Abstract
The predominant mode of growth of bacteria in the environment is within sessile, matrix-enclosed communities known as biofilms. Biofilms often complicate chronic and difficult-to-treat infections by protecting bacteria from the immune system, decreasing antibiotic efficacy, and dispersing planktonic cells to distant body sites. While the biology of bacterial biofilms has become a major focus of microbial research, the regulatory mechanisms of biofilm development remain poorly defined and those of dispersal are unknown. Here we establish that the RNA binding global regulatory protein CsrA (carbon storage regulator) of Escherichia coli K-12 serves as both a repressor of biofilm formation and an activator of biofilm dispersal under a variety of culture conditions. Ectopic expression of the E. coli K-12 csrA gene repressed biofilm formation by related bacterial pathogens. A csrA knockout mutation enhanced biofilm formation in E. coli strains that were defective for extracellular, surface, or regulatory factors previously implicated in biofilm formation. In contrast, this csrA mutation did not affect biofilm formation by a glgA (glycogen synthase) knockout mutant. Complementation studies with glg genes provided further genetic evidence that the effects of CsrA on biofilm formation are mediated largely through the regulation of intracellular glycogen biosynthesis and catabolism. Finally, the expression of a chromosomally encoded csrA′-′lacZ translational fusion was dynamically regulated during biofilm formation in a pattern consistent with its role as a repressor. We propose that global regulation of central carbon flux by CsrA is an extremely important feature of E. coli biofilm development.
Bacteria have evolved elaborate mechanisms for adhering to and colonizing solid surfaces, thereby establishing microbial communities known as biofilms (13). Biofilms represent a distinct lifestyle for bacteria which provides protection from deleterious conditions. The impact of microbial biofilms is pervasive throughout the biosphere. In medicine, biofilms are known to complicate the majority of chronic and difficult-to-treat bacterial infections, including prostatitis, biliary tract infections, and urinary catheter cystitis caused by Escherichia coli (14). Thus, the biology of bacterial biofilms has become a major focus of microbial research.
Studies of the biophysical, structural, and chemical properties of biofilms have culminated in our present concept of the mature biofilm as a complex community exhibiting channels and pillars that may facilitate nutrient exchange and waste removal (13). A biofilm is initiated by the attachment of individual cells to a surface followed by migration and replication to form microcolonies which eventually produce a mature biofilm (32, 35). A variety of extracellular molecules and surface organelles participate in biofilm development. In E. coli, flagella, type I pili, and curli fimbriae are involved in attachment and adherence (35, 46). In Pseudomonas, flagella and type IV pili have been implicated (32). It is interesting that although motility is involved in the early stages of biofilm formation, it is unfavorable for maintenance of a mature biofilm and flagellar gene expression is decreased in the biofilms of both E. coli and Pseudomonas aeruginosa (discussed in reference 47). The extracellular polysaccharide colanic acid is needed to generate the typical three-dimensional structure of E. coli biofilm, although a biofilm is still formed in its absence (17). In contrast, the extracellular polyuronide alginate is essential for the formation of a mature biofilm in P. aeruginosa (35).
The regulatory mechanisms that guide biofilm development have also come under recent scrutiny. The social behavior known as quorum sensing, whereby the concentration of a diffusible autoinducer provides a regulatory signal in response to population density, is essential for the construction of a mature biofilm of P. aeruginosa (19). The Crc protein (catabolite repression control) is also required, in part because it activates the synthesis of type IV pili (33). A two-component response regulator, GacA, was also recently implicated, although the basis of this effect is unknown (34). Intracellular polyphosphate has pleiotropic effects on a variety of extracellular components and regulatory factors in P. aeruginosa which participate in biofilm formation (37, 38). In E. coli, OmpR (outer membrane protein regulator) and RpoS or ςs (a sigma factor needed for the transcription of stationary phase genes) are apparently involved (1, 46). Nutritional cues, e.g., carbon and iron availability in Pseudomonas fluorescens and P. aeruginosa, respectively, also significantly affect biofilm formation (9, 35). Clearly, the regulation of biofilm development is highly complex and remains poorly understood in any species.
The dispersal or shedding of planktonic cells from a biofilm may be essential to permit bacteria to escape the confines of the biofilm in order to colonize new locations (14). Direct microscopic observation has revealed that physiological and environmental signals influence the dispersal process (5). Biofilm dispersal in P. aeruginosa and P. fluorescens has been postulated to involve the degradation of alginate (2, 12). Furthermore, it has been suggested that dispersal of cells from a biofilm may be cell cycle mediated in E. coli (3). The differential expression of chitinase genes among a subpopulation of marine bacteria has been postulated to promote detachment of cells from biofilm (7). In summary, the processes involved in biofilm dispersal are not clearly defined in any species, and no regulatory pathway has been shown to be capable of inducing biofilm dispersal.
It was previously observed, under a single growth condition, that a csrA disruption caused E. coli to adhere to culture tubes, forming a coating suggestive of a biofilm (40). The csrA gene encodes a global regulatory protein, CsrA (carbon storage regulator), which represses several metabolic pathways that are induced in the stationary phase of growth, including glycogen biosynthesis and catabolism and gluconeogenesis (40, 43, 50). Conversely, CsrA activates glycolysis, acetate metabolism, and motility (43, 48, 49). CsrA is an RNA binding protein that regulates gene expression posttranscriptionally by binding to mRNA transcripts of regulated genes and either increasing or decreasing their decay rates (28, 48). Furthermore, ∼18 subunits of CsrA bind to an untranslated RNA molecule, CsrB, which antagonizes CsrA activity (27). A highly repeated sequence element found in the loop segments of CsrB hairpins may mediate this binding (27, 39). Homologues of csrA are widely distributed among eubacteria, including numerous human pathogens, but are not apparent in eucaryotes (39). The csrA gene of Salmonella enterica serovar Typhimurium represses genes that are involved in the invasion of mammalian gastrointestinal mucosa (4), and the csrA homolog, rsmA, of plant pathogenic Erwinia species represses the production of proteins involved in both plant disease and host response (15, 16). Thus, there is evidence that CsrA homologues of eubacteria are important in regulating host-microbe interactions. The purpose of the present study was to investigate the regulatory role of CsrA in biofilm development.
MATERIALS AND METHODS
Bacterial strains and media.
The strains, phage, and plasmids used in these experiments are listed in Table 1. Bacteria were routinely cultured at 37°C in Luria-Bertani (LB) medium (30), while cultures for biofilm assays were generally grown at 26°C. Biofilm assays were typically carried out in colony-forming antigen (CFA) medium containing (per liter) 10 g of Casamino Acids (Difco), 1.5 g of yeast extract (Difco), 50 mg of MgSO4, and 5 mg of MnCl2, pH 7.4 (20), or were carried out in artificial urine medium containing (per liter) 0.65 g of CaCl2 H2O, 0.65 g of MgCl2 6H20, 4.6 g of NaCl2, 2.3 g of Na2SO4, 0.65 g of sodium citrate, 0.20 g of sodium oxalate, 2.8 g of KH2PO4, 1.6 g of KCl, 2.0 g of NH4Cl, 12 g of urea, 1.1 g of creatinine, and 1% tryptic soy broth (BBL, Cockeysville, Md.) (31). Glycogen biosynthesis was assessed using Kornberg agar medium (29). M63 salts solution contained (per liter) 13.6 g of KH2PO4, 2 g of (NH4)2SO4, 0.5 mg of FeSO4 7H20, and 1 ml of 1 M MgSO4, pH 7.0. Antibiotics were used as required for biofilm studies at the following concentrations: ampicillin, 200 μg/ml; chloramphenicol, 20 μg/ml; kanamycin, 100 μg/ml; and tetracycline, 10 μg/ml. Ampicillin and kanamycin were used at 50 and 40 μg/ml, respectively, during the construction of the csrA′-′lacZ fusion.
TABLE 1.
Bacterial strains, phage, and plasmids used in this study
| Strain, phage, or plasmid | Relevant genotype or characteristics | Reference or source |
|---|---|---|
| E. coli K-12 strains | ||
| MG1655 | F− λ− | Michael Cashel |
| CF7789 | MG1655 Δ lacI-Z(MluI) | Michael Cashel |
| KSA712 | CF7789 Δ (λ att-lom)::bla φ(csrA′-′lacZ)1 (hyb) Ampr Kans | This study |
| AAECO72 | MG1655 Δ fimB-H | Ian Blomfield |
| DHB6521 | F− λInch1(Kanr)Δlac(MS265) mel NalrsupF58 (=suIII+) | 11 |
| JM101 | supE thiΔ (lac-proAB)[F′ traD36 proAB lacIqZΔM115] | 44 |
| MC4100 | F− Δ(argF-lac)U169 rpsL relA flhD deoC ptsF rbsR | 23 |
| W3110 | F−mcrA mcrB IN(rrnD-rrnE)1 λ− | 44 |
| RG1-B | MG1655 csrB::cam (a precise deletion of csrB) | 22 |
| MM5057 | Δ motB urvC-279::Tn10 | Mike Manson |
| MHR 204 | MC4100 csgA2::Tn105 | 23 |
| RH106 | MC4100 rpoS::Tn10 | R. Hengge-Aronis |
| SG20043 | MC4100 cpsE::Tn10 | Valerie Stout |
| TK821 | MC4100 ompR331::Tn10 | 44 |
| TR1-5 or TRa | csrA::kanR | 40 |
| glgAb | glgA::kanR | This study |
| DJ1 | MG1655 csgA2::Tn105 | This study |
| DJ2 | TRMG1655 csgA2::Tn105 | This study |
| DJ3 | MG1655 cpsE::Tn10 | This study |
| DJ4 | TRMG cpsE::Tn10 | This study |
| DJ6 | TRAAECO72 | This study |
| DJ7 | AAECO72 csgA2::Tn105 | This study |
| DJ8 | TRAAECO72 csgA2::Tn105 | This study |
| DJ9 | AAECO72 cpsE::Tn10 | This study |
| DJ10 | TRAAECO72 cpsE::Tn10 | This study |
| DJ11 | MG1655 csgA2::Tn105 cpsE::Tn10 | This study |
| DJ12 | TR MG1655 csgA2::Tn105 cpsE::Tn10 | This study |
| DJ13 | AAECO72 csgA2::Tn105 cpsE::Tn10 | This study |
| DJ14 | TRAAECO72 csgA2::Tn105 cpsE::Tn10 | This study |
| DJ21 | MG1655 ΔmotB uvrC-279::Tn10 | This study |
| DJ22 | AAECO72 ΔmotB uvrC-279::Tn10 | This study |
| DJ24 | TR ΔmotB uvrC-279::Tn10 | This study |
| DJ25 | TRAAECO72 ΔmotB uvrC 279::Tn10 | This study |
| DJ30 | MG1655 rpoS::Tn10 | This study |
| DJ31 | TR MG1655 rpoS::Tn10 | This study |
| DJ32 | MG1655 rpoS::Tn10 csgA2::Tn105 | This study |
| DJ33 | TR MG1655 rpoS::Tn10 csgA2::Tn105 | This study |
| DJ34 | AAECO72 rpoS::Tn10 | This study |
| DJ35 | TR AAECO72 rpoS::Tn10 | This study |
| DJ36 | AAECO72 csgA2::Tn105 rpoS::Tn10 | This study |
| DJ37 | TRAAECO72 csgA2::Tn105 rpoS::Tn10 | This study |
| DJ40 | MG1655 ompR333::Tn10 | This study |
| DJ41 | TR MG1655 ompR333::Tn10 | This study |
| DJ42 | MG1655 ompR333::Tn10 csgA2::Tn105 | This study |
| DJ43 | TR MG1655 ompR333::Tn10 csgA2::Tn105 | This study |
| DJ44 | AAECO72 ompR333::Tn10 | This study |
| DJ45 | TR AAECO72 ompR333::Tn10 | This study |
| DJ46 | AAECO72 ompR333::Tn10 csgA2::Tn105 | This study |
| DJ47 | TR AAECO72 ompR333::Tn10 csgA2::Tn105 | This study |
| Clinical strains | ||
| C. freundii P5 | 24 | |
| E. coli P18 | 24 | |
| E. coli O157:H7 EF302 | 26 | |
| S. enterica serovar Typhimurium ATCC 14028 | 4 | |
| Bacteriophages | ||
| P1vir | Strictly lytic P1; forms clear plaques | Carol Gross |
| λInCh1 | For chromosomal integration of genes | 11 |
| Plasmids | ||
| pUC19 | Cloning vector, Ampr | 44 |
| pBR322 | Cloning vector, Ampr Tetr | 44 |
| pCSR10 | E. coli csrA gene in pUC19, Ampr | 40 |
| pSTCSR5 | S. enterica serovar Typhimurium csrA in pUC19, GenBank accession no. 276860, Ampr | This study |
| pCSRH6-19 | Produces recombinant CsrA under IPTG induction, Ampr | 27 |
| pCSRB-SF | Minimal csrB in pUC18, Ampr | 27 |
| pOP12 | Contains asd-glgCAP′ in pBR322 Tetr | 25 |
| pOP245 | glgA in pBR322, Tetr | 25 |
| pJF02 | glgP in pUC19, Ampr | 51 |
| pMLB1034 | For construction of ′lacZ translational fusions, Ampr | 41 |
| pTR151P1 | PstI subclone from pTR151, csrA::kanR allele in pUC19, Ampr | 40 |
Indicates that the wild-type csrA allele has been replaced by csrA::kanR.
Strains names with the suffix glgA contained a glgA::kanR insertion.
Quantitative biofilm assay.
Overnight cultures were inoculated 1:100 into fresh medium. In the microtiter plate assay, inoculated cultures were grown in a 96-well polystyrene microtiter plate. Growth of planktonic cells was determined by absorbance at 600 nm or total protein assay. Biofilm was measured by discarding the medium, rinsing the wells with water (three times), and staining bound cells with crystal violet (BBL) (32). The dye was solubilized with 33% acetic acid (EM Science, Gibbstown, N.J.), and absorbance at 630 nm was determined using a microtiter plate reader (DynaTech, Chantilly, Va.). For each experiment, background staining was corrected by subtracting the crystal violet bound to uninoculated controls. All comparative analyses were conducted by incubating strains within the same microtiter plate to minimize variability. To confirm that observed effects on biofilm formation in microtiter wells were not surface specific, cultures were grown and tested simultaneously in new borosilicate glass test tubes (18 mm). Each experiment was performed at least in triplicate, and the data were analyzed by Tukey Multigroup Analysis (StatView-SAS Institute Inc., Cary, N.C.).
Molecular and genetic techniques.
P1 transduction or cotransduction of resistance markers, subcloning, and molecular genetic techniques were performed by standard procedures (30, 44). The construction of a chromosomal csrA′-′lacZ translational fusion first involved the preparation of a plasmid containing an in-frame csrA′-′lacZ fusion, pCAZ1, by subcloning a 0.4-kb blunt-ended EcoRI-BglII restriction fragment from pTR151P1 into the SmaI site of pMLB1034. This fusion was transferred to λInCh1, the recombinant phage was moved into the chromosome, and most of the λ DNA was eliminated to generate a stable chromosomal fusion, which was confirmed by PCR analysis, as previously described (11). The fusion was transduced into E. coli K-12 strain CF7789 for expression studies.
β-Galactosidase, protein, and glycogen assays.
Cultures for the β-galactosidase assays were grown in borosilicate test tubes at 26°C, and planktonic cells were separated from adherent cells. The biofilm was suspended in CFA medium with pipetting to disrupt cell aggregates. β-Galactosidase activity was determined in 10-min assays, as previously described (41). Cell protein was obtained by precipitation with ice-cold 10% trichloroacetic acid and was quantified by using the bicinchoninic acid method with bovine serum albumin as the standard protein (45). Glycogen phenotypes were determined by staining colonies with iodine vapor (27).
Microscopy.
Sterile microscope coverslips were aseptically placed into sterile petri dishes along with 15 ml of a freshly inoculated culture. The coverslips were removed at various times, gently rinsed with phosphate buffer (per liter, 11 g of K2HPO4 and 8.5 g of KH2PO4, adjusted to pH 7.4 with NaOH), stained with 30 μg of acridine orange/ml (K&K Laboratories, Inc., Plainville, N.Y.), rinsed again, and mounted to the microscope slide with Immu-Mount (Shandon, Pittsburgh, Pa.).
A Nikon Microphot-FXA microscope with a 40× Plan Apo objective lens was used for Nomarski interference microscopy. All images were collected with a Roper Sensys cold-slow scan CCD camera (Roper Scientific Co., Phoenix, Ariz.) and the imaging program Image-Pro Lab software for MacIntosh (Scanalytics, Fairfax, Va.). A Zeiss LSM 410 Confocal Microscope with a 40× 1.2 N.A. C-Apochromat objective lens was used to generate a topographical image and cross-sections through the csrA mutant biofilm. Optical sections were collected at 2-μm steps through a sample depth of 20 μm using 488 nm and 510 to 525 nm excitation and emission wave lengths, respectively.
RESULTS
Biofilm formation in csrA mutant and wild-type strains.
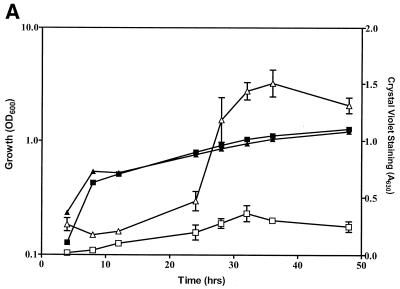
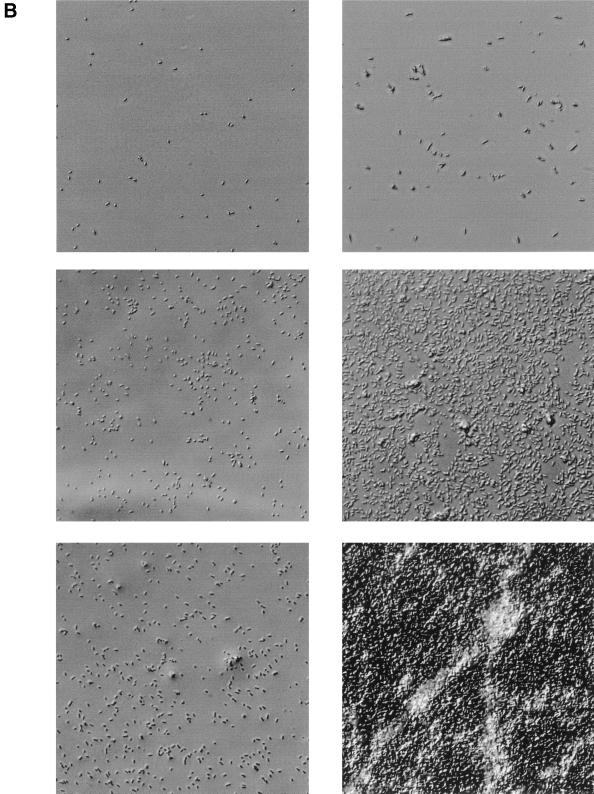
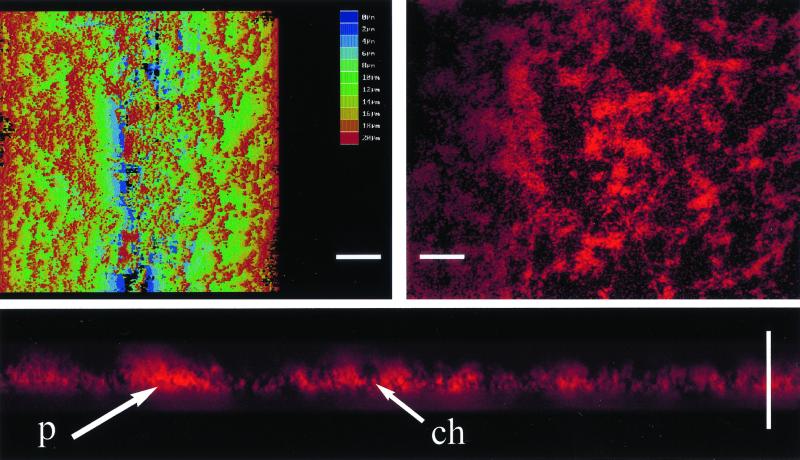
To quantitatively assess the role of CsrA in biofilm development, biofilm growth was monitored by using an assay based on crystal violet staining, while planktonic growth in the same cultures was monitored by absorbance. The wild-type E. coli K-12 strain MG1655 and its isogenic csrA mutant exhibited similar growth in liquid CFA medium, as shown in a representative experiment (Fig. 1A). In each strain, biofilm development was somewhat delayed until cultures were in the stationary phase of growth. Thereafter, biofilm accumulated much more rapidly and extensively in the csrA mutant. Further examination of biofilms formed on microscope coverslips by Nomarski interference microscopy also confirmed that biofilm formation was more prolific in the csrA mutant (Fig. 1B). In order to determine whether the biofilm formed by the csrA mutant exhibited features of a typical mature biofilm, scanning confocal laser microscopy was utilized. A topographical image of the mutant revealed a biofilm ∼20 μm thick at 24 h of growth (Fig. 2). A cross-section of this biofilm exhibited pillars and channels, characteristic of a mature biofilm. Thus, the increased adherence to abiotic surfaces caused by csrA disruption appears to reflect normal, though accelerated, biofilm formation. This stimulatory effect of a csrA mutation on biofilm formation was observed in every growth medium that we have examined. These include morpholinepropanesulfonic acid medium (40), CFA with or without 0.2% glucose or glycerol, Kornberg with 0.5% glucose, LB with or without 0.2% glucose, or M63 salts containing 0.2% glucose (data not shown). It was also observed under anaerobiosis (40). Likewise, every E. coli K-12 parental strain that has been tested exhibited this effect of csrA, including MC4100, W3110, MG1655, JM101, BW3414, and others (data not shown). Thus, the consequences of CsrA effects on biofilm formation, unlike those of certain other factors (18), do not appear to be restricted to specific growth conditions or strains.
FIG. 1.
Biofilm formation by wild-type and csrA mutant strains of E. coli. (A) Growth in polystyrene microtiter wells of planktonic cells of the wild-type strain MG1655 (filled squares) or its csrA mutant (filled triangles). Biofilm formation in the same wells is shown as open squares or triangles, respectively. (B) Nomarski interference of biofilm formed by MG1655 in the left panels (10, 16, and 24 h, top to bottom) or its csrA mutant in the right panels (3, 10, and 24 h). OD600, optical density at 600 nm.
FIG. 2.
Laser confocal microscopy of biofilm produced by a csrA mutant. A topographical image of the biofilm formed by TR1-5MG1655 is shown in the left panel (white scale bar, 14 μm; virtual color code depicts biofilm height above the microscope slide, from 0 to 20 μm, in 2-μm increments), along with a 2-μm-thick cross-section at a depth of 6 μm in the right panel (scale bar, 10 μm) and a cross-section of a sagital view tilted (Q = 45°, F = 30°) in the bottom panel (scale bar, 20 μm), as visualized by confocal microscopy. Examples of apparent pillars (p) and channels (ch) are indicated.
Overexpression of csrA in E. coli K-12 and clinical isolates.
Since a disruption in csrA dramatically increased biofilm formation, the effects of csrA overexpression on biofilm formation was examined. Ectopic expression of csrA from a multicopy plasmid completely inhibited biofilm formation in both wild-type E. coli K-12 and its csrA mutant relative to the plasmid vector controls (Fig. 3A).
FIG. 3.
Effects of csrA on 24-h biofilms grown in microtiter wells. (A) MG1655 (bars 1 to 3) or its csrA mutant (bars 4 to 6) containing plasmids pCSR10 (csrA) (bars 2 and 5) or pUC19 (bars 3 and 6). (B) Urinary tract pathogens E. coli P18 (bars 1 to 3) and C. freundii P5 (bars 4 to 6) containing plasmids pCSR10 (csrA) (bars 2 and 5) or pUC19 (bars 3 and 6). (C) Food-borne pathogens E. coli O157:H7 (bars 1 to 3) and S. enterica serovar Typhimurium ATCC 14028 (bars 4 to 6) containing plasmids pCSR10 (csrA) (bar 2), pSTCSR5 (S. enterica serovar Typhimurium csrA) (bar 5), or pUC19 (bars 3 and 6). Each bar shows the averages and standard errors of three separate experiments, and asterisks denote significant differences between strains (P < 0.0001).
Because biofilms are known to complicate a variety of infections, we examined whether csrA overexpression would inhibit biofilm formation in several clinical isolates. Two strains isolated from colonized urinary catheters, E. coli P18 and Citrobacter freundii P5, were tested by using artificial urine medium for cultivation to mimic their host environment (Fig. 3B). In these strains, overexpression of csrA was also inhibitory to biofilm formation. Biofilm formation was modestly inhibited in the food-borne pathogens E. coli O157:H7 strain EF302, an enterohemorrhagic isolate, and S. enterica serovar Typhimurium ATCC 14028, a diarrheal pathogen (Fig. 3C). Note that in the latter experiment, S. enterica serovar Typhimurium overexpressed its own csrA homolog. Thus, ectopic expression of csrA inhibited biofilm formation in these pathogenic relatives of E. coli, most significantly in the clinical isolates that apparently utilize biofilm formation as a virulence factor.
CsrB RNA is an antagonist of CsrA, apparently due to its ability to sequester ∼18 subunits of this protein (27). As a result, a csrB null mutation yields a phenotype similar to csrA overexpression, and csrB overexpression simulates a csrA disruption (22, 27). As predicted, biofilm formation was deficient in a csrB null mutant of E. coli K-12, relative to its isogenic parent, and was stimulated by a multicopy plasmid clone of csrB, pCSRB-SF, relative to a plasmid vector control (Table 2).
TABLE 2.
Effects of csrB on biofilm formationa
| Strains (csrB genotype) | Results with plasmid
|
||
|---|---|---|---|
| None | pCSRB-SF | pUC19 | |
| MG1655 (csrB+) | 0.170 ± 0.012 | 1.50 ± 0.172* | 0.067 ± 0.011 |
| RG1-B (csrB::cam) | 0.029 ± 0.003* | 0.21 ± 0.016* | 0.010 ± 0.003 |
Biofilm formation in CFA medium was measured at 24 h by crystal violet staining in microtiter wells (A630). Each result represents the mean values ± standard errors of three independent experiments, each conducted with triplicate samples. Statistical differences (P < 0.0001) were noted (*) between MG1655 and its csrB mutant, RG1-B, and between strains that contained either a multicopy csrB plasmid, pCSRB-SF, or the pUC19 control plasmid.
CsrA effects on biofilm formation by strains defective for extracellular and/or surface molecules or regulatory factors.
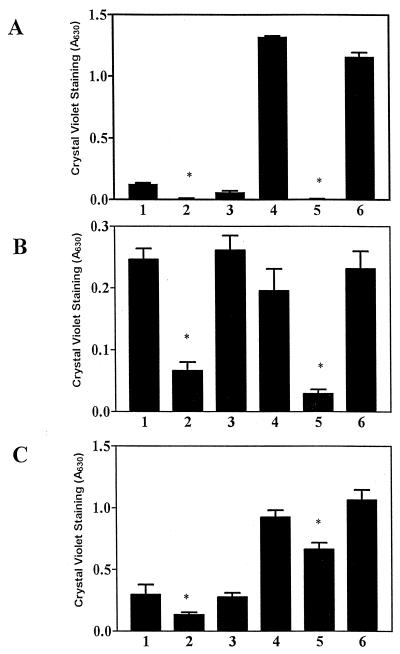
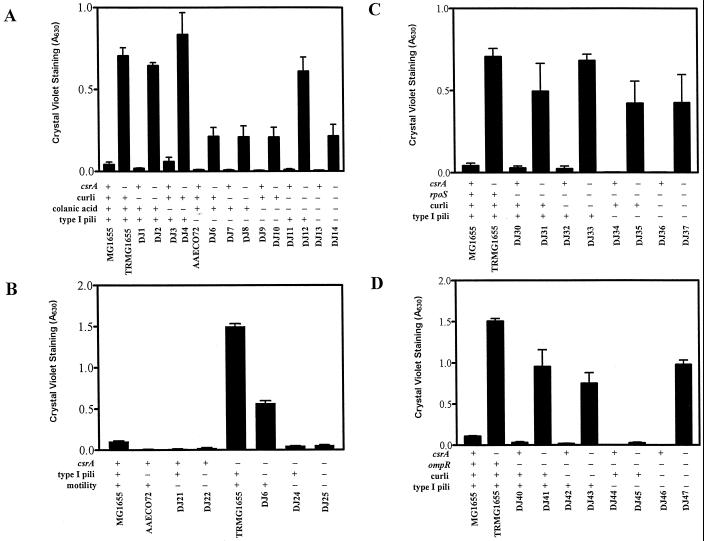
To address the mechanism by which CsrA represses biofilm formation in E. coli, its effects were examined in strains lacking one or more of the extracellular and/or surface factors that have been reported to participate in biofilm formation. Results using strains lacking curli fimbriae, colanic acid, and/or type I pili are shown in Fig. 4A. These results confirm that curli fimbriae and type I pili play significant roles in biofilm formation in the parent strain, while colanic acid does not quantitatively affect biofilm formation. Biofilm formation by all of the isogenic csrA mutants was substantially greater than that of the wild-type parent, MG1655. Furthermore, in the csrA mutant background only the loss of type I pili significantly decreased biofilm formation. Interestingly, the loss of curli fimbriae in the csrA mutant lacking colanic acid and type I pili reproducibly resulted in increased biofilm (Fig. 4A). Growth curves conducted on these strains indicated that the differences in biofilm formation exhibited by these strains were not due to growth defects (data not shown). Both flagella and motility are needed for initial cell attachment during biofilm formation in E. coli, although chemotaxis is not required (35). Therefore, the effect of a ΔmotB mutation, which renders cells nonmotile, was tested. In the csrA wild-type strains, a disruption in motility virtually eliminated biofilm formation (Fig. 4B). In a csrA mutant, biofilm was decreased by the motB mutation but was not eliminated (Fig. 4B). In the csrA motB mutant there was no additional effect of a type I pilus deletion. These studies revealed that a csrA mutant forms biofilm in the absence of each of these four previously reported extracellular and/or surface factors.
FIG. 4.
Effects of extracellular and/or surface factors and global regulators on biofilm formation in E. coli strain MG1655 and its isogenic csrA mutant. Crystal violet staining of 24-h biofilms formed in microtiter wells by mutants disrupted in curli fimbriae, colanic acid, and/or type I pili (A); type I pili and/or motility (B); RpoS, curli fimbriae, and/or type I pili (C); or OmpR, curli fimbriae, and/or type I pili (D). Each bar shows the averages and standard errors of three separate experiments (P < 0.0001).
RpoS and OmpR have been reported to regulate biofilm formation (1, 46). We observed that an rpoS disruption had only a modest effect on biofilm formation in the parental strain and exhibited no significant effect in the csrA mutant (Fig. 4C). While the addition of a curli (csgA) knockout mutation in the rpoS background yielded no additional effect, the loss of both type I pili and rpoS eliminated biofilm formation in the csrA wild-type strain. Nevertheless, these two mutations had minimal effects in the csrA mutant. Even the loss of curli fimbriae, type I pili, and rpoS only modestly decreased biofilm formation in the csrA mutant; biofilm formation by this strain was severalfold greater than that in the prototrophic parent, MG1655. OmpR did have significant effects on biofilm formation in the parent strain but only modestly affected the csrA mutant (Fig. 4D). Likewise, the disruption of both ompR and curli fimbriae caused only an ∼50% decrease in biofilm formation in the csrA mutant (Fig. 4D). However, the disruption of ompR and type I fimbriae almost eliminated biofilm formation in the csrA mutant. Interestingly, the further disruption of curli fimbriae increased biofilm in the latter strain (Fig. 4D), similar to its effect in the type I pili and colanic acid-deficient csrA mutant (Fig. 4A). This result again suggests that under certain situations, curli fimbriae may interfere with the function of some other factor(s) involved in biofilm formation. We conclude that the effects of CsrA are mediated independently of and are quantitatively more significant than those of OmpR or RpoS under these in vitro growth conditions.
Effects of CsrA are largely mediated through glycogen synthesis and catabolism.
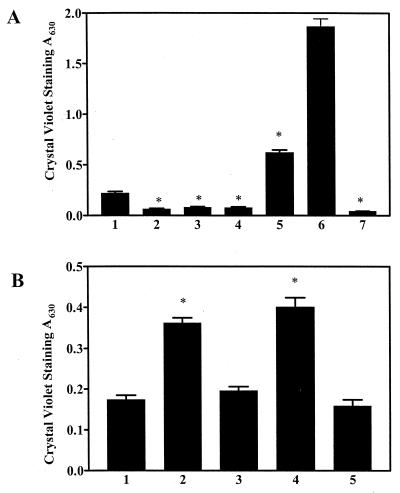
The csrA gene was first recognized as a repressor of glycogen biosynthesis (40). Subsequent studies revealed that CsrA coordinately regulates glycogen biosynthesis in the early stationary phase of growth and its subsequent catabolism; both processes are highly accelerated in a csrA mutant (50). This occurs because CsrA posttranscriptionally represses the glgCAP operon, which includes the biosynthetic genes glgC (ADP-glucose synthetase) and glgA (glycogen synthase) as well as the gene encoding the catabolic enzyme glycogen phosphorylase (glgP) (50). Therefore, the role of intracellular glycogen in biofilm formation was examined. Because glycogen synthesis is favored by the presence of excess carbon under nutrient limitation, e.g., nitrogen (36, 42), we tested MG1655 and its glgA mutant for glycogen accumulation on CFA medium. Weak accumulation by MG1655 was observed (data not shown). A glgA transposon insertion caused both csrA wild-type and mutant strains to become severely defective for biofilm formation (Fig. 5A, lanes 1 and 2 and lanes 6 and 7). Furthermore, biofilm formation did not differ quantitatively for csrA wild-type or mutant strains containing the glgA disruption. As expected, the glgA-carrying plasmid, pOP245, complemented the glycogen biosynthesis defect of this strain (data not shown). In contrast, pOP245 failed to restore biofilm formation (Fig. 5A, lane 3). The possibility that glycogen catabolism might also be required for biofilm formation and the fact that the glgA insertion had a polar effect on glgP expression were examined by complementation experiments with the glgP-carrying plasmid pJF02. While complementation by glgP alone did not restore biofilm formation (Fig. 5A, lane 4), both glgA and glgP resulted in even more biofilm production than that in the prototrophic parent (Fig. 5A, lane 5), indicating that glycogen biosynthesis and its subsequent turnover are both required for optimal biofilm formation.
FIG. 5.
Effects of glycogen synthesis and catabolism on biofilm formation at 24 h. Isogenic derivatives of strain MG1655, defective for glgA, csrA, or both, were compared. Plasmids pOP245, pJF02, or pOP12 carry glgA, glgP, or asd-glgBXCAP′ (deleted for most of glgP), respectively. (A) Effects of a polar glgA mutation. The strain identities for bars 1 to 7 were MG1655, glgA mutant, glgA mutant containing pOP245, pJF02, or both plasmids, the csrA mutant, and the csrA glgA double mutant, respectively. (B) Overexpression of glycogen biosynthetic or catabolic genes. Lanes 1 to 5 show MG1655 containing either no plasmid, pOP12, pBR322 (vector control), pJF02, or pUC19 (vector control), respectively. Each bar shows the averages and standard errors of three separate experiments. The asterisks denote significant differences with respect to the parent strain (P < 0.0001).
Because a csrA mutant overexpresses the glycogen biosynthetic genes as well as the gene encoding the catabolic enzyme glycogen phosphorylase (50), the relative significance of each of these metabolic pathways was examined. Figure 5B demonstrates that ectopic expression of either the glycogen biosynthetic genes or glgP in a csrA wild-type strain significantly enhanced biofilm formation. This experiment provided genetic evidence that an increase in glycogen biosynthesis or its subsequent catabolism improves biofilm formation and that both of these effects of CsrA are relevant. Based on these findings, we propose that intracellular glycogen, which is synthesized in the early stationary phase of growth and subsequently metabolized, serves as a carbon and/or energy source for the formation of one or more adhesins or other factors in the stationary phase of growth which are required for biofilm formation.
Biofilm dispersal.
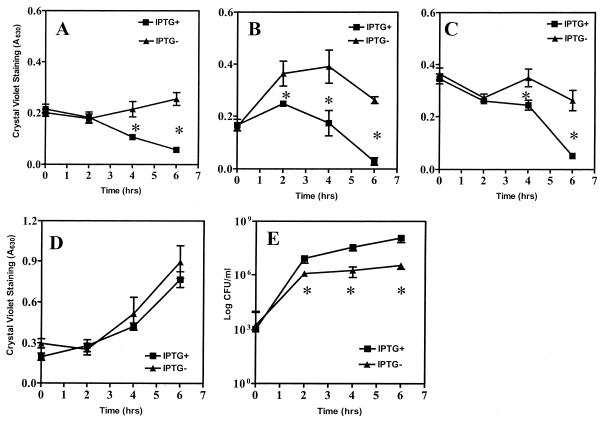
Biofilm can serve as a nidus of infection from which planktonic cells can spread or disperse to other sites in the body (14). To examine the potential role of CsrA in biofilm dispersal, a strain was constructed in which csrA expression could be induced with IPTG (isopropyl-β-d-thiogalactopyranoside), TR1-5JM101[pCSRH6-19]. The csrA-inducible phenotype of this strain was confirmed by the observation that glycogen accumulation is inhibited by the addition of IPTG to the growth medium (data not shown). This strain was allowed to grow for 24 h in CFA medium in borosilicate tubes in the absence of IPTG. Then IPTG (1 mM final concentration) or sterile water was added directly to the culture medium, along with ampicillin to maintain plasmid selection, and biofilm was monitored in quadruplicate cultures by the crystal violet assay. Induction of csrA resulted in the release of the biofilm over the following 4 to 6 h (Fig. 6A). Because nutrient limitation, metabolite accumulation, or other conditions in the spent medium might have affected the results, the dispersal experiment was repeated by removing the spent medium from a 24-h biofilm and gently rinsing it with and incubating it further in fresh sterile CFA medium containing ampicillin with or without IPTG. Again, csrA induction caused the biofilm to disperse (Fig. 6B). Next a 24-h biofilm was rinsed and the spent medium was replaced with M63 salts solution containing ampicillin with or without IPTG. This medium lacked a carbon and/or energy source for growth. In this experiment csrA induction also caused biofilm to disperse over the following few hours (Fig. 6C). Microscopic examination of cells released from the biofilms revealed that a small proportion of cells had become motile in the fresh CFA medium but that cells in spent medium or M63 salts were uniformly nonmotile (data not shown). Control experiments showed that IPTG did not affect biofilm formation or dispersal in strains lacking the inducible csrA gene (data not shown). Furthermore, the growth curves of the csrA-inducible strain were similar in the presence or absence of induction (data not shown). These experiments, conducted under three different physiological conditions (Fig. 6A to C), indicated that csrA expression can serve as a general signal for biofilm dispersal in E. coli. Conversely, when 0.2% glucose and ampicillin with or without IPTG were added directly to the spent medium, the preformed biofilm increased substantially in both the induced and uninduced cultures (Fig. 6D), suggesting that this nutrient can override the regulatory effect of CsrA on biofilm dispersal. A final experiment was conducted to monitor the planktonic cells that were released in response to csrA induction. Using the conditions given in the legend to Fig. 6C, cells released from the biofilm following induction were serially diluted and plated onto Kornberg medium (Fig. 6E). Because the induction step of this experiment was conducted in M63 salts, little or no cell division should have occurred during the experiment. Plating efficiency at 4 to 6 h was ∼1 log10 greater from the IPTG-induced culture versus that of the control, in relatively good agreement with data from the crystal violet assay. This experiment established that csrA induction releases viable planktonic cells from the biofilm rather than dispersing the biofilm by causing cell death or lysis.
FIG. 6.
Dispersal of bacteria from preformed biofilm by csrA induction. A strain containing an IPTG-inducible csrA gene, TRJM101[pCSRH6-19], was allowed to form a 24-h biofilm, whereafter IPTG (1 mM final concentration) or sterile water (containing ampicillin in each case) was added directly to the medium (A), the medium was discarded and fresh CFA containing ampicillin with or without IPTG was added (B), the medium was replaced with a M63 salts solution containing ampicillin with or without IPTG (C), or 0.2% glucose and ampicillin with or without IPTG was added directly to the spent media (D). Biofilm remaining at the indicated times following csrA induction is shown. Each value represents an average of at least two independent experiments, with quadruplicate samples, and asterisks denote significant differences between induced and uninduced cultures (P < 0.0001). (E) The medium at 24 h was replaced with M63 salts solution containing ampicillin with or without IPTG (as for panel C), and planktonic cells that were released were recovered without disturbing the biofilm, were serially diluted, and were plated onto Kornberg medium. Plating efficiency (CFU/milliliter) was determined from two separate experiments, each including 3 countable plates (>30 but <300 colonies) for each time point. Each of the data points depicts the averages and standard errors of the two experiments; asterisks denote significant differences between the induced and uninduced cultures (P < 0.0001).
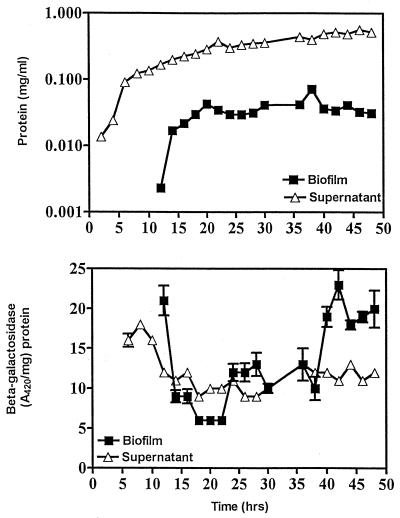
CsrA expression during biofilm development.
The above experiments revealed that CsrA plays an important regulatory role in E. coli biofilm formation. To assess whether csrA expression is modulated during biofilm development, a chromosomal csrA′-′lacZ translational fusion was constructed and β-galactosidase activity encoded by this fusion was monitored in planktonic and sessile cells. The results depict data from triplicate cultures, each assayed in duplicate reactions (Fig. 7). β-Galactosidase activity in planktonic cells declined immediately prior to the appearance of the biofilm and for the following few hours, whereafter it remained relatively constant. In the biofilm a sharp decline in activity was observed during the first few hours of growth such that activity was ultimately lower than that in the unattached cells. As biofilm growth ceased β-galactosidase activity increased moderately, and after 1.5 to 2 days of incubation it was consistently observed to increase to prebiofilm levels. Thus, csrA expression is dynamically regulated during the course of biofilm development.
FIG. 7.
Expression of a csrA′-′lacZ translational fusion in biofilm and planktonic cells of strain KSA712. Closed squares and open triangles represent determinations conducted on biofilm and planktonic cells, respectively. Average values (± standard errors), as determined from three cultures assayed with duplicate samples, are shown. Error bars are not visible where the standard error was less than the area occupied by a given symbol.
DISCUSSION
Biofilm formation is a complex developmental process, and studies of its regulation have begun to reveal a variety of influences. The present investigation demonstrates that the RNA binding protein CsrA has a dramatic effect on E. coli biofilm formation under a variety of growth conditions. A csrA mutant of E. coli K-12 produces a biofilm that differentiates into a complex multicellular structure. To our knowledge CsrA is the only repressor of biofilm formation to have been reported thus far. CsrA effects on biofilm formation are quantitatively greater than those of either of the regulatory factors that have been previously studied in E. coli, RpoS, or OmpR (1, 46). Nevertheless, biofilm formation is but one of the many physiological properties that are regulated by CsrA or its homologs in gram-negative bacteria (reviewed in reference 38; also see references 4, 8, and 16). As predicted by previous findings, which revealed that CsrB RNA antagonizes CsrA activity (22, 27), CsrB was found to activate biofilm formation.
Ectopic expression studies provided evidence that CsrA also represses biofilm formation in pathogenic relatives of E. coli K-12, notably in uropathogenic strains of E. coli and C. freundii that were isolated from colonized urinary catheters. It is conceivable that elevated intracellular levels of CsrA could permit this protein to interact with nontarget RNA molecules. Thus, we acknowledge that in the absence of other data, overexpression studies should be interpreted with caution. However, the experiments with the csrA knockout mutants of E. coli K-12 clearly establish a role for CsrA in biofilm formation in this nonpathogenic bacterium and increase our confidence in the results of csrA overexpression analyses in the related pathogens.
Biofilm formation was stimulated by csrA disruption even in the absence of one or more of the extracellular or surface factors that are known to participate in this process. This finding allows that CsrA may regulate, directly or indirectly, the production of another factor(s). However, it does not exclude a role for CsrA in the production of these same structural elements. On the contrary, CsrA stabilizes the flhDC message, which is essential for motility under various growth conditions (48). Interestingly, a csrA mutant is motile in CFA medium, which was primarily utilized in the present studies, due to the relatively high level of csrA-independent expression of flhDC in this medium (48). CsrA also influences the expression of genes involved in the synthesis of both curli and type I pili under certain conditions (manuscript in preparation). Thus, CsrA may affect a variety of properties that influence biofilm formation.
This study provides compelling genetic evidence that the primary effect of CsrA on biofilm formation in E. coli is through its regulatory role in the metabolism of glycogen. In a csrA mutant, a glgA (glycogen synthase) disruption was the most drastic down mutation that was examined in these studies. In addition, this was the only mutation that resulted in essentially identical amounts of biofilm being formed in csrA wild-type and mutant strains. Complementation studies further established the requirement for glycogen phosphorylase (GlgP), indicating that glycogen must be catabolized to promote biofilm formation. Previous studies have established that CsrA coordinately regulates both glycogen synthesis and catabolism (50). Thus, a primary role of CsrA in biofilm formation is to influence the flux of carbon into glycogen and the subsequent conversion of glycogen into glucose-1-phosphate by glycogen phosphorylase. The mechanism by which glycogen affects biofilm formation remains to be elucidated. Because both synthesis and turnover are required, we propose that glycogen serves as a carbon and energy source for the stationary-phase biosynthesis of one or more essential adhesins or other factors. Glycogen mutants are fully motile (data not shown), indicating that the loss of motility does not account for the effect of glycogen on biofilm formation. During the course of this study Bonafonte et al. reported that glycogen levels are positively correlated with biofilm formation in Salmonella enterica serovar Enteritidis (10). Thus, it is likely that intracellular glycogen plays an important role in biofilm formation in the Enterobacteriaceae.
Based on our results, the participation of additional regulators of carbon metabolism in biofilm formation should be examined in other eubacteria. This would help to determine whether the redirection of central carbon flux, through glycogen or perhaps other energy storage compounds, represents a general principle of bacterial biofilm formation. Perhaps the requirement for Crc in P. aeruginosa biofilm formation rests not only on its role in activating type IV pilus formation (33) but also on its influence on central carbon flux. Interestingly, the glycogen-like intracellular polymer of Streptococcus mutans has long been recognized as a virulence factor for the formation of dental caries (21). This has been assumed to result from the prolonged secretion of organic acids generated from the metabolism of this polymer. However, the possibility that it provides an endogenous carbon and energy source for the synthesis of the extracellular glucans, which are required for surface attachment and oral biofilm formation, should now be considered.
The induction of csrA within a preformed biofilm caused its dispersal under a variety of conditions. To our knowledge this is the first such demonstration of extensive biofilm dispersal in response to a regulatory gene or signal in any species. It has not escaped our attention that the ability to induce biofilm dispersal by csrA induction offers a useful approach for investigating the genetic and biochemical mechanisms of dispersal. We emphasize that there was no reason to suppose a priori that CsrA should affect both biofilm formation and dispersal, since the synthesis and the degradation or inactivation of a structure(s) that stabilize the biofilm should be accomplished by distinct mechanisms and thus require the expression of distinct sets of genes. Because CsrA affects both of these processes, compounds that mimic CsrA activity or increase csrA expression might both inhibit biofilm formation and also disperse antibiotic-sensitive planktonic cells from preexisting biofilm, thereby offering potential therapeutic applications. The concept of identifying compounds that modify gene expression in order to attenuate bacterial virulence mechanisms has been considered previously (reviewed in reference 6). A CsrA mimic should not be expected to affect the metabolism of eucaryotic hosts, which lack apparent csrA homologs. It was interesting to find that the addition of glucose during induction apparently blocked biofilm dispersal by CsrA. This indicates that nutritional cues may play a critical role in biofilm dispersal, as they do in biofilm formation (9, 35).
Although csrA disruption and overexpression studies demonstrated that CsrA has a powerful influence on biofilm development, they did not provide insight into the means by which the cell modulates this potential of CsrA. The finding that the expression of a csrA′-′lacZ translational fusion was dynamically regulated during the course of biofilm formation suggests that the intracellular availability of the CsrA protein itself may be a key feature of these processes. Thus, CsrA levels may provide a switch to direct biofilm formation and/or dispersal. Perhaps IPTG induction of csrA expression (Fig. 6) represents an extreme example of a typically more subtle process that signals the release of cells from a biofilm. We did not observe a statistically significant release of biofilm during the elevation in csrA′-′lacZ expression at ∼1.5 to 2 days of growth. Nevertheless, it is possible that this increase in csrA expression predisposes the biofilm to disperse in response to other factors or has subtle effects on the equilibrium between planktonic and sessile cells. Finally, a biofilm is not a uniform microenvironment. Therefore, the examination of csrA expression within individual cells of a developing biofilm may provide further insight into the role of CsrA in biofilm formation and dispersal.
Acknowledgments
We thank I. Blomfield, M. Cashel, J. W. Foster, M. Hammar, J. R. Johnson, M. Manson, and V. Stout for providing strains and M. Inouye for plasmid pJF02. We also thank Julian Borejdo for help with confocal microscopy.
This work was supported by a grant from the National Science Foundation (MCB-9726197).
REFERENCES
- 1.Adams, J. L., and R. J. McLean. 1999. Impact of rpoS deletion on Escherichia coli biofilms. Appl. Environ. Microbiol. 65:4285–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, D. G., B. Ruiz, C. San Jose, A. Jaspe, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179–184. [DOI] [PubMed] [Google Scholar]
- 3.Allison, D. G., D. J. Evans, M. R. Brown, and P. Gilbert. 1990. Possible involvement of the division cycle in dispersal of Escherichia coli from biofilms. J. Bacteriol. 172:1667–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altier, C., M. Suyemoto, and S. D. Lawhon. 2000. Regulation of Salmonella enterica serovar Typhimurium invasion genes by csrA. Infect. Immun. 68:6790–6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Applegate, D. H., and J. D. Bryers. 1991. Effects of carbon and oxygen limitations and calcium concentrations on biofilm removal processes. Biotech. Bioeng. 37:17–25. [DOI] [PubMed] [Google Scholar]
- 6.Barrett, J. F., and J. A. Hoch. 1998. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baty, A. M., III, C. C. Eastburn, S. Techkarnjanaruk, A. E. Goodman, and G. G. Geesey. 2000. Spatial and temporal variation in chitinolytic gene expression and bacterial biomass production during chitin degradation. Appl. Environ. Microbiol. 66:3574–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073–14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollinger, N., D. J. Hassett, B. H. Iglewski, J. W. Costerton, and T. R. McDerrmott. 2001. Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J. Bacteriol. 183:1990–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonafonte, M. A., C. Solano, B. Sesma, M. Alvarez, L. Montuenga, D. Garcia-Ros, and C. Gamazo. 2000. The relationship between glycogen synthesis, biofilm formation and virulence in Salmonella enteritidis. FEMS Microbiol. Lett. 191:31–36. [DOI] [PubMed] [Google Scholar]
- 11.Boyd, D., D. S. Weiss, J. C. Chen, and J. Beckwith. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd, A., and A. M. Charkrabarty. 1994. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:2355–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745. [DOI] [PubMed] [Google Scholar]
- 14.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. [DOI] [PubMed] [Google Scholar]
- 15.Cui, Y., A. Mukherjee, C. Korsi, Y. Liu, and A. K. Chatterjee. 1999. rsmC of the soft-rotting bacterium Erwinina carotovora subsp. carotovora negatively controls extracellular enzyme and harpinEcc production and virulence by modulating levels of regulatory RNA (rsmB) and RNA-binding protein (RsmA). J. Bacteriol. 181:6042–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui, Y., A. Chatterjee, Y. Liu, K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanyol)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinina spp. J. Bacteriol. 177:5108–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danese, P., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danese, P., L. A. Pratt, S. L. Dove, and R. Kolter. 2000. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol. Microbiol. 37:424–432. [DOI] [PubMed] [Google Scholar]
- 19.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295–298. [DOI] [PubMed] [Google Scholar]
- 20.Evans, D. G., D. J. Evans, Jr., and W. Tjoa. 1977. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect. Immun. 18:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons, R. J., and J. van Houte. 1975. Dental caries. Annu. Rev. Med. 26:121–136. [DOI] [PubMed] [Google Scholar]
- 22.Gudapaty, S., K. Suzuki, X. Wang, P. Babitzke, and T. Romeo. 2001. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J. Bacteriol. 183:6017–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammar, M., A. Arnquivist, Z. Bian, A. Olsén, and S. Nomark. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18:661–670. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, J. R., P. Delvari, and M. Azar. 1999. Activities of a nitrofurazone-containing urinary catheter and a silver hydrogel catheter against multidrug-resistant bacteria characteristic of catheter-associated urinary tract infection. Antimicrob. Agents Chemother. 43:2990–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar, A., C. E. Larsen, and J. Preiss. 1986. Biosynthesis of bacterial glycogen. Primary structure of Escherichia coli ADP-glucose: α-1,4-glucan, 4-glucosyltransferase as deduced from the nucleotide sequence of the glgA gene. J. Biol. Chem. 261:16256–16259. [PubMed] [Google Scholar]
- 26.Lin, J., M. P. Smith, K. C. Chapin, H. S. Baik, G. N. Bennett, and J. W. Foster. 1996. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 62:3094–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, M. Y., G. Gui, B. Wei, J. F. Preston III, L. Oakford, U. Yuksel, D. P. Geidroc, and T. Romeo. 1997. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272:17502–17510. [DOI] [PubMed] [Google Scholar]
- 28.Liu, M. Y., and T. Romeo. 1997. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J. Bacteriol. 179:4639–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, M. Y., H. Yang, and T. Romeo. 1995. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on mRNA stability. J. Bacteriol. 177:2663–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Minuth, J. N., D. M. Musher, and S. B. Thorsteinsson. 1976. Inhibition of the antibacterial activity of gentamicin by urine. J. Infect. Dis. 133:14–21. [DOI] [PubMed] [Google Scholar]
- 32.O’Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304. [DOI] [PubMed] [Google Scholar]
- 33.O’Toole, G. A., K. A. Gibbs, P. W. Hager, P. V. Phibbs, Jr., and R. Kolter. 2000. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 182:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkins, M. D., H. Ceri, D. G. Storey. 2001. Pseudomonas aeruginosa GacA, a factor in multihost virulence, is also essential for biofilm formation. Mol. Microbiol. 40:1215–1226. [DOI] [PubMed] [Google Scholar]
- 35.Pratt, L. A., and R. Kolter. 1999. Genetic analyses of bacterial biofilm formation. Curr. Opin. Microbiol. 2:598–603. [DOI] [PubMed] [Google Scholar]
- 36.Preiss, J., and T. Romeo. 1994. Molecular biology and regulatory aspects of glycogen biosynthesis in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 47:301–327. [DOI] [PubMed] [Google Scholar]
- 37.Rashid, M. H., and A. Kornberg. 2000. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid, M. H., K. Rumbaugh, L. Passador, D. G. Davies, A. N. Hamood, B. H. Iglewski, and A. Kornberg. 2000. Polyphosphate kinase is essential for biofilm development, quorum sensing and virulence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 97:9636–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321–1330. [DOI] [PubMed] [Google Scholar]
- 40.Romeo, T., M. Gong, M. Y. Liu, and A-M. Brun-Zinkernagel. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 175:4744–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romeo, T., J. Black, and J. Preiss. 1990. Genetic regulation of glycogen synthesis in Escherichia coli: in vivo effects of the catabolite repression and stringent response systems in glg gene expression. Curr. Microbiol. 21:131–137. [Google Scholar]
- 42.Romeo, T., and J. Preiss. 1989. Genetic regulation of glycogen biosynthesis in Escherichia coli: in vitro effects of cyclic AMP and guanosine 5′-diphosphate 3′-diphosphate and analysis of in vivo transcripts. J. Bacteriol. 171:2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabnis, N., H. Yang, and T. Romeo. 1995. Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA. J. Biol. Chem. 270:29096–29104. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olsen, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85. [DOI] [PubMed] [Google Scholar]
- 46.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejueune. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei, B. L., A.-M. Brun-Zinkernagel, J. W. Simecka, B. M. Prüβ, P. Babitzke, and T. Romeo. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245–256. [DOI] [PubMed] [Google Scholar]
- 49.Wei, B., S. Shin, D. LaPorte, A. J. Wolfe, and T. Romeo. 2000. Global regulatory mutations in csrA and rpoS cause severe central carbon stress in Escherichia coli in the presence of acetate. J. Bacteriol. 182:1632–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, H., M. Y. Liu, and T. Romeo. 1996. Coordinate genetic regulation of glycogen catabolism and biosynthesis in Escherichia coli via the CsrA gene product. J. Bacteriol. 178:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu, F., Y. Jen, E. Takeuchi, M. Inouye, H. Nakayama, M. Tagaya, and T. Fukui. 1988. Alpha-glucan phosphorylase from Escherichia coli: cloning of the gene, and purification and characterization of the protein. J. Biol. Chem. 263:13706–13711. [PubMed] [Google Scholar]