Abstract
To investigate the involvement of RelA in the regulation of Legionella pneumophila virulence, a deletion substitution was constructed in the relA gene. The relA knockout resulted in an undetectable level of ppGpp in the cells during the stationary phase, but the original level was restored when the relA gene product was supplied on a plasmid. The effect of the relA mutation was examined with two systems that are known to be expressed during the stationary phase in L. pneumophila. Pigment production was found to be dependent on the relA gene product, and only one-half as much pigment was produced by the relA mutant as by the wild-type strain. Flagellum gene expression was also found to be dependent on the relA gene product, as determined with a flaA::lacZ fusion. However, the relA gene product was found to be dispensable for intracellular growth both in HL-60-derived human macrophages and in the protozoan host Acanthamoeba castellanii. To determine the involvement of the relA gene product in expression of L. pneumophila genes required for intracellular growth (icm/dot genes), nine icm::lacZ fusions were constructed, and expression of these fusions in the wild-type strain was compared with their expression in relA mutant strains. Expression of only one of the icm::lacZ fusions was moderately reduced in the relA mutant strain. Expression of the nine icm::lacZ fusions was also examined in a strain containing an insertion in the gene that codes for the stationary-phase sigma factor RpoS, and similar results were obtained. We concluded that RelA is dispensable for intracellular growth of L. pneumophila in the two hosts examined and that both RelA and RpoS play minor roles in L. pneumophila icm/dot gene expression.
Legionella pneumophila, the causative agent of Legionnaires’ disease, is a facultatively intracellular pathogen. L. pneumophila is able to infect, multiply within, and kill human macrophages, as well as free-living amoebae (27, 34). The bacteria are taken up by regular phagocytosis or by a special mechanism termed coiling phagocytosis (6, 25). The bacteria are then found within a specialized phagosome that does not fuse with lysosomes or acidify (6, 23, 26), and the specialized phagosome undergoes several recruitment events that include association with smooth vesicles, mitochondria, and rough endoplasmic reticulum (1, 24, 46). The bacteria multiply within the specialized phagosome until the cell eventually lyses, releasing bacteria that can start new rounds of infection (27, 34).
Two regions of genes required for human macrophage killing and intracellular multiplication have been discovered in L. pneumophila (reviewed in references 39 and 48). Region I contains seven genes (icmV, -W, and -X and dotA, -B, -C, and -D) (5, 7, 30, 47), and region II contains 17 genes (icmT, -S, -R, -Q, -P, -O, -N, -M, -L, -K, -E, -G, -C, -D, -J, -B, and -F) (3, 32, 37, 38, 47). Most of these genes have also been shown to be required for intracellular growth in the protozoan host Acanthamoeba castellanii (41). Fourteen of the Icm/Dot proteins (IcmT, -P, -O, -L, -K, -G, -C, -D, -J, and -B and DotA, -B, -C, and -D) have been found to exhibit significant sequence similarity with Tra/Trb proteins from the IncI plasmids colIb-P9 and R64 (28, 42).
At this time, there is very little information regarding regulation of L. pneumophila virulence and no information regarding regulation of icm/dot gene expression. So far, the stationary-phase sigma factor RpoS (encoded by the rpoS gene) has been shown to be involved in L. pneumophila virulence, and a strain containing a knockout in this gene lost the ability to grow in the protozoan host A. castellanii (17) and was attenuated for intracellular growth in murine bone marrow-derived macrophages (4). However, this gene has been found to be dispensable for growth in HL-60-derived human macrophages and in THP-1 cells (17). It has been suggested that another factor that is related to the stationary phase, the relA gene product, is involved in regulation of L. pneumophila pathogenicity (18). RelA is a ppGpp synthatase that plays a major role in the Escherichia coli stringent response (10). The stringent response is a global response that occurs as a consequence of the binding of uncharged tRNA to ribosomes, which activates the RelA enzyme. As a result of this activation, ppGpp accumulates in the cells, which leads to rapid inhibition of stable RNA synthesis and accumulation of the stationary sigma factor (RpoS) (10, 16). In other bacteria, the relA gene product has been shown to coordinate entry into the stationary phase with several systems, such as fruiting body formation in Myxococcus xanthus (19) and morphological differentiation and antibiotic production in Streptomyces coelicolor (12). It has been suggested that in L. pneumophila this gene product coordinates virulence with entry into the stationary phase (18).
We were interested in determining the precise role of the relA gene product in L. pneumophila pathogenesis and icm/dot gene expression. To do this, we constructed a deletion substitution in the relA gene on the L. pneumophila chromosome and tested its effect on L. pneumophila intracellular growth and icm/dot gene expression. The mutation in the relA gene had no effect on L. pneumophila intracellular growth in HL-60-derived human macrophages or A. castellanii. A reduction in the expression of only one of nine icm::lacZ fusions was observed in the relA insertion mutant, and a similar result was obtained with an rpoS insertion mutant.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and media.
The L. pneumophila strains used in this work were JR32, a streptomycin-resistant, restriction-negative mutant of L. pneumophila Philadelphia-1 which is a wild-type strain in terms of intracellular growth (35); 25D, a mutant that cannot grow intracellularly (22); LM1376, an rpoS insertion mutant which is a JR32 derivative (17); and relA deletion substitution mutants GS-RelA and GS-RelA2 (two independent isolates of the relA mutant, which originated from two different electroporations), which are also JR32 derivatives. The plasmids and primers used in this work are described in Tables 1 and 2, respectively. The bacterial media, plates, and antibiotic concentrations used have been described previously (38).
TABLE 1.
Plasmids used in this study
| Plasmid | Features | Reference or source |
|---|---|---|
| pAB-1 | lacZ gene under Ptac control in pMMB207 | 36 |
| pGS-flaA-01 | L. pneumophila flaA regulatory region in pUC18 | This study |
| pGS-flaA-02 | L. pneumophila flaA regulatory region in pMC1403 | This study |
| pGS-flaA-03 | L. pneumophila flaA regulatory region in pGS-lac-01 | This study |
| pGS-lac-01 | pAB-1 with a promoterless lacZ gene | This study |
| pGS-reg-F1 | Regulatory region of icmF in pUC18 | This study |
| pGS-reg-F2 | Regulatory region of icmF in pMC1403 | This study |
| pGS-reg-F3 | Regulatory region of icmF in pGS-lac-01 | This study |
| pGS-reg-M1 | Regulatory region of icmM in pUC18 | This study |
| pGS-reg-M2 | Regulatory region of icmM in pMC1403 | This study |
| pGS-reg-M3 | Regulatory region of icmM in pGS-lac-01 | This study |
| pGS-relA-01 | L. pneumophila relA gene in pUC-18 | This study |
| pGS-relA-01-Km | pGS-RelA-01 with the Km cassette in the relA gene | This study |
| pGS-relA-01-Km-GR | Insert of pGS-RelA-01-Km in pLAW344 | This study |
| pGS-relA-05 | L. pneumophila relA in pMMB207αb | This study |
| pKP-Q-1 | Regulatory region of icmQ in pUC18 | This study |
| pKP-Q-21 | Regulatory region of icmQ in pMC1403 | This study |
| pLAW344 | sacB MCS oriT(RK2) CmroriR(ColE1) Apr | 50 |
| pMC1403 | Promoterless lacZ in pBR322 | 8 |
| pMMB207αb | RSF1010 derivative, IncQ lacIq CmroriT MCS | 38 |
| pOG-J-103 | Regulatory region of icmJ in pUC18 | This study |
| pOG-J-109 | Regulatory region of icmJ in pMC1403 | This study |
| pOG-J-122 | Regulatory region of icmJ in pGS-lac-01 | This study |
| pOG-P-105 | Regulatory region of icmP in pUC18 | This study |
| pOG-P-108 | Regulatory region of icmP in pMC1403 | This study |
| pOG-P-121 | Regulatory region of icmP in pGS-lac-01 | This study |
| pOG-Q-126 | Regulatory region of icmQ in pGS-lac-01 | This study |
| pOG-R-125 | Regulatory region of icmR in pGS-lac-01 | This study |
| pOG-T-104 | Regulatory region of icmT in pUC18 | This study |
| pOG-T-107 | Regulatory region of icmT in pMC1403 | This study |
| pOG-T-120 | Regulatory region of icmT in pGS-lac-01 | This study |
| pOG-V-110 | Regulatory region of icmV in pMC1403 | This study |
| pOG-V-123 | Regulatory region of icmV in pGS-lac-01 | This study |
| pOG-VW-106 | Regulatory region of icmVW in pUC18 | This study |
| pOG-W-111 | Regulatory region of icmW in pMC1403 | This study |
| pOG-W-124 | Regulatory region of icmW in pGS-lac-01 | This study |
| pSS-R-1 | Regulatory region of icmR in pUC18 | This study |
| pSS-R-27 | Regulatory region of icmR in pMC1403 | This study |
| pUC18 | oriR(ColE1) MCS Apr | 53 |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| flaA-Up | CAAAAAAGCGCTTTCGGAAC |
| flaA-Down | GGGATCCCCGTTGATTACTTGAGCCATAATTTTAGTC |
| icmF-F | GAACAAGGAGCAAGTATTTC |
| icmF-R | GGATCCCCGTATTGCTCAGTTGTCATTATATTAC |
| icmJ-F | TAAAGAGAATACCGCTTTACCC |
| icmJ-R | GGGATCCCCTCGTTGTTGATTATCCGCCAT |
| icmM-F | TGGTCTCAGGGGGTTGATAG |
| icmM-R | GGATCCCCCCATGTTTCTCGACTCATTTTTAC |
| icmP-F | TATATCGATACTCCAATGGCC |
| icmP-R | GGGATCCCCTTGCTGCTGTTGTTGTGCCAT |
| icmQ-F | AGCCATGATGAACGTGGTTTC |
| icmQ-R | GGGATCCCCATCAGTATTATTACCCATTATTAC |
| icmR-F | CCCTGGATGAGTTAATGTATG |
| icmR-R | GGGATCCCCCGAGAGTTGATCTTTCATAAC |
| icmT-F | TCTCCAATGGATTAAGTCCGG |
| icmT-R | GGGATCCCCTGCTGAAAATCCACCTGCCAT |
| icmVW-F | GGGATCCCCTGATCCTGATTTCTTTTTCATATT |
| icmVW-R | GGGATCCCCTTCATGGCTTAAATCAGGCAT |
| relA-F | ATGGTAAGAGTAAAAGATACGACTCCG |
| relA-R | CTATAATTGCCTTCTTGCTTCCAG |
| relA-Up-EI | GCCGGAATTCCATTGGCGCAGATGTTATGG |
| relA-Down-Bam | CGGGGGATCCTATTGAGCGAACACAGAGCG |
Construction of plasmid for allelic exchange.
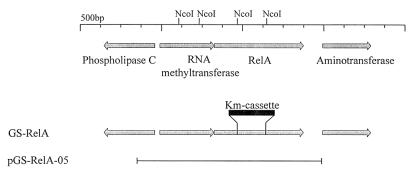
Primers relA-F and relA-R (Table 2) were designed by using L. pneumophila genome sequence information (http://genome3.cpmc.columbia.edu/∼legion/index.html). Primers relA-F and relA-R overlap the relA start codon and stop codon, respectively, and they were used to amplify a 2.2-kb DNA fragment containing the relA gene; this fragment was cloned into pUC18 digested with HincII to generate pGS-relA-01. To knock out the relA gene, the kanamycin resistance cassette (Pharmacia) was cloned into it, instead of an internal 721-bp NcoI fragment, to generate pGS-relA-01-Km (Fig. 1). Plasmid pGS-relA-01-Km was digested with SphI and BamHI, and after fill-in the insert was cloned into the EcoRV site of the allelic exchange vector pLAW344 to form pGS-relA-01-Km-GR. This plasmid was used for allelic exchange, as previously described (38). The resulting strains (GS-RelA and GS-RelA2) contain the first 183 amino acids of the RelA protein, as well as its last 311 amino acids. The internal 240 amino acids were deleted during construction, and the kanamycin resistance cassette was cloned instead of these amino acids. Several isolates were analyzed by PCR to confirm that the right change occurred on the chromosome (data not shown).
FIG. 1.
Linkage map of the relA locus. The arrows indicate coding regions. The site of the kanamycin cassette in the GS-RelA deletion substitution mutant is indicated. The restriction enzyme NcoI sites used for construction of the relA deletion substitution are shown. The line indicates the region covered by the complementing plasmid pGS-RelA-05. (The organization of the genes in the region was based on the incomplete sequence of the L. pneumophila genome; therefore, the identities of the three genes near relA are tentative.)
Construction of plasmid for RelA complementation.
To construct a relA-complementing clone, primers relA-Up-EI and relA-Down-Bam were used. A sequence analysis of the upstream region of the relA gene showed that an RNA methyltransferase homologous gene was located immediately upstream of this gene (Fig. 1). As we assumed that the two genes form an operon, PCR amplification was used to obtain both genes. Two primers were used to amplify a 3.8-kb DNA fragment that was subsequently digested with EcoRI and BamHI and cloned into the same sites in vector pMMB207αb to form pGS-relA-05. This plasmid was used for complementation.
Construction of lacZ fusions.
The promoterless lacZ vector pMC1403 (8) and plasmid pAB1 (36) were used to construct an L. pneumophila promoterless lacZ vector, pGS-lac-01. Plasmid pAB-1 was digested with SacI and XmnI, and plasmid pMC1403 was digested with SacI and ApaI (in both plasmids the SacI site is located in the middle of the lacZ gene). The 10-kb SacI-XmnI fragment of pAB-1 containing the pMMB207 vector and part of the lacZ gene was ligated with the 2.3-kb SacI-ApaI fragment of pMC1403 containing part of the promoterless lacZ gene in order to generate pGS-lac-01. pGS-lac-01 did not exhibit a significant level of β-galactosidase activity in either E. coli or L. pneumophila during the exponential and stationary phases (data not shown), and it was used to construct the icm::lacZ and flaA::lacZ fusions described below.
The icm genes for which icm::lacZ fusions were constructed were chosen on the basis of the complementation analysis that was performed previously (32, 37, 38). The location of the upstream primer (F primer) was chosen on the basis of the gene organization of the region, and at least 150 bp upstream from the first methionine of each gene was included. The downstream primer (R primer) was designed to generate an in-frame fusion between the first seven amino acids of the icm gene and the lacZ reporter gene. In addition, a lacZ fusion to the flaA gene regulatory region was constructed. To construct the icm::lacZ and flaA::lacZ fusions, the regulatory region of the icmT, -R, -Q, -P, -M, -J, -F, -V, and -W and flaA genes (in the case of icmF the fusion constructed was a fusion to the gene located upstream of it designated 47:8g [13]; these two genes are probably located on the same transcriptional unit) was amplified by PCR with the primers described in Table 2. The fragments generated were cloned into pUC-18 digested with SmaI to generate pOG-T-104, pSS-R-1, pKP-Q-1, pOG-P-105, pGS-reg-M1, pOG-J-103, pGS-reg-F1, pOG-VW-106 (for both icmV and icmW), and pGS-flaA-01, respectively. All of the plasmids were sequenced to confirm that no changes were made during PCR amplification. These plasmids were digested with BamHI (one site was located in the R primer, and one site was located in the vector) and cloned into pMC1403 digested with BamHI to obtain plasmids pOG-T-107, pSS-R-27, pKP-Q-21, pOG-P-108, pGS-reg-M2, pOG-J-109, pGS-reg-F2, pOG-V-110, pOG-W-111, and pGS-flaA-02, respectively. Clones that were found to be blue on plates containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were tested to determine the orientations of their inserts. In all of the blue clones tested only the orientation expected to generate an active fusion was found. These plasmids were digested with EcoRI and SacI (the EcoRI site was located upstream of the BamHI cloning site into which the regulatory regions were cloned, and the SacI site was located inside the lacZ gene) and cloned into pGS-lac-01 digested with the same enzymes to obtain plasmids pOG-T-120, pOG-R-125, pOG-Q-126, pOG-P-121, pGS-reg-M3, pOG-J-122, pGS-reg-F3, pOG-V-123, pOG-W-124, and pGS-flaA-03, respectively. These plasmids were used for experiments with L. pneumophila in which the effect of the relA insertion mutation on icm and flaA gene expression was determined relative to expression in the wild-type strain.
Measurement of ppGpp production in L. pneumophila.
ppGpp production was analyzed by thin-layer chromatography (TLC) essentially as described previously (9). L. pneumophila exponential cultures grown in regular AYE (ACES yeast extract) medium were labeled with carrier-free [32P]phosphoric acid (500 μCi ml−1; ICN Pharmaceuticals) for about 6 h before sampling. Samples were obtained during the early stationary phase (optical density at 600 nm [OD600], 2.6), the stationary phase (OD600, 3.1) and the late stationary phase (OD600, 3.6). At each time a 50-μl aliquot was extracted with 50 μl of 13 M formic acid, and 4 μl of extract was applied to a polyethyleneimine-cellulose TLC sheet (Baker) and developed for 40 min with 1.5 M KH2PO4 (pH 3.4). The optical densities of nonradioactive cultures grown under the same conditions were determined. The locations of ppGpp and GTP on the TLC autoradiogram were determined by using the results of a previous analysis of ppGpp in E. coli (9) and L. pneumophila (18) in which the same protocol was used. The relative amounts of ppGpp were determined with a Phosphor-Imager (Fuji BAS1000).
Intracellular growth in HL-60-derived macrophages.
Intracellular growth assays with HL-60-derived macrophages were performed like assays described previously (40). Wells of a 24-well microtiter dish containing 2 × 106 differentiated HL-60-derived macrophages were used for infection. L. pneumophila cells were added to the wells at a multiplicity of infection of approximately 0.1, and the infected HL-60-derived macrophages were incubated for 1 h at 37°C under 5% CO2. Then the wells were washed three times, and 0.6 ml of RPMI medium containing 2 mM Gln and 10% normal human serum was added to each well. The supernatant of each well was sampled at intervals of about 24 h, and the numbers of CFU were determined by plating samples on ACES buffered charcoal yeast extract (ABCYE) plates.
Intracellular growth in A. castellanii..
Intracellular growth assays with A. castellanii were performed like assays described previously (41). A total of 1.5 × 105 amoebae in PYG were added to wells of a 24-well microtiter dish, and the amoebae were incubated for 1 h at 37°C so that they could adhere. Then the PYG was aspirated, the wells were washed once with 0.5 ml of warm (37°C) Ac buffer, and 0.5 ml of warm Acanthamoebae buffer (Ac buffer) was added to each well. Then L. pneumophila in Ac buffer was added to the wells at a multiplicity of infection of approximately 0.1. The plate was incubated for 30 min at 37°C, and then the Ac buffer was aspirated, the wells were washed three times with 0.5 ml of warm Ac buffer, and 0.6 ml of warm Ac buffer was added to each well. The supernatant in each well was sampled at intervals of about 24 h, and the numbers of CFU were determined by plating samples on ABCYE plates.
Pigmentation measurements.
L. pneumophila strains were grown on ABCYE plates containing chloramphenicol for 48 h. The bacteria were scraped off the plates and suspended in AYE broth, and the OD600 in AYE broth containing chloramphenicol was adjusted to 0.1. Then 44-ml cultures were prepared, and 1.5-ml portions of the cultures were placed into tubes and grown for 14 to 16 h on a roller drum. Starting when the cultures reached an OD600 of about 3, triplicate samples were removed at intervals of 2 h, and pigment production and bacterial density were determined. One milliliter of culture was centrifuged for 10 min at 20,000 × g, and the supernatant was transferred to another tube. The bacterial pellet was resuspended in 1 ml of M63 medium (31), and a 1/10 dilution was prepared before the OD600 was measured. Pigmentation was analyzed by determining the absorbance of the culture supernatant at 550 nm (50).
β-Galactosidase assays.
β-Galactosidase assays were performed as described elsewhere (31). L. pneumophila strains were grown on ABCYE plates containing chloramphenicol for 48 h. The bacteria were scraped off the plates and suspended in AYE broth, and the OD600 was adjusted to 0.1 in AYE broth. The resulting cultures were grown on a roller drum for 17 to 18 h until the OD600 was about 3.2 (stationary phase). The assays were performed with 20 or 50 μl of culture, and the substrate for lacZ hydrolysis was o-nitrophenyl-β-d-galactopyranoside.
Sodium sensitivity.
The sodium sensitivities of the wild type (JR32) and a mutant (GS-RelA) were quantified by growing the bacteria for 72 h on ABCYE plates, scraping the bacteria off the plates, and adjusting the OD600 of each preparation to 4. Then eight 10-fold serial dilutions were plated on ABCYE plates containing or not containing 100 mM NaCl. Sodium sensitivity was determined by comparing the numbers of bacteria growing on the plates. Nonvirulent mutant 25D was used as a sodium resistance control strain.
RESULTS
Construction of an L. pneumophila relA deletion substitution mutant.
Previously, it was proposed that the relA gene product is involved in pathogenicity of L. pneumophila (18). To test this proposal directly, we decided to construct a deletion substitution in the relA gene and test its effect on pathogenicity and icm gene expression. Using a BLAST search (2) with the sequences available from the L. pneumophila genome project (http://genome3.cpmc.columbia.edu/∼legion/index.html), we found the L. pneumophila relA homolog. The RelA protein was found to be 44% identical and 63% similar to E. coli RelA (the sequence information is based on an incomplete L. pneumophila genome sequence, and it might contain errors that change the levels of identity and similarity slightly). Unlike the E. coli gene, the L. pneumophila relA gene is probably located on the same transcriptional unit together with an RNA methyltransferase gene (Fig. 1). A similar gene organization has been found in other bacteria, such as Haemophilus influenzae (15), Vibrio cholerae (20), Pseudomonas aeruginosa (45). By using the standard allelic exchange procedure (see Materials and Methods) a deletion substitution in the relA gene was constructed by cloning the kanamycin resistance cassette instead of the middle part of the relA gene (Fig. 1). The strain generated (GS-RelA) grew well in AYE medium and had the same growth rate as wild-type strain JR32, as determined by optical density and CFU analyses (data not shown).
RelA is required for ppGpp accumulation during the stationary phase.
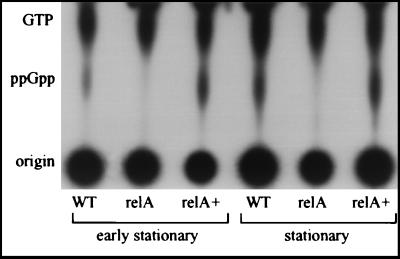
As described above, RelA is a ppGpp synthatase that under certain conditions (amino acid starvation, carbon starvation, entry into the stationary phase, etc.) converts GTP to ppGpp. One phenotype of a relA knockout strain that was expected was a change in ppGpp accumulation during the stationary phase. To test this hypothesis, we analyzed accumulation of ppGpp in the relA knockout strain (GS-RelA) and compared it to accumulation of ppGpp in wild-type strain JR32. As Fig. 2 shows, during the early stationary phase (OD600, 2.6) a small amount of ppGpp was present in the wild-type strain but not in the relA mutant. A clearer result was obtained during the stationary phase (OD600, 3.1), when a large amount of ppGpp was present in the wild-type strain but not in the mutant strain. A result similar to the result obtained for the stationary phase was obtained for the late stationary phase (OD600, 3.6) (data not shown). When we analyzed ppGpp accumulation in the mutant strain (GS-RelA) containing the relA complementing plasmid (pGS-RelA-05), clear complementation of ppGpp production was observed (Fig. 2). Moreover, a higher level of ppGpp was present in the mutant strain containing the complementing plasmid than in the wild-type strain (a 30% increase in the amount of ppGpp was observed in the early stationary phase, and a 50% increase was observed in the stationary phase). This result was expected because the relA gene product was supplied on a plasmid and higher levels of RelA may have been present in the cells. The lack of detectable levels of ppGpp in the relA knockout strain clearly indicates that like the relA gene product of E. coli, the relA gene product of L. pneumophila is the major ppGpp synthatase.
FIG. 2.
RelA is required for ppGpp production in L. pneumophila. The amounts of ppGpp in wild-type strain JR32 (WT), relA mutant strain GS-RelA (relA), and mutant strain GS-RelA complemented with a plasmid (pGS-relA-05) containing the relA gene product (relA+) were compared. The nucleotide pools were analyzed during the early stationary phase (OD600, 2.6) and the stationary phase (OD600, 3.1), as described in Materials and Methods. Results similar to the results shown for the stationary phase were obtained for the late stationary phase (OD600, 3.6) (data not shown). The results of one representative experiment of two independent experiments are shown.
RelA influences pigment formation during the stationary phase.
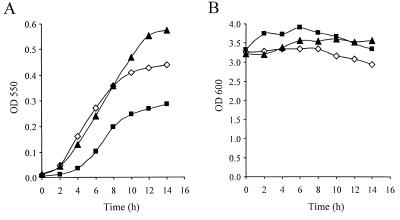
L. pneumophila forms a brown pigment during the stationary phase of growth, which was shown to be dispensable for intracellular growth (44, 50, 51). Previously, the stationary-phase sigma factor RpoS was found to be dispensable for pigment formation, even though the pigment was produced during the stationary phase (17). Because the relA gene product in E. coli and other bacteria is known to be involved in regulation of gene expression during the stationary phase (10, 12), we were interested in determining whether pigment production is regulated by relA. Therefore, pigment production in wild-type strain JR32 and pigment production in the relA mutant GS-RelA during the stationary phase were compared, and a very clear difference was observed (Fig. 3). Compared to the wild-type strain, the relA mutant (GS-RelA) began to produce pigment later, and the maximal amount of pigment produced by the mutant after 12 to 14 h during the stationary phase was about one-half the maximal amount produced by the wild type. To confirm that the phenotype observed was due to insertion in the relA gene and not to a secondary mutation, a plasmid containing the relA gene (pGS-relA-05) was used for complementation. As Fig. 3A shows, when the plasmid containing the relA gene product (pGS-RelA-05) was introduced into the GS-RelA strain, pigment began to form at the same time that it began to form in the wild-type strain, and the maximal amount of pigment produced was greater than the maximal amount produced by the wild-type strain. This result was consistent with the result obtained when ppGpp accumulation was determined, which showed that higher levels of ppGpp were produced in the complemented relA mutant strain than in the wild-type strain (Fig. 2).
FIG. 3.
Pigment production during the stationary phase is dependent on the relA gene product. Bacteria were grown to an OD600 of 3 (14 to16 h of growth, starting from an OD600 of 0.1), and after this (zero time) pigment production (A) and bacterial density (B) were measured at intervals of 2 h. Pigment production was measured by determining the OD550 after centrifugation, and bacterial density was measured by determining the OD600. Symbols: ◊, wild-type strain JR32 containing the vector pMMB207αb; ▪, RelA mutant strain GS-RelA containing the vector pMMB207αb; ▴, GS-RelA complemented with plasmid pGS-RelA-05. The experiment was done three times, and similar results were obtained in all of the experiments. The largest standard deviation for the pigment production measurements was ±0.015.
RelA affects flaA gene expression during the stationary phase.
It is well known that flagellum formation in L. pneumophila is correlated with entry into the stationary phase (33), and it has been shown that the flagellum subunit gene (flaA) requires the stationary-phase sigma factor for maximal expression (4). In addition, it has been shown previously that overproduction of a truncated form of the E. coli relA gene product in L. pneumophila results in expression of the gene encoding the flagellum subunit (flaA) (18). To test the effect of the relA insertion on expression of the gene coding for the flagellum subunit (flaA) during the stationary phase, a translational fusion between flaA and the lacZ reporter gene was constructed. When this flaA::lacZ fusion was introduced into the wild-type strain, a very high level of expression (6,607 ± 490 Miller units) was observed during the stationary phase (OD600, 3.2). When the same fusion was introduced into the relA mutant (GS-RelA), a dramatic reduction in the β-galactosidase level (1,827 ± 160 Miller units) compared to the level in wild-type strain JR32 was observed (a similar result was obtained during the late stationary phase [OD600, 3.6]). The β-galactosidase levels of these two strains were found to be significantly different (P > 0.0001) as determined by a standard t test (calculated with data from six independent experiments). This result indicates that the gene coding for the flagellum subunit (flaA) is regulated by the relA gene product, but it is still expressed at a relatively high level in the absence of relA. This result was expected as it is known that flagellum-related genes are regulated by the flagellum sigma factor rpoF (21) and by the stationary-phase sigma factor rpoS (4), which probably continue to express these genes in the relA mutant. To further confirm the effect of the relA knockout on flagellum gene expression, the motilities of the relA mutant and the wild-type strain were determined microscopically. Between 60 and 70% of the relA mutant bacteria were found to be motile during the stationary phase, whereas close to 100% of the wild-type bacteria were motile.
RelA is not required for intracellular growth in eukaryotic hosts.
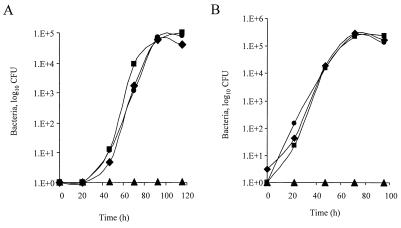
To examine the importance of the L. pneumophila RelA protein for intracellular multiplication, the ability of strain GS-RelA to multiply in HL-60-derived macrophages was examined by measuring the change in the number of bacterial CFU in the supernatant of wells containing HL-60-derived macrophages infected with different strains of L. pneumophila. L. pneumophila wild-type strain JR32 and mutant strain 25D, which is not able to multiply intracellularly (22), were used as controls. As Fig. 4A shows, the RelA mutant strains (GS-RelA and GS-RelA2) replicated to the same degree as JR32. Therefore, we concluded that RelA is not required for L. pneumophila replication in HL-60-derived macrophages. Then the ability of strain GS-RelA to kill HL-60-derived macrophages was tested by using a cytotoxicity assay (38). The results obtained clearly indicate that strain GS-RelA is able to kill HL-60-derived macrophages to the same extent as the wild-type strain (data not shown).
FIG. 4.
RelA is dispensable for growth in eukaryotic hosts. Intracellular growth experiments with HL-60-derived human macrophages (A) and with the protozoan host A. castellanii (B) were performed as described in Materials and Methods. The experiments were done three times, and similar results were obtained in all of the experiments. Symbols: ▪, JR32; ▴,25D; ⧫ and •, independent isolates of the relA deletion substitution mutant (GS-RelA and GS-RelA2, respectively).
L. pneumophila normally replicates in the environment within protozoan hosts, such as A. castellanii (14). Therefore, the abilities of the wild-type and relA mutant strains to multiply in amoebae were tested. Strains JR32, 25D, GS-RelA, and GS-RelA2 were used to infect A. castellanii, and as Fig. 4B shows, the RelA mutant strains (GS-RelA and GS-RelA2) and the wild-type strain (JR32) replicated to the same extent. We concluded that the relA gene product is dispensable for intracellular growth of L. pneumophila in the eukaryotic hosts examined.
Wild-type strains of L. pneumophila are known to be salt sensitive (11), and mutants that cannot grow intracellularly are salt resistant (35). These two phenotypes were shown to be correlated previously (35). Therefore, we tested the sensitivity to salt (100 mM NaCl) of the relA deletion substitution mutant; as expected from the intracellular growth experiments, this strain was found to be salt sensitive to the same extent as the wild-type strain (data not shown).
RelA has a minor effect on icm gene expression.
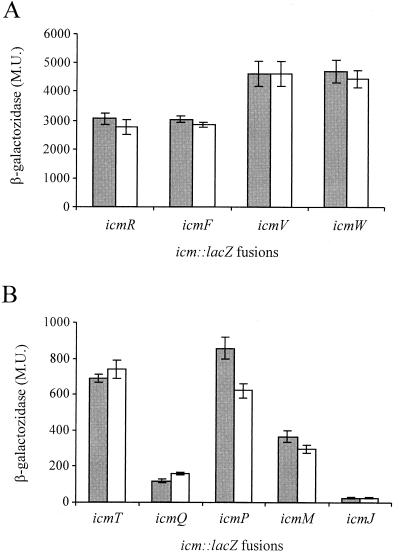
To further investigate the possible involvement of the relA gene product in intracellular multiplication of L. pneumophila, we examined the role of this gene product in icm gene expression. Based on the complementation analysis that was performed previously (32, 37, 38), nine icm::lacZ fusions (icmT::lacZ, icmR::lacZ, icmQ::lacZ, icmP::lacZ, icmM::lacZ, icmJ::lacZ, icmF::lacZ, icmV::lacZ, and icmW::lacZ) were constructed, and the levels of β-galactosidase expression in relA mutant strain GS-RelA and wild-type strain JR32 were compared. As Fig. 5 shows, the icm::lacZ fusions can be divided into two groups based on their levels of expression in the wild-type strain. Four icm::lacZ fusions (icmR::lacZ, icmF::lacZ, icmV::lacZ, and icmW::lacZ) exhibited high levels of expression of β-galactosidase, and these fusions were not affected by the relA mutation (Fig. 5A). In contrast, five icm::lacZ fusions (icmT::lacZ, icmQ::lacZ, icmP::lacZ, icmM::lacZ, and icmJ::lacZ) exhibited low levels of expression of β-galactosidase, and the level of expression of one of them (icmP::lacZ) was slightly reduced in the relA insertion mutant (Fig. 5B). The level of β-galactosidase expression of the icmP::lacZ fusion was reduced from 858 to 621 Miller units (P < 0.0001). Even with this lacZ fusion, the effect was small, and it was probably not enough to influence intracellular multiplication of the L. pneumophila relA mutant, as judged from Fig. 4. (It is possible that some minor effects of the relA knockout were missed because the icm::lacZ fusions were located on a plasmid.)
FIG. 5.
RelA has a minor effect on expression of one icm::lacZ fusion. Translational fusions between nine icm genes (icmT, icmR, icmQ, icmP, icmM, icmJ, icmF, icmW, and icmV) and the lacZ reporter gene were constructed and introduced into wild-type strain JR32 (shaded bars) and RelA insertion mutant GS-RelA (open bars). β-Galactosidase activity was measured as described in Materials and Methods. Four of the icm::lacZ fusions were found to have high β-galactosidase activities (A), and five had low activities (B). The data are expressed in Miller units (M.U.) and are averages based on at least three different experiments. Expression of icmP::lacZ in JR32 (858.47 Miller units) and expression of icmP::lacZ in GS-RelA (621.89 Miller units) were significantly different (P > 0.0001), as determined by the standard t test. Results similar to the results shown were obtained during the late stationary phase (OD600, 3.6) (data not shown).
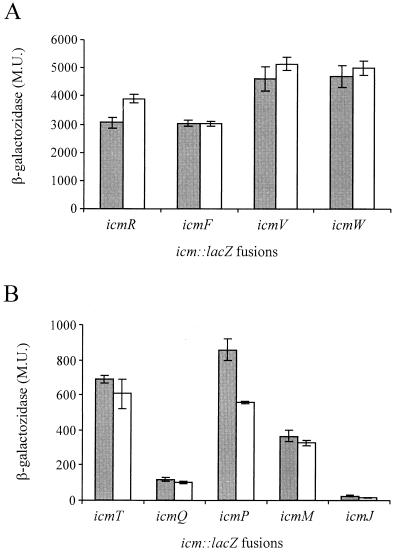
RpoS has a minor effect on icm gene expression.
The stationary-phase sigma factor RpoS is an additional factor that may be involved in regulation of genes required for intracellular growth of L. pneumophila. Unlike the relA gene product, the stationary-phase sigma factor has been shown to be required for L. pneumophila intracellular growth in A. castellanii (17) and to be partially required for growth in murine bone marrow-derived macrophages (4). However, it was also dispensable for intracellular growth in HL-60-derived human macrophages and in THP-1 cells (17). Due to these results and because both RelA and RpoS are involved in expression of genes during the stationary phase, we were interested in determining the involvement of the rpoS gene product in icm gene expression. The levels of expression of β-galactosidase of the nine icm::lacZ fusions described above in an rpoS mutant strain (LM1376) and wild-type strain JR32 were compared. As Fig. 6 shows, the rpoS mutation had a minor effect on expression of the icm::lacZ fusions, and expression of only two genes was changed. The level of expression of β-galactosidase of the icmR::lacZ fusion increased from 2,944 to 3,902 Miller units (P < 0.0001), and the level of expression of the icmP::lacZ fusion decreased from 858 to 554 Miller units (P < 0.0001). The effect of the rpoS mutation on the icmP::lacZ fusion was similar to the effect of the relA mutation on this fusion (Fig. 5B and 6B), and it might have been due to the effect of relA on rpoS gene expression, as shown in E. coli (16). The effect of RpoS and the effect of RelA on icm gene expression were found to be minor. These results are in agreement with the data showing that RpoS (17) and RelA (this study) are dispensable for L. pneumophila intracellular multiplication in human macrophages, a host in which the icm gene products have been shown to be indispensable for intracellular growth (37).
FIG. 6.
RpoS has a minor effect on expression of two icm::lacZ fusions. Translational fusions between nine icm genes (icmT, icmR, icmQ, icmP, icmM, icmJ, icmF, icmW, and icmV) and the lacZ reporter gene were constructed and introduced into wild-type strain JR32 (shaded bars) and RpoS insertion mutant LM1376 (open bars). β-Galactosidase activity was measured as described in Materials and Methods. Four of the icm::lacZ fusions were found to have high β-galactosidase activities (A), and five had low activities (B). The data are expressed in Miller units (M.U.) and are averages based on at least three different experiments. Expression of icmP::lacZ in JR32 (858.47 Miller units) and expression of icmP::lacZ in LM1376 (554.53 Miller units) were significantly different (P > 0.0001), as were expression of icmR::lacZ in JR32 (2,944.12 Miller units) and expression of icmR::lacZ in LM1376 (3,902.14 Miller units), as determined by the standard t test.
DISCUSSION
During the past several years 24 icm/dot genes required for intracellular multiplication and host cell killing have been found in L. pneumophila (39). Fourteen of these genes have been found to be homologous to the R64 tra/trb genes involved in conjugation (42), and several of the L. pneumophila icm/dot genes have been shown to participate in bacterial conjugation (40). Even though 24 icm/dot genes are known, there is very little information regarding their regulation and the time when they are expressed. Previous reports that addressed this issue indicated that L. pneumophila virulence is connected to the stationary phase of growth. It has been shown that the L. pneumophila rpoS gene product is required for intracellular growth in the protozoan host A. castellanii (17) and is partially required for intracellular growth in murine bone marrow-derived macrophages (4). However, it is dispensable for intracellular growth in HL-60-derived macrophages and THP-1 cells (17). Another report (18) indicated that L. pneumophila virulence is coordinated with entry into the stationary phase by ppGpp, the stringent response alarmone. In the same report, several phenotypic traits assumed to be connected with L. pneumophila virulence (such as sodium sensitivity, motility, and expression of the flagellum subunit) were shown to be coordinated with ppGpp production (18).
The stringent response in E. coli is initiated after starvation for amino acids that leads to inhibition of stable RNA, ribosome, and protein synthesis and for accumulation of ppGpp. This global response is a consequence of binding of uncharged tRNA to ribosomes that activates the relA gene product, a guanosine 3′,5′-bispyrophosphate (ppGpp) synthetase. It has been shown that accumulation of ppGpp is involved in several processes, such as activation of the rpoS sigma factor in E. coli (16, 29), fruiting body formation in M. xanthus (19), and antibiotic production in S. coelicolor (12). All of these processes appear to be activated during the stationary phase and to depend on the RelA enzymatic activity.
We examined the effect of L. pneumophila RelA on gene expression during the stationary phase and on intracellular multiplication. Two independent systems that are known to be expressed during the stationary phase in L. pneumophila cultures were found to be affected by the relA mutation. Pigment formation was dramatically affected by deletion of the relA gene, and only one-half as much pigment was produced in this strain during the stationary phase. This phenotype was complemented with a plasmid containing the relA gene, indicating that the relA deletion is the only mutation that influences pigment formation in this strain. In addition, expression of the gene coding for the flagellum subunit (flaA) was reduced more than threefold in the relA mutant compared to the wild-type strain (as determined by a flaA::lacZ fusion).
When the involvement of RelA in intracellular growth and icm gene expression was examined, it became clear that a deletion substitution in relA had no effect on intracellular multiplication in the two eukaryotic hosts tested. Moreover, only a minor reduction in β-galactosidase activity was observed in one of the nine icm::lacZ fusions that were tested. Expression of this fusion (icmP::lacZ) was reduced to a similar minor extent in an RpoS insertion mutant as well.
In addition to construction of the deletion substitution in relA, we also tried to construct a deletion in the L. pneumophila spoT homologous gene (the L. pneumophila spoT homolog was identified from the L. pneumophila genome sequence information by its homology to E. coli SpoT gene [52% identity and 70% similarity]). SpoT is the only known E. coli ppGpp pyrophosphohydrolase that also possesses ppGpp synthetase activity (9). Several attempts were made to construct such a deletion, but they were not successful. We also tried to construct this deletion in the background of the relA mutant strain but had no success (this was the only way in which a spoT null strain was constructed in E. coli [52]). It is very likely that the spoT gene of L. pneumophila is essential.
It is known that RelA is the major source of ppGpp in E. coli, and as was clearly shown here, this is also the case in L. pneumophila. The dramatic decreases in expression of two independent systems that were observed in the L. pneumophila RelA mutant probably were due to the lack of ppGpp in the cells during the stationary phase. The ppGpp data indicate that SpoT, by it self, does not produce detectable levels of ppGpp in the conditions examined and that it cannot overcome the deletion in relA. The fact that in a bacterial strain the relA mutation had a clear effect on ppGpp accumulation, pigment production, and flaA gene expression but no effect on intracellular growth clearly indicates that factors other than relA control icm gene expression.
As described above, the stationary-phase sigma factor RpoS was shown to be involved in L. pneumophila intracellular growth in A. castellanii, and it has also been suggested that the stringent response system participates together with RpoS in regulation of L. pneumophila virulence (4, 18). However, both RpoS and RelA were shown here to have minor effects on icm/dot gene expression. The icm/dot genes have been shown to be required for intracellular growth in all eukaryotic hosts examined, including HL-60- and U937-derived human macrophages (37, 49), murine bone marrow-derived macrophages (47), and the protozoan hosts A. castellanii (41) and Dictyostelium discoideum (43). Taking all of these results together, we believe that it is very likely that some of the virulence factors required for intracellular growth in amoebae are regulated by the rpoS sigma factor, but the major system that contributes to L. pneumophila intracellular growth (the icm/dot system) is probably controlled by other regulatory factors. These factors remain to be identified.
Acknowledgments
We thank Karen Pomeranz and Shai Senderovich for plasmid construction. We are grateful to Howard A. Shuman for carefully reading the manuscript.
This work was supported by a grant from the Israel Science Foundation and by the Tel-Aviv University internal research fund. G. Segal was supported by the Alon fellowship from the Israeli Ministry of Education.
REFERENCES
- 1.Abu Kwaik, Y. 1996. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl. Environ. Microbiol. 62:2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, H. L., J. P. Vogel, and R. R. Isberg. 1998. Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect. Immun. 66:950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachman, M. A., and M. S. Swanson. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol. Microbiol. 40:1201–1214. [DOI] [PubMed] [Google Scholar]
- 5.Berger, K. H., J. J. Merriam, and R. R. Isberg. 1994. Altered intracellular targeting properties associated with mutations in the Legionella dotA gene. Mol. Microbiol. 14:809–822. [DOI] [PubMed] [Google Scholar]
- 6.Bozue, J. A., and W. Johnson. 1996. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect. Immun. 64:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand, B. C., A. B. Sadosky, and H. A. Shuman. 1994. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol. Microbiol. 14:797–808. [DOI] [PubMed] [Google Scholar]
- 8.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cashel, M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants, p. 341–356. In K. W. Adolph (ed.), Methods in molecular genetics, vol. 3. Academic Press, New York, N.Y.
- 10.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1994. The stringent response, p. 1458–1496. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C.
- 11.Catrenich, C. E., and W. Johnson. 1989. Characterization of the selective inhibition of growth of virulent Legionella pneumophila by supplemented Mueller-Hinton medium. Infect. Immun. 57:1862–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraburtty, R., and M. Bibb. 1997. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J. Bacteriol. 179:5854–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein, P. H., M. A. Edelstein, F. Higa, and S. Falkow. 1999. Discovery of virulence genes of Legionella pneumophila by using signature tagged mutagenesis in a guinea pig pneumonia model. Proc. Natl. Acad. Sci. USA 96:8190–8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286–290. [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J. F. Tomb, B. A. Dougherty, J. M. Merrick, K. Mckenney, G. Sutton, W. Fitzhugh, C. Fields, G. J. D., J. Scott, R. Shirley, L. I. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, D. T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. Mcdonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496–512. [DOI] [PubMed] [Google Scholar]
- 16.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary-phase sigma factor sigma S is positively regulated by ppGpp. J. Bacteriol. 175:7982–7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hales, L. M., and H. A. Shuman. 1999. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J. Bacteriol. 181:4879–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721–731. [DOI] [PubMed] [Google Scholar]
- 19.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heuner, K., J. Hacker, and B. C. Brand. 1997. The alternative sigma factor sigma-28 of Legionella pneumophila restores flagellation and motility to an Escherichia coli fliA mutant. J. Bacteriol. 179:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, M. A. 1987. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J. Exp. Med. 166:1310–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz, M. A. 1983. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz, M. A. 1984. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27–33. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz, M. A., and F. R. Maxfield. 1984. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J. Cell Biol. 99:1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires’ disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 60:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komano, T., T. Yoshida, K. Narahara, and N. Furuya. 2000. The transfer region of IncI1 plasmid R64: similarities between R64 tra and Legionella icm/dot genes. Mol. Microbiol. 35:1348–1359. [DOI] [PubMed] [Google Scholar]
- 29.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marra, A., S. J. Blander, M. A. Horwitz, and H. A. Shuman. 1992. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. USA 89:9607–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular biology. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 32.Purcell, M. W., and H. A. Shuman. 1998. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66:2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowbotham, T. J. 1986. Current views on the relationships between amoebae, legionellae and man. Isr. J. Med. Sci. 22:678–689. [PubMed] [Google Scholar]
- 34.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadosky, A. B., J. W. Wilson, H. M. Steinman, and H. A. Shuman. 1994. The iron superoxide dismutase of Legionella pneumophila is essential for viability. J. Bacteriol. 176:3790–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segal, G., and H. A. Shuman. 1997. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun. 65:5057–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segal, G., and H. A. Shuman. 1998. How is the intracellular fate of the Legionella pneumophila phagosome determined? Trends Microbiol. 6:253–255. [DOI] [PubMed] [Google Scholar]
- 40.Segal, G., and H. A. Shuman. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components on IncQ plasmid RSF1010. Mol. Microbiol. 30:197–208. [DOI] [PubMed] [Google Scholar]
- 41.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segal, G., and H. A. Shuman. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669–670. [DOI] [PubMed] [Google Scholar]
- 43.Solomon, J. M., A. Rupper, J. A. Cardelli, and R. R. Isberg. 2000. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect. Immun. 68:2939–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinert, M., H. Engelhard, M. Flugel, E. Wintermeyer, and J. Hacker. 1995. The Lly protein protects Legionella pneumophila from light but does not directly influence its intracellular survival in Hartmannella vermiformis. Appl. Environ. Microbiol. 61:2428–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. [DOI] [PubMed] [Google Scholar]
- 46.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609–3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873–876. [DOI] [PubMed] [Google Scholar]
- 48.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30–34. [DOI] [PubMed] [Google Scholar]
- 49.Wiater, L. A., K. Dunn, F. R. Maxfield, and H. A. Shuman. 1998. Early events in phagosome establishment are required for intracellular survivel of Legionella pneumophila. Infect. Immun. 66:4450–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiater, L. A., A. B. Sadosky, and H. A. Shuman. 1994. Mutagenesis of Legionella pneumophila using Tn903dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641–653. [DOI] [PubMed] [Google Scholar]
- 51.Wintermeyer, E., M. Flugel, M. Ott, M. Steinert, U. Rdest, K. H. Mann, and J. Hacker. 1994. Sequence determination and mutational analysis of the lly locus of Legionella pneumophila. Infect. Immun. 62:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao, H., M. Kalman, K. Ikehara, S. Zemel, G. Glaser, and M. Cashel. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980–5990. [PubMed] [Google Scholar]
- 53.Yanish-Perron, C., J. Viera, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119. [DOI] [PubMed] [Google Scholar]