Abstract
VirB2 propilin is processed by the removal of a 47-amino-acid signal peptide to generate a 74-amino-acid peptide product in both Escherichia coli and Agrobacterium tumefaciens. The cleaved VirB2 protein is further cyclized to form the T pilin in A. tumefaciens but not in E. coli. Mutations in the signal peptidase cleavage sequence of VirB2 propilin cause the formation of aberrant T pilin and also severely attenuate virulence. No T pilus was observed in these mutants. The potential role of the exact VirB2 propilin cleavage and cyclization in T pilus biogenesis and virulence is discussed.
T pili are generated when Agrobacterium tumefaciens cells are induced by plant phenolic compounds such as acetosyringone. Induction leads to the expression of virulence (vir) genes located on a resident Ti plasmid (10). Of these vir genes, the 11 genes of the virB operon are involved in the synthesis and assembly of the T pilus (5). The T pilus is composed mainly of processed VirB2 protein (6), with VirB5 (12) and VirB7 (11) as associated components. Jones et al. (4) first characterized the processing steps of VirB2 that is cleaved into a 7.2-kDa product in both Escherichia coli and A. tumefaciens. Further studies by mass spectrometry (MS) demonstrated that the processed VirB2 product is composed of 74 amino acid residues linked by a peptide bond between the amino- and carboxyl-terminal residues to generate a cyclic peptide (the T pilin) (1). T pilin subunits are transported across the bacterial membranes and assembled into an exocellular, flexuous T pilus filament 10 nm in diameter protruding from the bacterial cell wall (5–7). Transport of T pilin subunits to the cell surface requires each of the 11 genes of the virB operon (5), while propilin cleavage can occur independently of any other gene of the Ti plasmid (1). The 47-residue signal peptide is cleaved off amino terminally from VirB2 propilin, most likely by a general signal peptidase I, LepB (1, 4, 13). Whether peptidyl cyclization of the resulting 74-residue peptide takes place simultaneously or is an individual reaction remains unclear.
In the present study, we examined the propilin cleavage and cyclization reactions that lead to T pilin formation in E. coli and A. tumefaciens and addressed the roles of these reactions in T pilus biogenesis and tumorigenesis.
T pilin is cyclized in A. tumefaciens but not in E. coli.
Based on immunoblotting and pulse-chase analyses, it was demonstrated that VirB2 propilin is cleaved in A. tumefaciens, as well as in E. coli (4). To determine whether the processed VirB2 protein is cyclized in E. coli like that in A. tumefaciens, we expressed virB2 under tac promoter control in both E. coli and A. tumefaciens as previously described (1). VirB2 was induced by isopropyl-β-d-thiogalactopyranoside (IPTG) in E. coli and A. tumefaciens (in the absence of the Ti plasmid), and cells were analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) MS and by immunoblotting with anti-VirB2 serum as previously described (1, 6). Briefly, 0.3 μl of either cell suspension or pilus preparation was cocrystallized with trans-3-indolylacrylic acid and analyzed with a Bruker Reflex-II time-of-flight spectrometer (Bruker-Daltonik). Processed VirB2 was detected at m/z 7,202.2 when expressed in E. coli but found at m/z 7,184.3 when expressed in A. tumefaciens (Fig. 1A). The difference in mass of 18 Da is explained by the loss of one molecule of H2O, caused by the formation of a new peptide bond (head-to-tail linkage) in the processed VirB2 protein, yielding the cyclized T pilus subunit (1). The results suggest that VirB2 is cleaved by a general signal peptidase in both E. coli and A. tumefaciens but that the product is only cyclized in A. tumefaciens. As cleavage and cyclization take place in the absence of the Ti plasmid, we concluded that Ti plasmid genes other than virB2 are dispensable for VirB2 propilin processing. Interestingly, the linear and cyclic molecules also show different mobilities when fractionated by Tricine-sodium dodecyl sulfate (SDS)-16.5% polyacrylamide gel electrophoresis (PAGE). As shown in Fig. 1B, the linear form of cleaved VirB2 (m/z 7,202.2) has a mobility slower than that of T pilin (m/z 7,184.3). A mass difference of 18 Da cannot be resolved by SDS-PAGE; the difference in mobility is likely due to a difference in peptide conformation between the linear and circularized forms of the molecules.
FIG. 1.
Cyclization of T pilin occurs in A. tumefaciens but not in E. coli. (A) MALDI-TOF MS data derived from sample preparations with whole cells of E. coli DH5α expressing VirB2 (pUCD4805) versus A. tumefaciens NT1REB expressing VirB2 (pUCD4813) in the absence of its Ti plasmid (1). Purified T pili and vectors served as controls. The signal at m/z 7,202.2 corresponds to linear processed VirB2, while the signal at m/z 7,184.3 corresponds to T pilin (indicated by arrows). (B) Immunoblot of linear VirB2 and T pilin. Whole-cell lysates from E. coli DH5α containing either pTTQ18 (vector) or pUCD4805 (VirB2) or from A. tumefaciens NT1REB containing either pUCD4813 (VirB2, no Ti) or pTiC58 were fractionated by Tricine-SDS-16.5% PAGE and analyzed by immunoblotting using anti-VirB2 antibody. Molecular masses of protein size markers (in kilodaltons) are indicated on the left. Positions of linear processed VirB2 and T pilin are marked by arrows.
For the conjugative R pilus of IncP plasmids, we have shown previously that propilin processing includes triple truncation and a cyclization step leading to the production of R pilus subunits in E. coli (1). Thereby, TraF, a plasmid-encoded protein, catalyzes the last step in this maturation cascade (3), acting as a leader peptidase-like protease in a catalytic dyad-like mechanism (2). In contrast, T pilin cyclization is independent from other Ti plasmid genes. The fact that VirB2 is processed in both A. tumefaciens and E. coli but is only cyclized in A. tumefaciens suggests that peptide cyclization might be catalyzed by a second enzyme that is present in A. tumefaciens but not in E. coli. However, the lack of a linear processed T pilin signal (m/z 7,202.2) in A. tumefaciens implies either that the reactions of the signal peptidase cleavage and peptide bond formation occur by two efficient, almost simultaneous steps or, alternatively, that these two reactions are catalyzed by the very same enzyme. We have examined the recently completed A. tumefaciens genome sequences by performing a BLAST search using the E. coli LepB (signal peptidase I) protein sequence as a string and found one copy of LepB encoded by the A. tumefaciens circular chromosome (accession number AAK86842). No additional TraF homolog was found on either the chromosomes or the cryptic plasmid other than the known TraF protein (accession number AAK91092) encoded by the Ti plasmid. The potential LepB homolog of chromosomal origin is a good candidate for VirB2 processing and cyclization. Alternatively, a second enzyme that has no sequence homology to LepB or TraF might be required for cyclization of the processed VirB2 protein into T pilin in A. tumefaciens.
Requirement of the signal peptidase recognition sequence for T pilin synthesis.
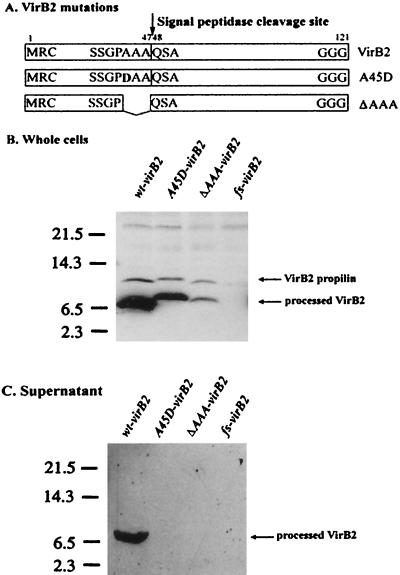
A signal peptidase cleavage within VirB2 was predicted to occur between Ala-47 (A47) and Gln-48 (Q48) (13) (Fig. 2A). When two virB2 mutants, one coding for an amino acid substitution (A45D) and the other coding for a three-amino-acid deletion (A45 to A47, ΔAAA) in the signal peptidase cleavage sequence, were induced and tested for virulence in a previous study, both mutants were avirulent and neither VirB2 propilin nor processed VirB2 was detected (4). We have now examined the effects of these mutations in A. tumefaciens strains grown under optimal T pilus induction conditions at 19°C (6) instead of at 28°C (4). After induction with acetosyringone in I medium at 19°C for 72 h (6), we were able to detect VirB2 products from both the A45D mutant and ΔAAA deletion mutants (Fig. 2B). No VirB2 signals were detected from a frameshift mutant used as a negative control. This frameshift mutant was created by PCR to generate a 1-bp deletion at nucleotide 119 of the virB2 open reading frame (TTT instead of TTTT) and introduced into Ti plasmid pJK270 by markerless gene replacement performed as previously described (4). Although mutant A45D and ΔAAA VirB2 proteins were produced and processed, they accumulated at lower levels than the wild-type protein, displaying electrophoretic mobilities slower than that of T pilin. In addition to processed VirB2, we were also able to detect the propilin although the propilins were not consistently detectable due to the quick turnover of the precursor. This result coincides with the previous observation that both A45D and ΔAAA mutants are also processed when they are expressed in E. coli (4). Although the VirB2 mutant proteins were processed, they appeared not to be exported out of A. tumefaciens cells, as judged by the absence of VirB2 in concentrated cell supernatants (containing the secreted and surface proteins) obtained by trichloroacetic acid precipitation performed as previously described (5) (Fig. 2C). Furthermore, T pili were not detected by electron microscopy of these mutants (data not shown). These data suggest that the VirB2 signal peptidase cleavage sequence plays a role important for correct processing to produce T pilin subunits for their export and assembly into T pili.
FIG. 2.
Processing and exportation analyses of VirB2 mutant proteins in A. tumefaciens. (A) Mutations in the signal peptidase cleavage sequences of VirB2. Proteins are drawn as boxes, the first and last amino acid residues are numbered, and the signal peptidase cleavage site A47-Q48 is indicated. The A45D mutant carries a substitution mutation that replaces Ala-45 (A). with Asp (D) (in bold), and the ΔAAA mutation is a deletion of three Ala residues at positions 45 to 47. (B and C) Strain NT1RE containing either pJK270 (wild type; wt-virB2), pUCD4605 (A45D-virB2), pUCD4606 (ΔAAA-virB2), or pUCD4607 (frameshift mutant, fs-virB2) was induced with acetosyringone in I medium at 19°C for 72 h (6). Proteins from whole-cell lysates (B) and from their concentrated supernatants (C) were fractionated by Tricine-SDS-16.5% PAGE and immunoblotted with anti-VirB2 antibody. Molecular masses of protein markers are shown on the left side of each immunoblot. Unprocessed and processed VirB2 proteins are indicated.
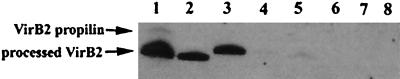
To determine the localization of mutant VirB2, total membrane fractions were isolated (13) and treated with 25 mM HEPES buffer (pH 7.6) containing 2% Sarkosyl (lauryl sarcosinate) on ice for 30 min and centrifuged at 40,000 × g and 4°C for 30 min (8, 14). The inner membrane proteins were present in the supernatant, and the outer membrane proteins were sedimented in the pellet. By immunoblotting analysis with anti-VirB2 antibody, A45D mutant proteins accumulated mainly in the inner membrane fraction (Sarkosyl soluble), with trace amounts appearing in the outer membrane fraction (Sarkosyl insoluble), and AAA mutant proteins were detected only in the inner membrane fraction (Fig. 3). Wild-type T pilin molecules associated with both the inner and outer membrane fractions and appeared to be exocellularly assembled into the T pilus filament.
FIG. 3.
Localization of mutant and wild-type VirB2 proteins in A. tumefaciens NT1RE. Strain NT1RE contained either pJK270 (wild-type virB2) (lanes 1 and 2), pUCD4605 (A45D-virB2) (lanes 3 and 4), pUCD4606 (ΔAAA-virB2) (lanes 5 and 6), or pUCD4607 (frameshift mutation virB2) (lanes 7 and 8). Isolated membrane vesicles from each strain were treated with 2% Sarkosyl to separate inner membrane (lanes 1, 3, 5, and 7)- and outer membrane (lanes 2, 4, 6, and 8)-associated proteins. Proteins were fractionated by Tricine-SDS-16.5% PAGE and analyzed by immunoblotting with anti-VirB2 antibody. Unprocessed and processed VirB2 proteins are indicated.
Intact A. tumefaciens NT1RE cells producing either wild-type or mutant VirB2 were induced with acetosyringone in I medium at 19°C for 72 h and analyzed by MALDI-TOF MS for detection of VirB2 products formed on the bacterial surface as described previously (1). Whereas a signal at m/z 7,184.3 (the m/z of T pilin) was detected from the wild-type strain, no signal matching the theoretical mass of the cleaved A45D or ΔAAA mutant protein was obtained (data not shown), suggesting that little mutant VirB2 is on the bacterial surface.
Virulence is severely affected by alterations in the signal peptide cleavage sequence.
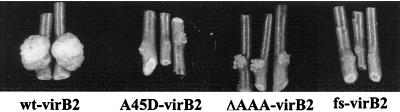
To determine the effects of mutations at the signal peptidase cleavage sequences of VirB2 on virulence, the tumor-inducing activity of wild-type and mutant virB2 strains was assessed by inoculation of Jimson weed (Datura stramonium) seedlings as previously described (4). Three independent assays were carried out with each strain by using three plant replicates. Tumors were induced by wild-type A. tumefaciens NT1RE(pJK270) but not by the mutant strains at 2 weeks postinoculation. However, tiny tumors (1 to 2 mm in diameter) were induced by both A45D and ΔAAA mutants when tumorigenesis was assessed at 4 to 5 weeks postinoculation (Fig. 4) The frameshift mutation completely inhibited tumor formation. The tiny-tumor formation might be caused by a reversion mutation of A45D or ΔAAA within a large population of avirulent mutant cells. The frameshift mutant consistently failed to produce tumors. Similar results were obtained in independent tests using a root disk assay performed with root tissue derived from Pastinaca sativa (parsnip) in accordance with published protocols (data not shown) (9).
FIG. 4.
Tumorigenicity assay on D. stramonium. Representative sections of the stem of each test plant inoculated with the indicated strain are shown at 4 to 5 weeks postinoculation. The inoculated strains of A. tumefaciens NT1RE contained either pJK270 (wild-type virB2 [wt-virB2]), pUCD4605 (A45D-virB2), pUCD4606 (ΔAAA-virB2), or pUCD4607 (virB2 frameshift mutant [fs-virB2]).
The signal peptidase cleavage sequence of VirB2 appears to play a critical role during virulence. Mutations near or at the signal peptide cleavage site (A47-Q48) result in severe attenuation of virulence. The avirulence of the frameshift mutant underlines the essential function of VirB2 in virulence. Within strains bearing either the A45D or the ΔAAA mutation, VirB2 processing still occurs but the processed product is degraded and not exported; thus, the biogenesis of the T pilus is hampered. Interestingly, the mutations did not completely abolish virulence since very tiny tumors arose at 4 to 5 weeks postinoculation, thus raising the question of the role of the T pilus during tumorigenesis. It is possible that a vestigial and partially functional pilus is formed only in planta and not on induction media. However, intriguingly, a nonpolar virB1 mutant also reveals a phenotype similar to that of the A45D and ΔAAA mutants. We have previously shown that the nonpolar virB1 mutant, which only causes the attenuation on tumorigenesis, is unable to export T pilin and assemble a T pilus (5). In conclusion, it is certain that VirB2 is the indispensable component of the VirB channel, which is essential for transport of the T DNA-protein complex from A. tumefaciens into the plant cell. However, the T pilus may not be required for T-DNA transfer but definitely plays an important role in sufficient T DNA-protein complex transport through the VirB channel or the T pilus is important but not essential for intimate contact (attachment) between the Agrobacterium cell and the host plant cell for T DNA transfer.
Acknowledgments
This work was supported in part by an NIH grant and a grant from Avenir Genetic Technologies, Inc., awarded to C.I.K. and by a grant from the Deutsche Forschungsgemeinschaft awarded to E.L.
REFERENCES
- 1.Eisenbrandt, R., M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado, and E. Lanka. 1999. Conjugative pili of IncP plasmids and the Ti plasmid T pilus are composed of cyclic subunits. J. Biol. Chem. 274:22548–22555. [DOI] [PubMed] [Google Scholar]
- 2.Eisenbrandt, R., M. Kalkum, R. Lurz, and E. Lanka. 2000. Maturation of IncP pilin precursors resembles the catalytic dyad-like mechanism of leader peptidases. J. Bacteriol. 182:6751–6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haase, J., and E. Lanka. 1997. A specific protease encoded by the conjugative DNA transfer systems of IncP and Ti plasmids is essential for pilus synthesis. J. Bacteriol. 179:5728–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones, A. L., E. M. Lai, K. Shirasu, and C. I. Kado. 1996. VirB2 is a processed pilin-like protein encoded by the Agrobacterium tumefaciens Ti plasmid. J. Bacteriol. 178:5706–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai, E. M., O. Chesnokova, L. M. Banta, and C. I. Kado. 2000. Genetic and environmental factors affecting T pilin export and T pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 182:3705–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai, E. M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 180:2711–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai, E. M., and C. I. Kado. 2000. The T pilus of Agrobacterium tumefaciens. Trends Microbiol. 8:361–369. [DOI] [PubMed] [Google Scholar]
- 8.Lamber, P. A. 1988. Isolation and purification of outer membrane proteins from Gram-negative bacteria, p.110–121. In I. Hancock and I. Poxton (ed.), Bacterial cell surface techniques. John Wiley & Sons, Inc., New York, N.Y.
- 9.Pueppke, S. G., and U. K. Benny. 1984. Adsorption of tumorigenic Agrobacterium tumefaciens cells to susceptible potato tuber tissues. Can. J. Microbiol. 30:1030–1037. [DOI] [PubMed] [Google Scholar]
- 10.Rogowsky, P. M., T. J. Close, J. A. Chimera, J. J. Shaw, and C. I. Kado. 1987. Regulation of the vir genes of Agrobacterium tumefaciens plasmid pTiC58. J. Bacteriol. 169:5101–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagulenko, V., E. Sagulenko, S. Jakubowski, E. Spudich, and P. J. Christie. 2001. VirB7 lipoprotein is exocellular and associates with the Agrobacterium tumefaciens T pilus. J. Bacteriol. 183:3642–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt-Eisenlohr, H., N. Domke, C. Angerer, G. Wanner, P. C. Zambryski, and C. Baron. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 181:7485–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirasu, K., and C. I. Kado. 1993. Membrane location of the Ti plasmid VirB proteins involved in the biosynthesis of a pilin-like conjugative structure on Agrobacterium tumefaciens. FEMS Microbiol. Lett. 111:287–294. [DOI] [PubMed] [Google Scholar]
- 14.Thorstenson, Y. R., G. A. Kuldau, and P. C. Zambryski. 1993. Subcellular localization of seven VirB proteins of Agrobacterium tumefaciens: implications for the formation of a T-DNA transport structure. J. Bacteriol. 175:5233–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]