompT hsdSB(rB− mB−) dcm gal (DE3) pLysS Cmr | Promega |
| Plasmids | ||
| pET11a | Apr, T7 expression vector | Stratagene |
| pUC18 | Apr, cloning vector | 53 |
| pPCR-Script Amp SK(+) | Apr, cloning vector | Stratagene |
| pKSC2 | 2.3-kb PstI fragment containing catF in pUC18 | This study |
| pMGTH | Same as pKSC2, opposite direction | This study |
| pKSC2-1 | 2.0-kb EcoRI fragment of pKSC2 in pUC18 | This study |
| pKSC2-2 | 1.1-kb HincII fragment of pKSC2 in pUC18 | This study |
| pKSC2-4 | 0.35-kb PstI/SmaI fragment of pKSC2 in pUC18 | This study |
| pKSC2-5 | 0.6-kb HincII fragment of pKSC2 in pUC18 | This study |
| pKSC2-6 | 0.45-kb HincII fragment of pKSC2 in pUC18 | This study |
| pMGTR11R | 2.3-kb EcoRI fragment containing catI and part of catJ | This study |
| pMGTR2 | Same as pMGTR11R, opposite direction | This study |
| pMGTR4 | 1.65-kb PCR product (ESTR1, ESTR2), blunt end in pPCR-Script Amp SK(+) | This study |
| pMGTR3 | Same as pMGTR4, opposite direction | This study |
| pESBLT8 | pMGTR4 digested with NdeI and BamHI, excised fragment transferred in pET11a/NdeI/BamHI | This study |
| pMGTR2H380 | 0.38-kb HincII fragment of pMGTR2 in pUC18 | This study |
| pMGTR2H550 | 0.55-kb HincII fragment of pMGTR2 in pUC18 | This study |
| pMGTR2H880 | 0.88-kb HincII fragment of pMGTR2 in pUC18 | This study |
| pMGELH3 | 3.6-kb EcoRI fragment containing catD in pUC18 | This study |
| pMGELH3E/S2 | 2.4-kb EcoRI/SmaI fragment of pMGELH3 in pUC18 | This study |
| pMGELH3Pst1 | 1.1-kb PstI fragment of pMGELH3 in pUC18 | This study |
| pMGELH3Apo1 | 0.7-kb ApoI fragment of pMGELH3 in pUC18 | This study |
| pMGELH3S/Su1 | 0.9-kb SalI/SmaI fragment of pMGELH3 in pUC18 | This study |
| pMGELH3S/So2 | 1.6-kb SalI/SmaI fragment of pMGELH3 in pUC18 | This study |
| pMGELHExpr7 | 1.1-kb PCR product (MG-ELH-1, MG-ELH-2) with generated NheI/BamHI restriction sites, digested with NheI/BamHI, and transferred in pET11a/NheI/BamHI | This study |
Plasmid preparation, DNA manipulation, and sequencing.
DNA manipulations such as subcloning, digestion, ligation, and transformation were performed according to standard procedures (44). DNA sequencing was performed by MWG-BIOTECH AG, Ebersberg, Germany.
PCR amplification.
PCR amplification experiments were performed with genomic DNA of Pseudomonas sp. strain B13 as the template. Primers CATF1 (5′-GGCTGGCGCTTCATCAA-3′) and CATF2 (5′-AAGGCTTCGTT[GC]AG[CT]TC[AG]AT-3′), which corresponded to regions conserved in the 3-oxoadipyl-CoA thiolase gene (catF) of Acinetobacter calcoaceticus (28) and pcaF of P. putida PCH722 (21, 35) (bases 336 to 452 and 959 to 978 of pcaF, respectively), were designed. PCR with primers CATF1 and CATF2 was performed with AmpliTaq DNA polymerase (Perkin-Elmer, Norwalk, Conn.) and an annealing temperature of 48°C.
For construction of an expression plasmid with transferase gene forward primer ESTR1 (5′-CGAGACCGAGCATATGGCTGAACTCCTGACC-3′) was designed with an NdeI site (underlined) and the start codon of catI (italics) and reverse primer ESTR2 (5′-GTGCGCACGGGATCCAGATGTAGACTTCGCG-3′) with a BamHI site (underlined) and the stop codon of catJ (italics). PCR with primers ESTR1 and ESTR2 was carried out with the Advantage cDNA Polymerase Mix (Clontech, Palo Alto, Calif.) and an annealing temperature of 68°C.
An expression plasmid for the hydrolase was made with forward primer MG-ELH-1 (5′-GTTCCCGATGGCTAGCGTCAAACTCG-3′), with a constructed NheI site (underlined; boldface G indicates a change from C in the original sequence of strain B13 necessary to create the restriction site) and the start codon of catD (italics), and reverse primer MG-ELH-2 (5′-CTGTGTGAACGGATCCGCCAGACCAT-3′), with a BamHI site (underlined; boldface G indicates a change from C in the original sequence necessary to create the restriction site). The PCR was performed with the Advantage-GC cDNA Polymerase Mix (Clontech) and an annealing temperature of 68°C. All other conditions were used as suggested by the manufacturers.
Southern hybridization and labeling.
Genomic DNA from Pseudomonas sp. strain B13 was digested and separated on agarose gel (0.8%) and blotted onto nylon membranes (Hybond N+; Amersham Pharmacia Biotech, Buckinghamshire, England). Specific probes for hybridization were recovered from agarose gels and labeled using Rediprime II and Redivue [32P]dCTP (Amersham Pharmacia Biotech).
Preparation of cell extracts and enzyme assays.
Preparation of cell extracts and enzyme assays were carried out as reported previously (26).
Sequence analysis.
BLAST searches for screening homologous proteins (1) were made by using the BLAST, version 2.0, software (http://www.ncbi.nlm.nhi.gov/BLAST). For search of consensus sequences the PROSITE database (http://www.expasy.ch/prosite) was used (4). The sequences were compiled and aligned by using ClustalX software (version 1.8) (48). Percentages in Table 2 were obtained with GeneDoc (version 2.5) (www.cris.com/∼ketchup/genedoc.shtml) using multiple alignments from ClustalX.
TABLE 2.
Genes and gene products
| Gene | Position in sequence (nt) | Function in strain B13 | Related protein | Enzyme activity detecteda | Source | Identity (%)b | Accession no.c | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| ORF1 | 83–496 | Unknown | Unknown, PA1353 | − | P. aeruginosa PAO1 | 60 | gb AAG04742 | 46 |
| Unknown, PhnB | − | E. coli | 30 | gb AAA24338 | 8 | |||
| catI | 813–1661 | Subunit A, 3-oxoadipate: succinyl-CoA transferase | Probable CoA transferase | − | P. aeruginosa PAO1 | 87 | gb AAG03615 | 46 |
| Acetate-CoA transferase | − | M. loti | 59 | gb BAB50895 | 24 | |||
| Glutaconate-CoA transferase | + | Acidaminococcus fermentans | 30 | emb CAA57199 | 31 | |||
| Glutaconate-CoA transferase | − | Sulfolobus solfataricus | 23 | gb AAK41344 | ||||
| Unknown protein | − | Comamonas testosteroni | 20 | dbj BAB15810 | ||||
| Hypothetical protein Rv3551 | − | Mycobacterium tuberculosis | 20 | emb CAB05066 | 9 | |||
| Glutaconate-CoA transferase | − | Archaeoglobus fulgidus | 19 | gb AAB90043 | 27 | |||
| CoA transferase | − | Deinococcus radiodurans | 18 | gb AAF12247 | 51 | |||
| Hypothetical 3-oxoadipate-CoA transferase | − | Bacillus subtilis | 18 | dbj BAA11705 | 54 | |||
| 3-Oxoadipate-CoA transferase | − | Helicobacter pylori 26695 | 17 | gb AAD07743 | 49 | |||
| 3-Oxoadipate-CoA transferase | − | Caulobacter crescentus | 17 | gb AAK24376 | 36 | |||
| 3-Oxoadipate:succinyl-CoA transferase | + | Sphingomonas sp. strain RW1 | 16 | emb CAA51372 | 2, 3, 18 | |||
| catJ | 1661–2443 | Subunit B, 3-oxoadipate: succinyl-CoA transferase | Probable CoA transferase | − | P. aeruginosa PAO1 | 91 | gb AAG03616 | 46 |
| Acetoacetyl-CoA transferase | − | M. loti | 61 | dbj BAB50894 | 24 | |||
| Glutaconate-CoA transferase | + | Acidaminococcus fermentans | 30 | emb CAA57200 | 31 | |||
| Unknown protein | − | Comamonas testosteroni | 27 | dbj BAB15811 | ||||
| Glutaconate-CoA transferase | − | Sulfolobus solfataricus | 22 | gb AAK41345 | ||||
| 3-Oxoadipate-CoA transferase | − | H. pylori 26695 | 20 | gb AAD07744 | 49 | |||
| 3-Oxoadipate-CoA transferase | − | Caulobacter crescentus | 20 | gb AAK24377 | 36 | |||
| 3-Oxoadipate:succinyl-CoA transferase | + | Sphingomonas sp. strain RW1 | 20 | emb CAA51373 | 2, 3, 18 | |||
| 3-Oxoacid CoA transferase | − | H. pylori | 20 | emb CAA03917 | ||||
| catF | 2440–3645 | 3-Oxoadipyl-CoA thiolase | Probable acyl-CoA thiolase, PA3589 | − | P. aeruginosa PAO1 | 85 | pir G83197 | 46 |
| 3-Oxoadipyl-CoA thiolase | + | P. putida | 82 | sp Q51956 | 21 | |||
| 3-Oxoadipyl-CoA thiolase | − | Burkholderia pseudomallei | 72 | gb AAG12159 | ||||
| 3-Oxoadipyl-CoA thiolase | − | Caulobacter crescentus | 72 | gb AAK23095 | 36 | |||
| 3-Oxoadipyl-CoA thiolase | − | M. loti | 66 | dbj BAB50893 | 24 | |||
| 3-Oxoadipyl-CoA thiolase, PcaF | − | Acinetobacter calcoaceticus ADP1 | 65 | sp Q43974 | 28 | |||
| 3-Oxoadipyl-CoA thiolase, CatF | − | Acinetobacter calcoaceticus ADP1 | 65 | sp Q43935 | 45 | |||
| Acetyl-CoA acetyltransferase | − | E. coli | 64 | emb CAA66099 | 16 | |||
| 3-Oxoadipyl-CoA thiolase | − | Streptomyces coelicolor A3(2) | 55 | emb CAB89028 | 42 | |||
| Acetyl-CoA acetyltransferase | − | Bacillus halodurans | 55 | dbj BAB03924 | 47 | |||
| Ketothiolase | − | P. putida U | 53 | gb AAC24332 | 37 | |||
| Acetyl-CoA acetyltransferase | − | Sphingomonas sp. strain RW1 | 51 | emb CAA51374 | 2, 3, 18 | |||
| 3-Oxoadipyl-CoA thiolase | − | Streptomyces coelicolor | 50 | pir T35019 | ||||
| 3-Oxoadipyl-CoA thiolase, PA0228 | − | P. aeruginosa PAO1 | 50 | pir D83618 | 46 | |||
| 3-Oxoadipyl-CoA thiolase | − | Deinococcus radiodurans | 47 | pir G75598 | 51 | |||
| catD | 3782–4570 | 3-Oxoadipate enol-lactone hydrolase | 3-Oxoadipate enol-lactone hydrolase | − | P. aeruginosa PAO1 | 74 | gb AAG03620 | 46 |
| PcaD-like protein | − | P. putida DOT-T1 | 61 | gb AAD39558 | 41 | |||
| 3-Oxoadipate enol-lactone hydrolase, CatD2 | − | R. eutropha | 54 | gb AAG42026 | ||||
| 3-Oxoadipate enol-lactone hydrolase, CatD1 | − | R. eutropha | 50 | gb AAG42037 | ||||
| Probable hydrolase, PA0480 | − | P. aeruginosa PAO1 | 48 | gb AAG03869 | 46 | |||
| 3-Oxoadipate enol-lactone hydrolase | − | Bradyrhizobium japonicum | 45 | emb CAA71271 | 30 | |||
| Probable 3-oxoadipate enol-lactone hydrolase | − | Leishmania major | 45 | emb CAC02005 | ||||
| 3-Oxoadipate enol-lactone hydrolase | + | Frateuria sp. | 44 | dbj BAA75208 | 34 | |||
| 3-Oxoadipate enol-lactone hydrolase II | − | Acinetobacter calcoaceticus ADP1 | 44 | gb AAC46435 | 10, 11 | |||
| Probable 3-oxoadipate enol-lactone hydrolase | − | M. loti | 42 | dbj BAB49603 | 24 | |||
| 3-Oxoadipate enol-lactone hydrolase I | − | Acinetobacter calcoaceticus ADP1 | 39 | gb AAC37150 | 19, 20 | |||
| 3-Oxoadipate enol-lactone hydrolased | − | Rhodococcus opacus | 25 | gb AAC38246 | 14 | |||
| 4-Carboxymuconolactone decarboxylased | − | Caulobacter crescentus | 25 | gb AAK24382 | 36 | |||
| ORF6 | 4706–5888 | Unknown | Unknown, PA1997 | − | P. aeruginosa PAO1 | 81 | gb AAG05385 | 46 |
| Acetoacetyl-CoA synthetase | + | S. meliloti | 67 | gb AAC64548 | 6 |
+, enzyme activity determined with cloned gene, showing evidence that nucleotide sequence codes the respective enzyme; −, absence of direct proof for responsibility of ORF for enzyme (enzyme named because of sequence alignment).
Percentage of amino acids that are identical when sequences are aligned with sequences listed in all nonredundant databases.
gb, GenBank; emb, EMBL Data Library; dbj, DDBJ; sp, Swiss-Prot; pir, National Biomedical Research Foundation, Protein Information Resource.
Enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity.
Nucleotide sequence accession number.
The nucleotide sequences determined in this work have been deposited under GenBank accession no. AY044272.
RESULTS AND DISCUSSION
Cloning of the genes of the lower 3-oxoadipate modified ortho pathway.
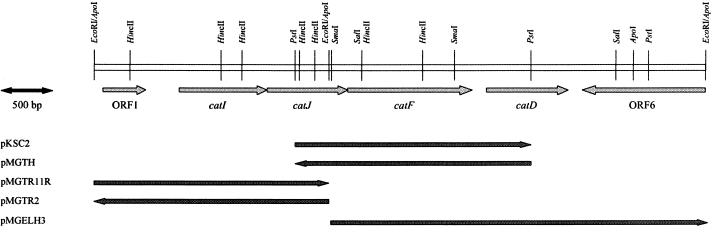
A PCR was run by making use of primers CATF1 and CATF2, which corresponded to regions conserved in the 3-oxoadipyl-CoA thiolase gene (catF) of Acinetobacter calcoaceticus (28) and pcaF of P. putida PCH722 (21, 35). This resulted in a 539-bp fragment containing a part of catF. This PCR product was used as a probe in Southern hybridization experiments, and a 2.3-kb PstI fragment of genomic DNA of Pseudomonas sp. strain B13 was found to hybridize with the probe. This fragment was cloned in pUC18/PstI to give clones pKSC2 and pMGTH, which harbor the same insert but in the opposite direction. Thiolase activity of 0.27 U/mg of protein was found in cells harboring clone pKSC2; there was no activity in clone pMGTH and E. coli DH5α harboring the vector without any insert. The DNA sequence of pKSC2 comprises one incomplete open reading frame (ORF). The deduced protein sequence was found to have highest identity with glutaconate-CoA transferase of Acidaminococcus fermentans (31). Since the N-terminal protein sequences of both subunits of the purified 3-oxoadipate:succinyl-CoA transferase of Pseudomonas sp. strain B13 also showed the highest homology with that of CoA transferase (26), it was thought that the incomplete ORF might be part of the transferase gene(s). The complete ORF4, termed catF, extends from positions 511 to 1716, i.e., an overlapping of 4 bp with catJ. It codes for a protein of 401 amino acid residues with a predicted mass of 41,678 Da. The N-terminal amino acid sequence of the thiolase obtained from the purified enzyme (26) was identical to the sequence deduced from the cloning.
Downstream of catF an additional incomplete ORF, which was similar to that encoding PcaD, was found, and thus it is assumed that it codes the first part of 3-oxoadipate enol-lactone hydrolase.
Clones bearing sequences upstream and downstream of catF were generated to obtain complete genes encoding transferase and hydrolase.
The 326-bp PstI/EcoRI fragment of the 2.3-kb PstI fragment was used as probe in Southern hybridization experiments to find the transferase gene. A 2.3-kb EcoRI fragment of genomic DNA of Pseudomonas sp. strain B13 hybridized with the probe. Cloning of the fragment into pUC18/EcoRI gave clones pMGTR11R and pMGTR2, which have inserts with opposite orientations. To have a functional transferase, a 1.7-kb PCR product, plasmid pMGTR4, was prepared by the use of a PCR product obtained with primers ESTR1 and ESTR2. Cloning into the NdeI/BamHI site of expression vector pET11a allowed the isolation of a clone with plasmid pESBLT8. Enzyme assays with crude extracts of this clone revealed strong activity of 3-oxoadipate:succinyl-CoA transferase compared to those of the controls. An activity of 4.6 U/mg of protein was detected, while no activity was present when plasmid pET11a lacked the insert.
The DNA sequence of pMGTR2 comprises one ORF (ORF1), from position 83 to 496, with unknown function.
Two complete ORFs were found on pMGTR2 plus the 5′ part of pKSC2. By comparing the N-terminal amino acid sequences of the subunits of the transferase obtained from the purified enzyme (26) with our deduced amino acid sequences, we identified each translational start site. Amino acid sequences obtained from cloning and enzyme purification were nearly identical, with only one difference in each subunit, which might be the result of an error in the analysis of the protein sequences. ORF2 (catI) encodes a polypeptide of 282 amino acids and starts at nucleotide 813. ORF3 (catJ) encodes a polypeptide of 260 amino acids and begins at nucleotide 1661. Both ORFs were oriented in the same direction and showed an overlapping of 1 bp, which needs −1 translational frameshifting (15).
The same 539-bp PCR product, containing a part of catF, which allowed the detection of the whole thiolase gene (see above), was used as a probe in Southern hybridization experiments to find the complete catD gene. A 3.6-kb EcoRI fragment of genomic DNA of Pseudomonas sp. strain B13 hybridized with the probe. Cloning the fragment into pUC18/EcoRI gave clone pMGELH3.
Cloning a PCR product, obtained with primers MG-ELH-1 and MG-ELH-2, into the NdeI/BamHI site of expression vector pET11a in E. coli XL10-Gold Kan allowed the isolation of plasmid pMGELHExpr7. This was transferred into expression strain E. coli BL21(DE3)pLysS. Enzyme assays with crude extracts of this clone revealed strong activity of 3-oxoadipate enol-lactone hydrolase compared to those of the controls. An activity of 32.1 U/mg of protein was detected, while no activity was present in pMGELH3, containing the 3.6-kb EcoRI fragment.
Downstream of catD an incomplete ORF of 1,183 bp, which is in the opposite direction to catIJF and catD, was found.
Sequence comparison.
By analyzing our sequences together with published sequences of CoA transferases, thiolases, and hydrolases, several important points can be made. There is significant sequence similarity of thiolases, in α-, β-, γ-, and ɛ-proteobacteria as well as low-GC and high-GC gram-positive bacteria, not only with those enzymes involved in the degradation of aromatic compounds (amino acids 83% identical to those of PcaF of P. putida and 67% identical to those of CatF and PcaF of Acinetobacter calcoaceticus) but also with other thiolases functioning for other purposes such as β-oxidation (Table 2).
The same is true for hydrolase CatD of strain B13, which shows high sequence identities: amino acids 61% identical to those of 3-oxoadipate enol-lactone hydrolase of P. putida and 74% identical to those of PcaD of P. aeruginosa PAO1.
In contrast, CoA transferases show a more diverse picture. The B13 subunits of the transferase have only low sequence similarity (less than 20%) to the 3-oxoadipate:succinyl-CoA transferases of Acinetobacter calcoaceticus and P. putida, as well as other CoA transferases. Some stretches of 30 and 29% identity to the glutaconate-CoA transferase from the strictly anaerobic bacterium Acidaminococcus fermentans were found. However, high sequence identities, 87 and 91%, to PcaI (PA0226) and PcaJ (PA0227) of P. aeruginosa PAO1 were observed, suggesting that these are also the subunits of the transferase.
The product of ORF1 and the PhnB protein of E. coli, an enzyme that is involved in phosphonate metabolism but whose exact function is not known, have 30% identical amino acids (8). A higher identity of the deduced amino acid sequence (60%) to protein PA1353 of P. aeruginosa PAO1 was observed.
The protein sequence deduced from the sequence downstream of catD, ORF6, was found to have high identity, 67%, to acetoacetyl-CoA synthetase of Sinorhizobium meliloti (6). The deduced sequence and protein PA1997 of P. aeruginosa PAO1, a protein with an AMP-binding motif, had 81% identical amino acids.
Operon structure.
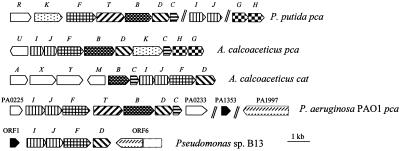
We identified the three overlapping 2.3-, 2.3-, and 3.6-kb DNA fragments from Pseudomonas sp. strain B13 (Fig. 2) encoding one enzyme with unknown function, 3-oxoadipate:succinyl-CoA transferase, 3-oxoadipyl-CoA thiolase, 3-oxoadipate enol-lactone hydrolase, and a part of an enzyme with an AMP-binding motif. All identified ORFs except ORF6 are oriented in the same direction. The overall organization of genes catI, catJ, and catF, encoding the lower pathway in Pseudomonas sp. strain B13, is identical to those of pca and cat clusters reported for Acinetobacter calcoaceticus and the pca cluster of P. aeruginosa PAO1 but different from the organization of the genes in P. putida (Fig. 3). In strain B13 the hydrolase gene follows the thiolase gene, a situation which is identical to that for the cat cluster in Acinetobacter calcoaceticus, while the gene is separated from pcaF by at least one gene in the other clusters. A comparison of the identical clusters of B13 and cat genes identified a much broader spacing between catF and catD in B13 than between catF and catD in Acinetobacter calcoaceticus. In addition, there is a promoter sequence in front of B13 catD which is absent between catF and catD in Acinetobacter calcoaceticus. Intergenic regions of 12 bp between the transferase and the thiolase genes of the pca and cat clusters in Acinetobacter calcoaceticus were seen (22), while an overlap of 4 bp between catJ and catF is present in Pseudomonas sp. strain B13. Spacing of 8 or 10 bp separated the genes of the subunits of the transferases in Acinetobacter calcoaceticus and in P. putida, respectively, while the B13 genes overlap with 1 bp. The data suggest that catI, catJ, and catF may constitute an operon in Pseudomonas sp. strain B13.
FIG. 2.
Schematic representation of the genetic organization of the ORFs that were identified on the overlapping 2.3-kb PstI, 2.3-kb EcoRI, and 3.6-kb EcoRI fragments. Relevant restriction sites present on the fragments are indicated. Arrows, positions and orientations of the different ORFs detected.
FIG. 3.
Organization of gene clusters for protocatechuate (pca) and (chloro)catechol (cat) metabolism in selected bacteria. Arrows, direction of transcription; double lines, genes separated by >10 kbp. The information was compiled from the following sources: Acinetobacter calcoaceticus, references 19, 20, 22, 28 and 45; P. putida, references 21, 22and 38; P. aeruginosa PAO1, reference 46; strain B13, this study. catA, catechol 1,2-dioxygenase gene; catB, muconate cycloisomerase gene; pcaB, carboxymuconate cycloisomerase gene; catC, muconolactone isomerase gene; pcaC, carboxymuconolactone decarboxylase gene; catD and pcaD, 3-oxoadipate enol-lactone hydrolase genes; catF and pcaF, thiolase genes; pcaHG, protocatechuate 3,4-dioxygenase genes; pcaIJ and catIJ, transferase genes; pcaK and pcaT, transport genes; pcaR, pcaU, and catM, regulator genes; catX and catY, genes of unknown function.
Signature patterns.
Parales and Harwood (38) reported the presence of a glycine cluster in the N-terminal amino acid sequence of PcaI of P. putida with strong similarity to the consensus sequence (Prosite PS01273: [DN]-[GN]-x[2]-[LIVMFA][3]-G-G-F-x[3]-G-x-P) described by Wierenga et al. (52). Other CoA transferases from very diverse organisms show the same sequence: Helicobacter pylori (12), pig heart mitochondria (29), Acinetobacter calcoaceticus (28), and Clostridium acetobutylicum (7). Since glycine clusters are part of ADP-binding βαβ folds (52), this region of subunit A was thought to be important for the function of the transferase by binding CoA. In B13 transferase subunit A the respective glycine cluster, the mononucleotide-binding motif, is only partly present (D-G-x-x-V-A-L-E-G-F-x-x-x-x-P). One important glycine is replaced by a glutamic acid residue and an additional glycine is missing, a situation which is identical to that for protein PA0226 in P. aeruginosa PAO1.
A second signature pattern for CoA transferases (Prosite PS01274: [LF]-[HQ]-S-E-N-G-[LIVF][2]-[GA]) was postulated on the basis of a sequence alignment of CoA transferase by Parales and Harwood (38). The alignment was of a region in the N terminus of subunit B, which contains a conserved glutamate that is involved in the catalytic mechanism according to the studies by Jencks (23). The short consensus sequence S-E-N-G was confirmed by Rochet and Bridger (43). An exception from that theme was the glutaconate-CoA transferase of Acidaminococcus fermentans, which had very low sequence similarity to the other CoA transferases and in which the consensus S-E-N-G was not detected (31). Instead of that the important glutamic acid residue, identified by chemical methods (5) and by site-directed mutagenesis (32), was found in the short motif E-S-G in the N-terminal part of the enzyme. The consensus motif S-E-N-G is also absent in CatJ of Pseudomonas sp. strain B13, but some stretches of similarity with the glutaconate-CoA transferase were found, especially the E-S-G motif with the glutamic acid residue at position 51. This short motif has also been observed in protein PA0227 of P. aeruginosa PAO1.
The hallmarks of proteins belonging to the thiolase family are three signature patterns two of which are based on the regions around the biologically important cysteines (Prosite PS00098: [LIVM]-[NST]-x[2]-C-[SAGLI]-[ST]-[SAG]-[LIVMFYNS]-x-[STAG]-[LIVM]-x[6]-[LIVM]; PS00099: [AG]-[LIVMA]-[STAGCLIVM]-[STAG]-[LIVMA]-C-x-[AG]-x-[AG]-x-[AG]-x-[SAG]). The first conserved cysteine residue located in the N-terminal section of the enzymes is involved in the formation of an acyl-enzyme intermediate; the second, located at the C-terminal extremity, is the active-site base involved in deprotonation in the condensation reaction. The third motif (Prosite PS00737: N-x[2]-G-G-x-[LIVM]-[SA]-x-G-H-P-x-[GA]-x-[ST]-G) is based on a highly conserved region in the C-terminal part of these proteins.
Sequence comparison indicated that the three conserved motifs are present in CatF of strain B13. The comparison suggests that Cys-91 of the B13 enzyme is the active-site cysteine residue and that the consensus sequence of the active site is L-N-x-x-C-A-S-G-M-x-A-V-x-x-x-x-x-x-I and belongs to the PS00098 motif. The consensus sequence of PcaF of P. aeruginosa PAO1 showed an isoleucine instead of a valine. Cys-387, the second important cysteine of the active site of CatF in strain B13, is part of motif G-L-C-T-M-C-x-G-x-G-x-G-x-A, which hits the above consensus sequence PS00099. In the PAO1 consensus the first cysteine is replaced by an alanine. The thiolase signature (PS00737) was found at position 347 in CatF of strain B13: N-x-x-G-G-x-I-A-x-G-H-P-x-G-x-S-G.
A search for consensus sequences of CatD with other enzymes, even the counterparts of Acinetobacter calcoaceticus and P. putida, indicated no clearly conserved motif.
An putative AMP-binding domain signature (Prosite PS00455:[LIVMFY]-x[2]-[STG]-[STAG]-G-[ST]-[STEI]-[SG]-x-[PASLIVM]-[KR]) was found in the protein sequence deduced from ORF6 at amino acids 11 to 22: I-x-x-S-S-G-T-T-G-x-P-K. Therefore, the gene might code for the ATP- and CoA-dependent 3-oxoadipate-activating enzyme in strain B13.
Conclusion.
In general, the data presented here indicate that the sequences of enzymes functioning in the lower 3-oxoadipate pathway of strain B13 are highly similar to those of the counterparts of P. aeruginosa PAO1. In contrast, there were major differences between the subunits of the CoA-transferase of strain B13 on the one hand and those used by the other aromatic compound-degrading bacteria, P. putida and Acinetobacter calcoaceticus, on the other. With respect to the organization of the catIJFD gene cluster, the situations in Acinetobacter calcoaceticus and Pseudomonas sp. strain B13 are identical. This study illustrated again that nature’s patchwork assembly process has happened and will continue.
Acknowledgments
This work was financed by a grant from Deutsche Forschungsgemeinschaft and by the European Union, contract BIO4-CT97-2040.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armengaud, J., B. Happe, and K. N. Timmis. 1998. Genetic analysis of dioxin dioxygenase of Sphingomonas sp. strain RW1: catabolic genes dispersed on the genome. J. Bacteriol. 180:3954–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armengaud, J., K. N. Timmis, and R. M. Wittich. 1999. A functional 4-hydroxysalicylate/hydroxyquinol degradative pathway gene cluster is linked to the initial dibenzo-p-dioxin pathway genes in Sphingomonas sp. strain RW1. J. Bacteriol. 181:3452–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bairoch, A., P. Bucher, and K. Hofmann. 1997. The PROSITE database, its status in 1997. Nucleic Acids Res. 25:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckel, W., U. Dorn, and R. Semmler. 1981. Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur. J. Biochem. 118:315–321. [DOI] [PubMed] [Google Scholar]
- 6.Cai, G.-Q., B. T. Driscoll, and T. C. Charles. 2000. Requirement for the enzymes acetoacetyl coenzyme A synthetase and poly-3-hydroxybutyrate (PHB) synthase for growth of Sinorhizobium meliloti on PHB cycle intermediates. J. Bacteriol. 182:2113–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cary, J. W., D. J. Petersen, E. T. Papoutsakis, and G. N. Bennett. 1990. Cloning and expression of Clostridium acetobutylicum ATCC824 acetoacetyl-coenzyme A: acetate/butyrate:coenzyme A-transferase in Escherichia coli. Appl. Environ. Microbiol. 56:1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C. M., Q. Z. Ye, Z. M. Zhu, B. L. Wanner, and C. T. Walsh. 1990. Molecular biology of carbon-phosphorus bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and C-P lyase acitivity in Escherichia coli B. J. Biol. Chem. 265:4461–4471. [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry, III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, S. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, S. Skelton, S. Squares, R. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. [DOI] [PubMed] [Google Scholar]
- 10.Collier, L. S., G. L. Gaines, III, and E. L. Neidle. 1998. Regulation of benzoate degradation in Acinetobacter sp. strain ADP1 by BenM, a LysR-type transcriptional activator. J. Bacteriol. 180:2493–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collier, L. S., N. N. Nichols, and E. L. Neidle. 1997. benK encodes a hydrophobic permease-like protein involved in benzoate degradation by Acinetobacter sp. strain ADP1. J. Bacteriol. 179:5943–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corthésy-Theulaz, I. E., G. E. Bergonzelli, H. Henry, D. Bachmann, D. D. Schorderet, A. L. Blum, and L. N. Ornston. 1997. Cloning and characterization of Helicobacter pylori succinyl CoA:acetoacetate CoA-transferase, a novel prokaryotic member of the CoA-transferase family. J. Biol. Chem. 272:25659–25667. [DOI] [PubMed] [Google Scholar]
- 13.Dorn, E., M. Hellwig, W. Reineke, and H.-J. Knackmuss. 1974. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch. Microbiol. 99:61–70. [DOI] [PubMed] [Google Scholar]
- 14.Eulberg, D., S. Lakner, L. A. Golovleva, and M. Schlömann. 1998. Characterization of a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP: evidence for a merged enzyme with 4-carboxymuconolactone-decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. J. Bacteriol. 180:1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farabaugh, P. J. 1996. Programmed translational frameshifting. Microbiol. Rev. 60:103–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrandez, A., M. A. Prieto, J. L. Garcia, and E. Diaz. 1997. Molecular characterization of PadA, a phenylacetaldehyde dehydrogenase from Escherichia coli. FEBS Lett. 406:23–27. [DOI] [PubMed] [Google Scholar]
- 17.Frantz, B., and A. M. Chakrabarty. 1987. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorobenzoate degradation. Proc. Natl. Acad. Sci. USA 84:4460–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happe, B., L. D. Eltis, H. Poth, R. Hedderich, and K. N. Timmis. 1993. Characterization of 2,2′,3-trihydroxybiphenyl dioxygenase, an extradiol dioxygenase from the dibenzofuran- and dibenzo-p-dioxin-degrading bacterium Sphingomonas sp. strain RW1. J. Bacteriol. 175:7313–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartnett, C., E. L. Neidle, K. L. Ngai, and L. N. Ornston. 1990. DNA sequences of genes encoding Acinetobacter calcoaceticus protocatechuate 3,4-dioxygenase: evidence indicating shuffling of genes and of DNA sequences within genes during their evolutionary divergence. J. Bacteriol. 172:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartnett, G. B., and L. N. Ornston. 1994. Acquisition of apparent DNA slippage structures during extensive evolutionary divergence of pcaD and catD genes encoding identical catalytic activities in Acinetobacter calcoaceticus. Gene 142:23–29. [DOI] [PubMed] [Google Scholar]
- 21.Harwood, C. S., N. N. Nichols, M. K. Kim, J. L. Ditty, and R. E. Parales. 1994. Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J. Bacteriol. 176:6479–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harwood, C. S., and R. E. Parales. 1996. The β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50:553–590. [DOI] [PubMed] [Google Scholar]
- 23.Jencks, W. P. 1973. Coenzyme A transferases. In P. D. Boyer (ed.), The enzymes, p. 483–496. Academic Press, New York, N.Y.
- 24.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331–338. [DOI] [PubMed] [Google Scholar]
- 25.Kasberg, T., V. Seibert, M. Schlömann, and W. Reineke. 1997. Cloning, characterization, and sequence analysis of the clcE gene encoding the maleylacetate reductase of Pseudomonas sp. strain B13. J. Bacteriol. 179:3801–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaschabek, S. R., B. Kuhn, D. Müller, E. Schmidt, and W. Reineke. 2002. Degradation of chloroaromatics by Pseudomonas sp. B13: purification and characterization of a 3-oxoadipate:succinyl-CoA transferase and 3-oxoadipyl-CoA thiolase. J. Bacteriol. 184:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klenk, H. P., R. A. Clayton, J. Tomb, O. White, K. E. Nelson, K. A. Ketchum, R. J. Dodson, M. Gwinn, E. K. Hickey, J. D. Peterson, D. L. Richardson, A. R. Kerlavage, D. E. Graham, N. C. Kyrpides, R. D. Fleischmann, J. Quackenbush, N. H. Lee, G. G. Sutton, S. Gill, E. F. Kirkness, B. A. Dougherty, K. McKenney, M. D. Adams, B. Loftus, S. Peterson, C. I. Reich, L. K. McNeil, J. H. Badger, A. Glodek, L. Zhou, R. Overbeek, J. D. Gocayne, J. F. Weidman, L. McDonald, T. Utterback, M. D. Cotton, T. Spriggs, P. Artiach, B. P. Kaine, S. M. Sykes, P. W. Sadow, K. P. D’Andrea, C. Bowman, C. Fujii, S. A. Garland, T. M. Mason, G. J. Olsen, C. M. Fraser, H. O. Smith, C. R. Woese, and J. C. Venter. 1997. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364–370. [DOI] [PubMed] [Google Scholar]
- 28.Kowalchuk, G. A., G. B. Hartnett, A. Benson, J. E. Houghton, K. L. Ngai, and N. L. Ornston. 1994. Contrasting patterns of evolutionary divergence within the Acinetobacter calcoaceticus pca operon. Gene 146:23–30. [DOI] [PubMed] [Google Scholar]
- 29.Lin, T. W., and W. A. Bridger. 1992. Sequence of cDNA clone encoding pig heart mitochondrial CoA transferase. J. Biol. Chem. 267:975–978. [PubMed] [Google Scholar]
- 30.Lorite, M. J., J. Sanjuan, L. Velasco, J. Olivares, and E. J. Bedmar. 1998. Characterization of Bradyrhizobium japonicum pcaBDC genes involved in 4-hydroxybenzoate degradation. Biochim. Biophys. Acta 1397:257–261. [DOI] [PubMed] [Google Scholar]
- 31.Mack, M., K. Bendrat, O. Zelder, E. Eckel, D. Linder, and W. Buckel. 1994. Location of the two genes encoding glutaconate coenzyme A-transferase at the beginning of the hydroxyglutarate operon in Acidaminococcus fermentans. Eur. J. Biochem. 226:41–51. [DOI] [PubMed] [Google Scholar]
- 32.Mack, M., and W. Buckel. 1995. Identification of glutamate β54 as the covalent-catalytic residue in the active site of glutaconate CoA-transferase from Acidaminococcus fermentans. FEBS Lett. 357:145–148. [DOI] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Murakami, S., A. Takashima, J. Takemoto, S. Takenaka, R. Shinke, and K. Aoki. 1999. Cloning and sequence analysis of two catechol-degrading gene clusters from the aniline-assimilating bacterium Frateuria species ANA-18. Gene 226:189–198. [DOI] [PubMed] [Google Scholar]
- 35.Nichols, N. N., and C. S. Harwood. 1995. Repression of 4-hydroxybenzoate transport and degradation by benzoate: a new layer of regulatory control in the Pseudomonas putida β-ketoadipate pathway. J. Bacteriol. 177:7033–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nierman, W. C., T. V. Feldblyum, M. T. Laub, I. T. Paulsen, K. E. Nelson, J. Eisen, J. F. Heidelberg, M. R. K. Alley, N. Ohta, J. R. Maddock, I. Potocka, W. C. Nelson, A. Newton, C. Stephens, N. D. Phadke, B. Ely, R. T. DeBoy, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, J. F. Kolonay, J. Smit, M. Craven, H. Khouri, J. Shetty, K. Berry, T. Utterback, K. Tran, A. Wolf, J. Vamathevan, M. Ermolaeva, O. White, S. L. Salzberg, J. C. Venter, L. Shapiro, and C. M. Fraser. 2001. Complete genome sequence of Caulobacter crescentus. Proc. Natl. Acad. Sci. USA 98:4136–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivera, E. R., B. Minambres, B. Garcia, C. Muniz, M. A. Moreno, A. Ferrandez, E. Diaz, J. L. Garcia, and J. M. Luengo. 1998. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. USA 95:6419–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parales, R. E., and C. S. Harwood. 1992. Characterization of the genes encoding β-ketoadipate:succinyl-coenzyme A transferase in Pseudomonas putida. J. Bacteriol. 174:4657–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parales, R. E., and C. S. Harwood. 1993. Regulation of the pcaIJ genes for aromatic acid degradation in Pseudomonas putida. J. Bacteriol. 175:5829–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perkins, E. J., M. P. Gordon, O. Caceres, and P. F. Lurquin. 1990. Organization and sequence analysis of the 2,4-dichlorophenol hydroxylase and dichlorocatechol oxidative operons of plasmid pJP4. J. Bacteriol. 172:2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos, J. L., E. Duque, P. Godoy, and A. Segura. 1998. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J. Bacteriol. 180:3323–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map for the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77–96. [DOI] [PubMed] [Google Scholar]
- 43.Rochet, J.-C., and W. A. Bridger. 1994. Identification of glutamate 344 as the catalytic residue in the active site of pig heart CoA transferase. Protein Sci. 3:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Shanley, M. S., A. Harrison, R. E. Parales, G. Kowalchuk, D. J. Mitchell, and L. N. Ornston. 1994. Unusual G+C content and codon usage in catIJF, a segment of the ben-cat supra-operonic cluster in the Acinetobacter calcoaceticus chromosome. Gene 138:59–65. [DOI] [PubMed] [Google Scholar]
- 46.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrook-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. M. Lim, K. A. Smith, D. H. Spencer, G. K. S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. [DOI] [PubMed] [Google Scholar]
- 47.Takami, H., K. Nakasone, Y. Takaki, G. Maeno, R. Sasaki, N. Masui, F. Fuji, C. Hirama, Y. Nakamura, N. Ogasawara, S. Kuhara, and K. Horikoshi. 2000. Complete genome sequence of the alkaliphilic bacterium Bacillus halodurans and genomic sequence comparison with Bacillus subtilis. Nucleic Acids Res. 28:4317–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX window interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Aids Res. 24:4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomb, J.-F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539–547. (Erratum, 389:412.) [DOI] [PubMed] [Google Scholar]
- 50.van der Meer, J. R., R. W. van Neerven, E. J. de Vries, W. M. de Vos, and A. J. B. Zehnder. 1991. Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. J. Bacteriol. 173:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, R. J. Dodson, D. H. Haft, M. L. Gwinn, W. C. Nelson, D. L. Richardson, K. S. Moffat, H. Qin, L. Jiang, W. Pamphile, M. Crosby, M. Shen, J. J. Vamathevan, P. Lam, L. McDonald, T. Utterback, C. Zalewski, K. S. Makarova, L. Aravind, M. J. Daly, K. W. Minton, R. D. Fleischmann, K. A. Ketchum, K. E. Nelson, S. Salzberg, H. O. Smith, J. C. Venter, and C. M. Fraser. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wierenga, R. K., P. Terpstra, and W. G. J. Hol. 1986. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J. Mol. Biol. 187:101–107. [DOI] [PubMed] [Google Scholar]
- 53.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida, K., K. Shindo, H. Sano, S. Seki, M. Fujimura, N. Yanai, Y. Miwa, and Y. Fujita. 1996. Sequencing of a 65 kb region of the Bacillus subtilis genome containing the lic and cel loci, and creation of a 177 kb contig covering the gnt-sacXY region. Microbiology 142:3113–3123. [DOI] [PubMed] [Google Scholar]