Abstract
The gram-positive plant pathogen Rhodococcus fascians provokes leafy gall formation on a wide range of plants through secretion of signal molecules that interfere with the hormone balance of the host. Crucial virulence genes are located on a linear plasmid, and their expression is tightly controlled. A mutant with a mutation in a chromosomal locus that affected virulence was isolated. The mutation was located in gene vicA, which encodes a malate synthase and is functional in the glyoxylate shunt of the Krebs cycle. VicA is required for efficient in planta growth in symptomatic, but not in normal, plant tissue, indicating that the metabolic requirement of the bacteria or the nutritional environment in plants or both change during the interaction. We propose that induced hyperplasia on plants represents specific niches for the causative organisms as a result of physiological alterations in the symptomatic tissue. Hence, such interaction could be referred to as metabolic habitat modification.
The outcome of a plant-pathogen interaction results from the complex action of a whole set of genes, from both host and pathogen. The tools used by pathogens to provoke plant diseases are often well characterized, and a wide array of pathogenic approaches has been described, ranging from destructive to subtle strategies. The most destructive interactions are often lethal for the plant and result from the bacterial secretion of extracellular cell wall-degrading enzymes that literally digest the plant (for instance, soft rots by Pantoea species [1]) or from the obstruction of transport tissues leading to wilting (such as for Ralstonia solanacearum [23], Pantoea stewartii [28], and Corynebacterium species [29, 46]). A more subtle approach consists of disrupting the plant's hormone balance, resulting in the formation of hyperplasia without killing the host (such as crown galls by Agrobacterium tumefaciens [26] and galls by Pantoea agglomerans pv. gypsophilae [30] and Pseudomonas savastanoi pv. savastanoi [58]).
The most obvious reason why bacteria seek interaction with plants is nutrition. For the necrogenic pathogens, this nutritional benefit is clear. On the other hand, A. tumefaciens transfers a discrete piece of its tumor-inducing (Ti) plasmid, the T- DNA, which carries genes responsible for hormone and opine synthesis, to the plant nucleus (47). Opines are novel compounds whose structure depends on the bacterial strain that induces the crown gall. Only this strain carries the catabolic genes specific for that type of opine on its Ti plasmid (14). Hence, the bacterium that invests its energy into genetically transforming its host is rewarded in terms of specific nutritional compounds. However, for other hyperplasia-inducing bacteria such a benefit is not evident.
Rhodococcus fascians (20) belongs to the latter class of pathogens. It is a gram-positive actinomycete with a very broad host range, encompassing both monocots and dicots (3, 53). It is a well-adapted epiphyte and grows on the surface of the plant as well as inside tissues (6). Infection of decapitated plants leads to the formation of leafy galls accompanied with the loss of apical dominance, whereas infection of seedlings strongly inhibits growth, both of the roots and the foliage, and causes a thickening of the hypocotyl. To exert these effects on plants, R. fascians strain D188 requires the action of several genes located on the conjugative linear plasmid pFiD188 (7). At least three loci on pFiD188 determine a balanced virulence. The fas locus contains six genes that encode an isopentenyltransferase (IPT) and enzymes involved in the modification of the IPT product. Together they form the machinery for the synthesis of a signal molecule that is essential for symptom development (7, 8, 48, 49). The expression of the fas genes is controlled through a complex network encompassing both transcriptional and translational regulation (50). The other two loci, att and hyp, determine the severity of the symptoms, and mutations lead to attenuated virulence and hypervirulence, respectively (7). Whereas the actual role of the hyp locus in virulence remains to be determined, the att locus is a key factor in cell-cell signaling and hence in optimal virulence gene expression (31).
The strategy used by R. fascians to provoke leafy gall formation on its hosts is steadily being elucidated, but the selective advantage for the bacteria interacting with the plant remains unclear. Here, we report on the characterization of a chromosomal mutation that leads to a severe attenuation of virulence. We provide evidence why this mutant cannot induce wild-type leafy galls and give clues regarding the force driving R. fascians to interact with plants.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of extracts
The bacterial strains used are listed in Table 1. Escherichia coli was grown in Luria-Bertani broth at 37°C and was used for cloning and amplification of plasmids. R. fascians strains were grown in yeast extract broth (YEB) at 28°C. When necessary, the following antibiotics were added: carbenicillin (200 μg/ml), chloramphenicol (25 μg/ml), and phleomycin (1 μg/ml).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Marker(s)a | Description | Reference or source |
|---|---|---|---|
| E. coli | |||
| MC1061 | Δara-139 Δ(ara leu)7697 ΔlacX74 galU galK hsrR hsrM+rpsL+ StrA | 4 | |
| R. fascians | |||
| D188 | Wild type, virulent, Cdr | 11 | |
| D188-5 | Plasmid free, nonvirulent, Strr | 11 | |
| X798 | pDPX insertion mutant, diminished virulence, Cmr | This work | |
| Plasmids | |||
| pRF37 | Apr Phr | Bifunctional cloning vector | 12 |
| pDPX | Apr Cmr | Integrating promoter probe vector with xylE as reporter (Fig. 1) | This work |
| pUCDV5 | Apr | Plasmid containing pDPX flanking sequences rescued from total DNA from strain X798 with BglII | This work |
| pUCDV6 | Apr | pUC18 linearized with BamHI with a 5.2-kb BglII fragment from cosmid pJGV13807 | This work |
| pUCDV7 | Apr | pUC18 with a translational fusion of the vicA promoter (1.7-kb SphI) to uidA (2.5-kb SphI/XbaI) from pGUS1 as a 4.2-kb HindIII/XbaI fragment | 41; this work |
| pRFDV8 | Apr Phr | pRF37 with a 4.4-kb BglII/SphI fragment from pUCDV6 | This work |
| pJGV13807 | Apr | Cosmid containing vicA, selected from a partial Sau3A-generated library of total DNA of D188 in bifunctional cosmid vector pJGV9 | 10 |
Ph, phleomycin.
Growth curves on different media were determined by measuring the optical density at 600 nm (OD600) every 24 h over a period of 7 days. Control media were YEB or minimal A (0.45% K2HPO4, 0.1% KH2PO4, 0.025% Na3citrate · 2H2O, 0.01% MgSO4 · H2O, 0.01% thiamine) supplemented, when appropriate, with 0.05% (NH4)2SO4 and 0.5% glycerol, 0.4% glucose, or 0.5% acetate or with 2 mM amino acids. In the test media, the carbon source was plant or leafy gall extracts (1/20 [vol/vol]). To prepare such extracts, fresh tissue was crushed extensively with a pestle and centrifuged at 18,000 × g for 10 min in an Eppendorf centrifuge; the supernatant was collected, and the remaining tissue was crushed again. After another centrifugation, the pooled supernatant was sterilized through a Minisart filter (pore size, 0.2 μm).
pDPX and other plasmids
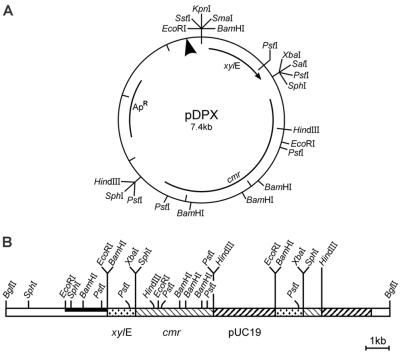
A list of the plasmids with relevant characteristics is given in Table 1. pDPX, a new integrating promoter probe vector, was constructed by cloning the xylE gene of Pseudomonas putida (59) from pYGV1 (19) as a 1.2-kb BamHI/XbaI fragment into pUC19 (39). The chloramphenicol resistance gene (cmr) from R. fascians was cloned in this intermediate as a 3.5-kb SphI fragment isolated from pRF41 (13), yielding pDPX (7.4 kb; see Fig. 1A). After this vector had been introduced into R. fascians, it was integrated illegitimately into the genome via a NarI-spanning sequence. This integration system differs from known site-specific recombination events and is probably mediated through a linearized plasmid intermediate (10). The integration generated a translational fusion between the promoterless xylE and the interrupted gene, which allowed the monitoring of both expression and functionality in virulence.
FIG. 1.
Physical maps of pDPX and pUCDV5. (A) Promoter probe pDPX. Restriction sites and the NarI-spanning illegitimate integration site (arrowhead) are given. (B) Plasmid pUCDV5. Parts of plasmid pDPX are duplicated in pUCDV5 because of a second, homologous recombination event that occurred after the first illegitimate integration; this second recombination was accompanied by an internal deletion of vector DNA. Relevant restriction sites are indicated. Open bar, R. fascians chromosomal DNA; black bar, probe. Locations of the promoterless xylE gene, cmr gene, and pUC19 vector DNA are shown.
R. fascians infection of plants
Sterile Nicotiana tabacum (L.) W38 seeds were germinated on half-strength MS medium (38) supplemented with 0.01% thiamine, 1% sucrose, and 0.8% agar. Seeds were inoculated with 20 μl of a late-logarithmic culture of R. fascians as soon as the radicles emerged. When 4 to 5 weeks old, plants were infected by applying a drop of concentrated R. fascians culture to the decapitated apical meristem. Phenotypes were scored after 3 weeks.
Expression analysis
For the qualitative analysis of xylE expression in the obtained mutants, bacteria were grown on solid medium and sprayed with 0.5 M catechol. After a few seconds an intense yellow color appeared in xylE-expressing colonies.
Quantitative expression was analyzed by measuring β-glucuronidase (GUS) activity. Cells were grown for 2 days in YEB, diluted 10-fold in YEB, collected by centrifugation after overnight growth, washed, and diluted 5-fold in JM medium (7). After test compounds had been added (40 μl of plant extract and 20 μl of gall extract per ml or 20 mM carbon sources) and the cells had been induced overnight, the bacteria were harvested, resuspended in 1 ml of GUS buffer (50 mM KPO4 [pH 7.5], 0.01% sodium dodecyl sulfate, 1 mM EDTA, 0.8% β-mercaptoethanol), and permeabilized with 30 μl of chloroform for 5 min, after which 1 mM para-nitrophenyl glucuronide was added. The reaction mixture was kept at 37°C until the yellowing was evident, and the reaction was stopped with 1 mM 2-amino-2-methyl-1,3-propanediol. The GUS activity is given as OD414 × 1,000/(OD600 × time [in minutes]).
Estimation of the glyoxylate concentration in the supernatant of R. fascians cultures
To estimate the amount of glyoxylate that accumulated in the supernatant of bacterial cultures, the formation of phenylhydrazones from produced aldo- and ketoacids and phenylhydrazine (16) was determined. In a final volume of 300 μl, the assay mixture contained 66.7 mM KPO4 (pH 6.85), 3.33 mM phenylhydrazine-HCl, 20 mM cysteine-HCl, and 160 μl of supernatant. After 5 min, the OD324 was determined and the hydrazone concentration was normalized to the amount of bacteria present in the culture (OD600).
Determination of Mas activity
Malate synthase (Mas) activity was assayed photometrically at 232 nm according to a modified method described by Dixon and Kornberg (16). In a final volume of 1 ml, the assay mixture contained 50 mM Tris-HCl (pH 7.6), 40 mM MgCl2, 0.24 mM acetyl-coenzyme A, and 2 mM glyoxylate. One unit of activity corresponded to 1 μmol of malate formed per min. For mutant X798, cells were grown on glycerol for 2 days and transferred to minimal A medium with 0.5% acetate for 2 days; these cells were subsequently used for the determination of Mas activity.
High-voltage electrophoretic analysis of extracts and amino acid analysis
Tissue extracts were prepared as described above, and 50-μl samples were added to 1 ml of minimal A medium without a carbon or nitrogen source. The bacterial strains were diluted 10-fold in these test media and grown overnight. The bacteria were removed by centrifugation, and the supernatant was dried under vacuum and resuspended in a small volume of water. The whole samples were spotted on thin cellulose layers and run for 1 h in 0.1 M sodium citrate (pH 5.5) at 300 V. After the thin layers were dried, amino-containing compounds were revealed by soaking the electropherograms in a solution of 0.2% ninhydrin, 1% acetic acid, and 1% pyridine in acetone. When the thin-layer chromatograms were dry, they were incubated at 80°C until purple coloring appeared.
From similar unstained thin-layer chromatograms, the respective regions with a clearly different ninhydrin staining were scraped off and resuspended in water. Amino acid analyses were performed with a device consisting of a 420A derivatizer, a 140A separation system, and a 920A data module with precolumn derivatization with phenyl isothiocyanate (Applied Biosystems, Foster City, Calif.).
Determination of CFU in infected tissue
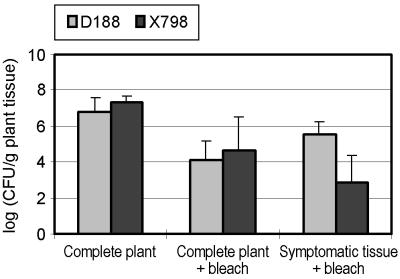
Five-week-old tobacco plants were infected by dipping in a bacterial suspension of approximately 107 CFU/ml, and 3 weeks after infection values of CFU per gram of plant tissue were determined. Bacterial populations were checked in three ways: complete plants were crushed to determine the total bacterial population, complete plants were treated with 6% sodium hypochloride (bleach) for 1 min followed by two washings in sterile water prior to crushing to better evaluate the contribution of internal bacterial populations to the complete population, and symptomatic tissue was bleached prior to crushing to assess the contribution of the internal bacterial population in galling tissues.
Extracts of crushed plants were diluted and plated on YEB without antibiotics. Values of CFU per gram of plant tissue were calculated, and the values were transformed logarithmically before statistical analysis. Values resulting from two independent experiments were pooled. Statistical analysis was done with the Statistica software (StatSoft, Tulsa, Okla.), and t tests were done to compare the populations of the two strains.
Other techniques
Standard methods for cloning, transformation, plasmid isolation, and DNA gel blot analysis were performed as described before (44).
The sequences of both strands of the 5.2-kb BglII insert of pUCDV6 were determined with automated dideoxy sequencing systems (A.L.F. DNA sequencer [Amersham Pharmacia Biotech, Little Chalfont, United Kingdom] and ABI377 DNA sequencer [Applied Biosystems]). Computer-assisted interpretation of the sequence was performed by the Sequence Analysis Software Package (version 10) of the Genetics Computer Group Inc. (GCG; Madison, Wis.). Homology searches with Swiss-Prot (release 39), Unique-PIR (release 65), and EMBL (release 64) databases were done using the FASTA algorithm (40), and sequences were aligned with PILEUP (GCG).
Current R. fascians techniques for plasmid isolation and transformation (12) and pulsed-field electrophoresis (7) have been described elsewhere.
Nucleotide sequence accession number
The sequence determined in this study has been deposited in the EMBL database under accession no. AJ301559.
RESULTS
Virulence in the chromosome: characterization of vic, a new virulence-related locus in R. fascians.
By mutagenesis experiments with randomly integrating vectors, three virulence loci had previously been isolated (7). Because these vectors proved to be very useful to isolate mutants, they were improved by introducing a promoterless reporter gene so that upon illegitimate integration into the genome not only was a mutation created but simultaneously, expression of the interrupted gene could be monitored. An integrating vector pDPX was constructed based on pUC19, the cmr gene of R. fascians as a marker, and the promoterless xylE gene of Pseudomonas putida as a reporter (Fig. 1A).
To isolate new virulence loci, pDPX was electroporated into wild-type strain D188 and transformants were selected on chloramphenicol-containing medium. The virulence phenotypes of 700 independent mutants were scored on tobacco seedlings. One mutant, strain X798, expressed xylE and showed severely deficient virulence. On seedlings, an extreme thickening of the hypocotyl was observed, but only limited or no inhibition of root development and leaf formation (Fig. 2B). As shown in Fig. 2C, strain X798 provoked symptoms on young decapitated plants with various degrees of severity. One-third of the plants had no visible symptoms (data not shown), another one-third showed very small shooty outgrowths without loss of apical dominance, and the remaining plants had elongated, loosely associated leafy galls.
FIG. 2.
Phenotypes of tobacco infected with strains D188 and X798. Shown are infection of a seedling with strains D188 (A) and X798 (B) and infection of a decapitated plant with strains X798 (C), D188 (D), and X798(pRFDV8) (E). Arrow (C), small shooty-like outgrowth. Bars, 25 mm (A and B) and 2 cm (C to E).
The locus was isolated by rescuing the pDPX-flanking border sequences from total DNA of strain X798 after digestion with BglII (an enzyme that does not cut in pDPX), self-ligation of the fragments, and transformation into E. coli. From the mapping of the resulting plasmid, pUCDV5 (Fig. 1B), it became clear that, after the first illegitimate integration, a second copy of pDPX recombined with the integrated copy, accompanied by an internal deletion of vector DNA. The wild-type locus was isolated from a genomic library of strain D188 (10) after screening with a 1.8-kb EcoRI fragment of the pUCDV5 insert (Fig. 1B). Ten overlapping cosmids that covered a region of approximately 40 kb were isolated and mapped. In each cosmid, two adjacent BamHI fragments (4.5 and 8.5 kb) hybridized to the probe (data not shown). The integration site of pDPX could be localized in a 5.2-kb BglII fragment, and this fragment was subcloned from cosmid pJGV13807 into pUC18 for further analysis, yielding plasmid pUCDV6.
To confirm that the phenotype of strain X798 resulted from the interruption of the isolated locus, a 4.4-kb BglII/partial SphI fragment from pUCDV6 was cloned into the E. coli-R. fascians shuttle vector pRF37 (12). The resulting plasmid, pRFDV8, was introduced into strain X798 by electroporation, and transformants were used to infect young decapitated tobacco plants. As shown in Fig. 2E, a full complementation to the wild-type phenotype was obtained with strain X798(pRFDV8).
The site of integration of pDPX into the genome of strain X798 was localized by DNA gel blot hybridization analysis on BamHI-digested total DNA of plasmid-free strain D188-5 and of strain D188 with the 1.8-kb EcoRI fragment from pUCDV5 as the probe. The same two fragments hybridized in both lanes, indicating that pDPX had integrated into the chromosome (data not shown), and the interrupted locus was named vic (for virulence in chromosome). This result was confirmed by DNA gel blot hybridization analysis with the same probe against DNA of strain D188 that had been subjected to pulsed-field electrophoresis to separate the chromosomal DNA from the linear plasmid DNA. No signal was obtained with the band corresponding to pFiD188 (data not shown).
Sequence analysis: the vic locus contains a Mas homologue.
The sequence of the 5.2-kb BglII fragment was determined and compared with the partial sequence obtained from pUCDV5. No deletion had accompanied the integration event, allowing the precise localization of the pDPX integration. This site was located in an open reading frame (ORF) with a high coding probability. The ORF (vicA, 2,175 bp) started with an ATG, ended with a TGA, and potentially encoded a protein of 725 amino acids (Fig. 3B). The ORF had an overall GC content of 63.8% and of 86.2% at the third position. Nine codons were not used in vicA: GTA for valine, AGG and AGA for arginine, ATA for isoleucine, TTA for leucine, TGT for cysteine, TCA and TCT for serine, and CCA for proline; all of these are rare codons in R. fascians as well as in Streptomyces and corynebacteria (33, 57). Data searches revealed that the encoded protein had high sequence similarity with Mas of Pseudomonas fluorescens (72% identity; EMBL accession no. Y11998), Mycobacterium tuberculosis (protein Rv1837c; 66% identity) (5), Mycobacterium leprae (66% identity; EMBL accession no. AL008609), and Corynebacterium glutamicum (60% identity) (43) and with MasG of E. coli (55% identity) (37). The sequence identity with other MasA homologues was low, approximately 20%. Typically, Mas is involved in the glyoxylate shunt of the Krebs cycle (27).
FIG. 3.
Physical map of the vic locus. (A) Fragment of the chromosome containing the vic locus. Relevant restriction sites and the integration site of pDPX in mutant X798 are given. (B) ORFs located on the 5.2-kb BglII fragment. (C) Fragments used to generate the complementing plasmid (pRFDV8) and the GUS fusion (pUCDV7).
Further analysis of the 5.2-kb BglII fragment revealed the presence of three translationally coupled ORFs upstream from the vicA gene that probably form an operon (Fig. 3B). The partial sequence of ORF1 (366 bp, 122 amino acids) potentially encoded a protein with homology to the carboxyl-terminal part of hypothetical protein Rv1842c of M. tuberculosis (58% identity and 72% similarity) (5), to a putative integral membrane protein of Streptomyces coelicolor (53% identity and 61% similarity) (42), and to hemolysins of several bacteria (2, 9, 18). The product of ORF2 (1,143 bp, 381 amino acids) showed the highest similarity to hypothetical protein Rv1841c of M. tuberculosis (48% identity and 64% similarity) (5) and to other putative integral membrane proteins (data not shown). ORF3 (915 bp, 305 amino acids) showed no similarity to known sequences and ended 63 bp upstream from vicA. ORF5 (525 bp, 175 amino acids) started 20 bp downstream from vicA; its product showed the highest similarity to hypothetical membrane proteins of M. tuberculosis (protein Rv1836c) (5) and M. leprae (EMBL accession no. AL008609) (32% identity and 48% similarity). Interestingly, the genetic organization of parts of the genome of R. fascians seems to be conserved compared with that of M. tuberculosis.
Expression of vicA is modulated depending on the available carbon sources.
Genes that encode shunt enzymes are normally expressed only during growth on C2 compounds. To monitor vicA gene expression, an integrating plasmid carrying a translational fusion between the 214-bp amino-terminal part of vicA and GUS was constructed (plasmid pUCDV7; Fig. 3C). The plasmid was introduced into strains D188-5 and D188 via electroporation, and recombinants were obtained on chloramphenicol-containing medium. The homologous recombination events in both strains were verified by DNA gel blot hybridization analysis (data not shown). Subsequently, the effect of several carbon sources on vicA gene expression was determined. Both strains, D188-5::pUCDV7 and D188::pUCDV7, were grown on YEB, washed, and resuspended in a defined medium. The GUS activity was measured following overnight incubation (see Materials and Methods). Although the expression of vicA was 3- to 23-fold higher in strain D188 than in D188-5, the expression patterns were similar and typical for a shunt gene (Table 2). Whereas vicA expression was induced by acetate, it was not affected by glycerol or glucose and it was repressed by malate in strain D188. Interestingly, the addition of pyruvate or plant or gall extracts also strongly induced vicA gene expression (Table 2). Because these additives are not C2 compounds, the observed induction of the vicA expression is atypical for a shunt gene.
TABLE 2.
Expression analysis of vicA in R. fascians strains D188 and D188-5
| Carbon source | GUS activitya in:
|
|
|---|---|---|
| D188-5 | D188 | |
| None | 10.4 ± 3.5 | 236.4 ± 17.0 |
| Sodium acetate | 56.2 ± 4.9 | 1,241.7 ± 41.7 |
| Glycerol | 25.6 ± 9.2 | 196.9 ± 70.9 |
| Glucose | 22.5 ± 11.0 | 158.2 ± 122.0 |
| Malate | 11.4 ± 1.3 | 34.4 ± 26.8 |
| Pyruvate | 62.3 ± 15.4 | 783.5 ± 120.7 |
| Plant extract | 69.3 ± 15.9 | 750.6 ± 83.3 |
| Gall extract | 56.4 ± 6.5 | 863.6 ± 76.1 |
GUS activity calculated as follows: (OD414 × 1,000)/(OD600 × time [in minutes]). The data are averages of three independent experiments.
The vicA gene is functional in the glyoxylate shunt.
Because of the sequence similarity and expression pattern of vicA, the putative role of vicA in the glyoxylate shunt was evaluated by monitoring the growth curves of strains D188-5, D188, and X798 on glycerol or acetate as the carbon source. Rates of growth on glycerol proved to be comparable for all three strains and significantly higher than those on acetate. However, strain X798 could not grow on acetate, in contrast to the two other strains (Fig. 4A). Moreover, when 5 mM glyoxylate was added at the beginning of growth on glycerol as the carbon source and the OD600 was measured after 2 days, it was found that the growth of strain D188 was not affected (OD600 = 1.2 under both conditions) whereas growth of strain X798 was repressed in the presence of glyoxylate (OD600 = 0.3 and 1.2 with and without glyoxylate, respectively).
FIG. 4.
Growth curves of R. fascians strains on different carbon sources. (A) Growth on glycerol (Gly) or acetate (Ac) as the sole carbon source. (B) Growth on minimal A medium with (NH4)2SO4 as the nitrogen source and leafy gall or plant extracts as the carbon source. The data are the averages of three independent experiments.
Growth under shunt-inducing conditions of Mas-deficient bacteria should lead to accumulation of glyoxylate. Glyoxylate and any keto- or aldoacid form adducts with phenylhydrazine (35). Therefore, during growth, samples of the supernatant were treated with phenylhydrazine and the formation of phenylhydrazones was monitored by measuring OD324. Throughout the growth curve, a much higher value at OD324 was determined for strain X798 than for strains D188 and D188-5 (Table 3). Whereas low values were obtained for strains D188 and D188-5, a very high absorbance was measured for strain X798 during growth on acetate and intermediate levels were measured during growth on glycerol.
TABLE 3.
Mas activity and estimate of glyoxylate accumulation during growth on different carbon sources
| Carbon source | Mas activity (U/mg of protein)a in strain:
|
Formation of phenylhydrazones from aldo- and ketoacidsb (OD324/OD600/ml of supernatant) in strain:
|
||||
|---|---|---|---|---|---|---|
| D188-5 | D188 | X798 | D188-5 | D188 | X798 | |
| Acetate | 198.9 ± 50.9 | 234.4 ± 133.1 | NDc | 0.2* | 0.3* | 12.4† |
| Glycerol | 52.1 ± 43.8 | 248.2 ± 71.7 | ND | 0.3* | 0.2* | 1.2‡ |
| Plant | 85.9 ± 26.9 | 103.5 ± 26.7 | ND | 0.4* | 0.4* | 1.1‡ |
| Gall | 137.3 ± 45.0 | 118.4 ± 21.0 | ND | 0.6* | 0.4* | 4.5§ |
The data are averages of three independent experiments.
For strains D188-5 and D188, the aldo- and ketoacid levels under the four growth conditions are not significantly different (P > 0.05; *); for mutant strain X798, there is a significant difference between aldo- and ketoacid levels on acetate (†), glycerol and plant (‡), and leafy gall (§) (P < 0.001). Also, between strains D188 and D188-5 and strain X798, there is a significant difference in aldo- and ketoacid concentrations (P < 0.001).
ND, not detected.
After 4 days of growth on either of the two carbon sources, the Mas activities of the three strains were measured (Table 3). On glycerol, the Mas activity was significantly higher in strain D188 than in strain D188-5. Whereas Mas activity was induced on acetate in strain D188-5, in strain D188 it was high for all carbon sources. In strain X798, no Mas activity could be detected.
Mas is required for growth in planta.
Although the above data indicate a clear role for vicA in the glyoxylate shunt, the expression data also suggest that vicA might play a dual role in R. fascians. Moreover, the question of why mutant X798 showed an attenuated virulence remained. In the R. fascians-plant interaction, persistent and metabolically active bacteria are required to maintain the leafy gall structure (53). It was hypothesized that in planta the bacteria would grow on nutritional sources whose metabolism required a functional Mas. Because mutant X798 had no functional Mas, glyoxylate would accumulate upon metabolism of these nutrients during the interaction with the plant. The glyoxylate accumulation would poison the bacteria and, hence, lead to a diminished virulence.
To test this hypothesis, growth of strains D188-5, D188, and X798 on a defined medium with plant and leafy gall extracts as the sole carbon source was evaluated. For independent extract preparations, the absolute growth levels varied strongly, which interfered with statistical analysis. Nevertheless, the obtained growth profiles were reproducible. Therefore, the results presented in Fig. 4B should be considered as qualitative rather than quantitative. Initially mutant strain X798 grew better on leafy gall extract than the other two strains, but the growth profile always showed a more steeply descending curve after the exponential growth phase (slope, −0.3 versus −0.1 under the other conditions), indicating that the bacteria died instead of entering stationary phase. Furthermore, the OD324 of the phenylhydrazones in the supernatant and Mas activity were determined during growth on plant and gall extracts as the sole carbon source. The data in Table 3 show that Mas activity was not detectable in mutant X798 and was not induced in strains D188 and D188-5. For mutant X798 only, the measured OD324 was intermediate and high upon growth on plant and leafy gall extracts, respectively (Table 3).
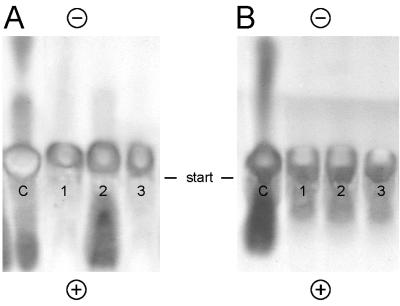
To further demonstrate the metabolic defect of mutant X798 under certain conditions, high-voltage electrophoretic analysis and ninhydrin staining of leafy gall extracts after growth of strains D188-5, D188, and X798 were performed (see Materials and Methods). As shown in Fig. 5A, the patterns of amino-containing molecules are clearly different. Several ninhydrin-positive products were used by strains D188-5 and D188 but not by strain X798. These compounds were eluted from the chromatograms, and chemical characterization showed that they corresponded to the common amino acids arginine, glutamic acid, aspartic acid, glutamine, and asparagine. However, when strain X798 was grown in the presence of these amino acids as the sole carbon and nitrogen sources in a defined medium, it removed these compounds from the medium (data not shown). Moreover, when extracts from A. tumefaciens-induced crown galls, which show an analogous pattern with respect to these common amino acids, were used to grow the three strains, no difference in utilization of ninhydrin-positive compounds could be observed (Fig. 5B).
FIG. 5.
Utilization of amino-containing compounds in leafy gall (A) and crown gall (B) extracts by strains D188 (lanes 1), X798 (lanes 2), and D188-5 (lanes 3). The cultures resulting from the catabolic tests were cleared by centrifugation, dried under vacuum, resuspended in a small volume of water, subjected to high-voltage thin-layer chromatography, and subsequently stained with ninhydrin. Lanes C, control without bacteria. − and +, cathode and anode, respectively.
Together, the above data suggest that in leafy gall extracts there are nutrients that can be used by R. fascians and that, upon uptake by strain X798, interfere with the general metabolism of the mutant because of a toxic accumulation of glyoxylate. If this model is correct, wild-type strain D188 and mutant strain X798 would differ in terms of in planta growth and/or survival. Therefore, plants were infected with the strains and after 3 weeks the total and internal population sizes of a complete plant were determined (see Materials and Methods). From Fig. 6, no difference between the colonization capacities of both strains (P > 0.05) can be seen. However, the numbers of bacteria inside symptomatic tissues were significantly lower for strain X798 than for strain D188 (P < 0.01; Fig. 6).
FIG. 6.
Colonization capacities of R. fascians strains D188 and X798.
DISCUSSION
A chromosomal mutant X798 of the phytopathogen R. fascians, which was strongly affected in its virulence on tobacco (Fig. 2), was isolated through the insertion of promoter probe vector pDPX (Fig. 1A). The mutation has been located in a gene, vicA, that is significantly homologous to genes that encode Mas. Mas functions in the glyoxylate shunt of the Krebs cycle and catalyzes the condensation of acetyl-coenzyme A and glyoxylate to form malate. Together with isocitrate lyase, the first enzyme of the shunt, it circumvents the loss of two carbons during the tricarboxylic acid cycle, when the organism is growing on C2 compounds as the sole carbon source (27). The shunt enzyme-encoding genes are often closely linked or organized as an operon (32, 43, 56). Because previously we had isolated the isocitrate lyase gene (icl) from R. fascians (54), we hybridized icl against the cosmids carrying vicA. No hybridization was observed, indicating that vicA and icl are not closely linked in R. fascians (data not shown).
In organisms such as E. coli (37), Candida tropicalis (24), and Saccharomyces cerevisiae (17, 22), two isozymes of Mas exist and in some cases they are regulated differently and are involved in different metabolic pathways, such as glyoxylate versus glycolate in E. coli and glyoxylate versus allantoin in S. cerevisiae. PCR analysis with vicA-based primers on total DNA also suggested the presence of a second, putative mas gene in R. fascians (data not shown). Until now, no additional data on the possible regulation and function of this gene have been available. However, vicA clearly is involved in the glyoxylate shunt: mutation of the gene leads to the inability to grow on acetate as the sole carbon source (Fig. 4A), to a complete loss of Mas activity, and to the accumulation of glyoxylate and possibly other aldo- or ketoacids upon growth on different carbon sources (Table 3). Moreover, when glyoxylate (5 mM) is added at the beginning of growth in a defined medium with glycerol (0.5%) as the carbon source, growth of wild-type strain D188 is not affected (100% growth irrespective of the presence of glyoxylate) whereas mutant X798 reaches only 30% of the growth obtained in the absence of glyoxylate. The expression of vicA is partly typical for a shunt gene (55); whereas acetate induces expression, other carbon sources, such as glucose and glycerol, do not (Table 2). Nevertheless, several data imply that, during infection, vicA is also involved in the metabolism of nutrients that, upon catabolism, release glyoxylate. When plant and gall extracts, both complex mixtures of carbon sources, as well as pyruvate are added, vicA is expressed. Interestingly, the expression level depends on the virulence plasmid (Table 2), suggesting that pFiD188 encodes a regulatory protein that either affects vicA expression positively or controls expression of a chromosomal nutrient uptake system. Another possibility is that a pFiD188-located gene codes for the uptake of nutrients catabolized through the vic locus. Another indication is that mutant X798 does not enter the stationary phase when grown on leafy gall extract as the sole carbon source but dies off (Fig. 4B), while under this condition glyoxylate and possibly other aldo- and ketoacids accumulate in the medium (Table 3). Finally, on leafy gall extracts, mutant X798 experiences a general metabolic defect (Fig. 5). Together, these data suggest that vicA may be required for in planta growth. Determination of the number of bacteria in infected tissues showed that mutant X798 can colonize the plant as well as strain D188; however, in symptomatic tissues the mutant is markedly deficient in its colonization capacities (Fig. 6).
Based on these data, we propose a model for the driving force of the interaction of R. fascians with its host and for the consequent role of vicA. Initially, R. fascians colonizes the surface of the plant. At a later stage R. fascians penetrates the plant tissue and colonizes mainly the intercellular spaces (6). At this point, the virulence genes are active and induce the formation of symptomatic tissues with a strong meristematic activity. This process alters the metabolic state of parts of the plant and the spectrum of compounds that can serve as nutritional factors for R. fascians. During the catabolism of these nutrients, glyoxylate is formed, and a functional VicA protein is essential for the removal of this toxic metabolite. In strain X798, this detoxification does not occur, glyoxylate accumulates, and the mutant bacteria stop growing or are killed. As a consequence, the mutants present in the leafy gall can no longer provide the signals essential for the maintenance of the gall and aberrant phenotypes are formed. Interestingly, the colonization and phenotypic data together imply that the bacteria inhabiting the nonsymptomatic parts of the plant are not involved in maintaining the leafy gall structure.
Although other mechanisms to explain the phenotype of strain X798, such as detoxification of plant defense molecules, could be envisaged, several reports support our model. In the interaction of Mycobacterium avium and M. tuberculosis with macrophages (25, 36), the glyoxylate cycle is very important in bacterial persistence and virulence. At the intracellular infection stage, the shunt enzymes are very active, indicating that a metabolic shift is required in the intracellular environment to meet the nutritional needs. Because a marked genomic conservation between R. fascians and M. tuberculosis was noticed, it is tempting to speculate that these closely related actinomycetes use similar strategies to interact with and persist in their respective hosts. Moreover, also in the interaction between Bradyrhizobium japonicum and soybean, a very high Mas activity in the intracellular bacteroids is measured (21, 34).
Nutritional requirements force soil bacteria to interact more or less intimately with plants. From an evolutionary point of view, this intimacy for nutritional sequestering occurred probably gradually, but the ultimate specialization to use nutritional mediators is currently represented uniquely by Agrobacterium strains (A. tumefaciens and A. rhizogenes). Genetic colonization of the host leads to a gratuitous production of novel compounds, known as opines, that are almost exclusively used by the crown gall-inducing bacteria that carry the respective catabolic genes on the Ti plasmid (15, 45). An appealing hypothesis on the origin of the opine concept was formulated by Vaudequin-Dransart et al. (51, 52). Primitive agrobacteria evolved to acquire the genes to catabolize an ancestral opine, which was produced by uninfected plants only at a certain stage in their development. With time, the bacteria succeeded in directing the biosynthesis of this ancestral opine at a stage convenient for the pathogen and not for the host through genetic transformation of the plant. However, in addition to this transformation approach, the mere induction of hyperplasia is sufficient to cause quantitative and/or qualitative changes in the plant metabolites that are available to the inciting bacteria, thus creating a specific niche. Our data illustrate that R. fascians uses this strategy to obtain particular nutrients from the plant and has a selective advantage over other plant-associated bacteria as a result. We propose that this concept should be broadened to all hyperplasia-inducing bacteria. In this more general form, the concept could be termed metabolic habitat modification.
Acknowledgments
Danny Vereecke and Karen Cornelis contributed equally to this work.
We thank Raimundo Villarroel and Jan Gielen for sequencing, Bart Devreese for amino acid analyses, Martine De Cock for help with the manuscript, and Karel Spruyt and Rebecca Verbanck for pictures and figures.
This work was supported by grants from the Interuniversity Poles of Attraction Programme (Belgian State, Prime Minister's Office—Federal Office for Scientific, Technical and Cultural Affairs; P4/15). D.V. and W.T. are indebted to the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-Technologisch Onderzoek in de Industrie for a postdoctoral fellowship. M.J. is a Research Associate of the Belgian “Fonds National de la Recherche Scientifique” (FNRS).
REFERENCES
- 1.Barras, F., F. van Gijsegem, and A. K. Chatterjee. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32:201-234. [Google Scholar]
- 2.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 3.Bradbury, J. F. 1986. Rhodococcus Zopf 1891, p. 185-187. In J. F. Bradbury (ed.), Guide to plant pathogenic bacteria. CAB International Mycological Institute, Slough, United Kingdom.
- 4.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M.-A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, K., T. Ritsema, J. Nijsse, M. Holsters, K. Goethals, and M. El Jaziri. 2001. The plant pathogen Rhodococcus fascians colonizes the exterior and interior of aerial parts of plants. Mol. Plant-Microbe Interact. 14:599-608. [DOI] [PubMed] [Google Scholar]
- 7.Crespi, M., E. Messens, A. B. Caplan, M. Van Montagu, and J. Desomer. 1992. Fasciation induction by the phytopathogen Rhodococcus fascians depends upon a linear plasmid encoding a cytokinin synthase gene. EMBO J. 11: 795-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespi, M., D. Vereecke, W. Temmerman, M. Van Montagu, and J. Desomer. 1994. The fas operon of Rhodococcus fascians encodes new genes required for efficient fasciation of host plants. J. Bacteriol. 176:2492-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deckert, G., P. V. Warren, T. Gaasterland, W. G. Young, A. L. Lenox, D. E. Graham, R. Overbeek, M. A. Snead, M. Keller, M. Aujay, R. Huber, R. A. Feldman, J. M. Short, G. J. Olsen, and R. V. Swanson. 1998. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature 392:353-358. [DOI] [PubMed] [Google Scholar]
- 10.Desomer, J., M. Crespi, and M. Van Montagu. 1991. Illegitimate integration of non-replicative vectors in the genome of Rhodococcus fascians upon electrotransformation as an insertional mutagenesis system. Mol. Microbiol. 5:2115-2124. [DOI] [PubMed] [Google Scholar]
- 11.Desomer, J., P. Dhaese, and M. Van Montagu. 1988. Conjugative transfer of cadmium resistance plasmids in Rhodococcus fascians strains. J. Bacteriol. 170:2401-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desomer, J., P. Dhaese, and M. Van Montagu. 1990. Transformation of Rhodococcus fascians by high-voltage electroporation and development of R. fascians cloning vectors. Appl. Environ. Microbiol. 56:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desomer, J., D. Vereecke, M. Crespi, and M. Van Montagu. 1992. The plasmid-encoded chloramphenicol resistance protein of Rhodococcus fascians is homologous to the transmembrane tetracycline efflux proteins. Mol. Microbiol. 6:2377-2385. [DOI] [PubMed] [Google Scholar]
- 14.Dessaux, Y., A. Petit, and J. Tempé. 1993. Chemistry and biochemistry of opines, chemical mediators of parasitism. Phytochemistry 34:31-38. [Google Scholar]
- 15.Dessaux, Y., A. Petit, S. K. Farrand, and P. J. Murphy. 1998. Opines and opin-like molecules involved in plant-rhizobiaceae interactions, p. 173-197. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 16.Dixon, G. H., and H. L. Kornberg. 1959. Assay methods of key enzymes of the glyoxylate cycle. Biochem. J. 72:3.
- 17.Fernandez, E., M. Fernandez, and R. Rodicio. 1993. Two structural genes are encoding malate synthase isoenzymes in Saccharomyces cerevisiae. FEBS Lett. 320:271-275. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-P. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Science 390:580-586. [DOI] [PubMed] [Google Scholar]
- 19.Gansemans, Y. 1987. Expressie van het xylE gen in Bacillus subtilis onder controle van het φ105 repressor-operator systeem. Dissertation. Universiteit Gent, Ghent, Belgium.
- 20.Goodfellow, M. 1984. Reclassification of Corynebacterium fascians (Tilford) Dowson in the genus Rhodococcus, as Rhodococcus fascians comb. nov. Syst. Appl. Microbiol. 5:225-229. [Google Scholar]
- 21.Green, L. S., D. B. Karr, and D. W. Emerich. 1998. Isocitrate dehydrogenase and glyoxylate cycle enzyme activities in Bradyrhizobium japonicum under various growth conditions. Arch. Microbiol. 169:445-451. [DOI] [PubMed] [Google Scholar]
- 22.Hartig, A., M. M. Simon, T. Schuster, J. R. Daugherty, H. S. Yoo, and T. G. Cooper. 1992. Differentially regulated malate synthase genes participate in carbon and nitrogen metabolism of S. cerevisiae. Nucleic Acids Res. 20:5677-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward, A. C. 1991. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29:65-87. [DOI] [PubMed] [Google Scholar]
- 24.Hikida, M., H. Atomi, Y. Fukuda, A. Aoki, T. Hishida, Y. Teranishi, M. Ueda, and A. Tanaka. 1991. Presence of two transcribed malate synthase genes in an n-alkane-utilizing yeast, Candida tropicalis. J. Biochem. 110:909-914. [DOI] [PubMed] [Google Scholar]
- 25.Höner zu Bentrup, K., A. Miczak, D. L. Swenson, and D. G. Russell. 1999. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 181:7161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooykaas, P. J. J., and A. G. M. Beijersbergen. 1994. The virulence system of Agrobacterium tumefaciens. Annu. Rev. Phytopathol. 32:157-179. [Google Scholar]
- 27.Kornberg, H. L. 1966. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 99:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh, J. A., and D. L. Coplin. 1992. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 46:307-346. [DOI] [PubMed] [Google Scholar]
- 29.Lelliott, R. A. 1966. The plant pathogenic coryneform bacteria. J. Appl. Bacteriol. 29:114-118. [Google Scholar]
- 30.Lichter, A., I. Barash, L. Valinsky, and S. Manulis. 1995. The genes involved in cytokinin biosynthesis in Erwinia herbicola pv. gypsophilae: characterization and role of gall formation. J. Bacteriol. 177:4457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maes, T., D. Vereecke, T. Ritsema, K. Cornelis, H. Ngo Thi Thu, M. Van Montagu, M. Holsters, and K. Goethals. 2001. The att locus of Rhodococcus fascians strain D188 is essential for full virulence on tobacco through the production of an autoregulatory compound. Mol. Microbiol. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 32.Maloy, S. R., and W. D. Nunn. 1982. Genetic regulation of the glyoxylate shunt in Escherichia coli K-12. J. Bacteriol. 149:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malumbres, M., J. A. Gil, and J. F. Martín. 1993. Codon preference in Corynebacteria. Gene 134:15-24. [DOI] [PubMed] [Google Scholar]
- 34.McDermott, T. R., S. M. Griffith, C. P. Vance, and P. H. Graham. 1989. Carbon metabolism in Bradyrhizobium japonicum bacteroids. FEMS Microbiol. Rev. 63:327-340. [Google Scholar]
- 35.McFadden, B. A., and W. V. Howes. 1960. The determination of glyoxylic acid in biological systems. Anal. Biochem. 1:240-248. [Google Scholar]
- 36.McKinney, J. D., K. Höner zu Bentrup, E. J. Muñoz-Elias, A. Miczak, B. Chen, W.-T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 37.Molina, I., M.-T. Pellicer, J. Badia, J. Aguilar, and L. Baldoma. 1994. Molecular characterization of Escherichia coli malate synthase G. Differentiation with malate synthase A isoenzyme. Eur. J. Biochem. 224:541-548. [DOI] [PubMed] [Google Scholar]
- 38.Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15:473-497. [Google Scholar]
- 39.Norrander, J., T. Kempe, and J. Messing. 1983. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26:101-106. [DOI] [PubMed] [Google Scholar]
- 40.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peleman, J., W. Boerjan, G. Engler, J. Seurinck, J. Botterman, T. Alliotte, M. Van Montagu, and D. Inzé. 1989. Strong cellular preference in the expression of a housekeeping gene of Arabidopsis thaliana encoding S-adenosylmethionine synthetase. Plant Cell 1:81-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redenbach, M., H. M. Kieser, D. Denapaite, A. Eichner, J. Cullum, H. Kinashi, and D. A. Hopwood. 1996. A set of ordered cosmids and a detailed genetic and physical map of the 8 Mb Streptomyces coelicolor A3(2) chromosome. Mol. Microbiol. 21:77-96. [DOI] [PubMed] [Google Scholar]
- 43.Reinscheid, D. J., B. J. Eikmanns, and H. Sahm. 1994. Malate synthase from Corynebacterium glutamicum: sequence analysis of the gene and biochemical characterization of the enzyme. Microbiology 140:3099-3108. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning, a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 45.Schell, J., M. Van Montagu, M. De Beuckeleer, M. De Block, A. Depicker, M. De Wilde, G. Engler, C. Genetello, J. P. Hernalsteens, M. Holsters, J. Seurinck, B. Silva, F. Van Vliet, and R. Villarroel. 1979. Interactions and DNA transfer between Agrobacterium tumefaciens, the Ti-plasmid and the plant host. Proc. R. Soc. Lond. B Biol. Sci. 204:251-266. [DOI] [PubMed] [Google Scholar]
- 46.Schuster, M. L., and C. C. Smith. 1983. Seed transmission and pathology of Corynebacterium flaccumfaciens in beans (Phaseolus vulgaris). Seed Sci. Technol. 11:867-875. [Google Scholar]
- 47.Sheng, J., and V. Citovsky. 1996. Agrobacterium-plant cell DNA transport: have virulence proteins, will travel. Plant Cell 8:1699-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Temmerman, W. 2000. Role of the fas locus in leafy gall development by the phytopathogen Rhodococcus fascians. Ph.D. thesis, Ghent University, Ghent, Belgium.
- 49.Temmerman, W., T. Ritsema, C. Simón-Mateo, M. Van Montagu, V. Mironov, D. Inzé, K. Goethals, and M. Holsters. 2001. The fas locus of the phytopathogen Rhodococcus fascians affects mitosis of tobacco BY-2 cells. FEBS Lett. 492:127-132. [DOI] [PubMed] [Google Scholar]
- 50.Temmerman, W., D. Vereecke, R. Dreesen, M. Van Montagu, M. Holsters, and K. Goethals. 2000. Leafy gall formation is controlled by fasR, an AraC-type regulatory gene, in Rhodococcus fascians. J. Bacteriol. 182:5832-5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaudequin-Dransart, V., A. Petit, W. S. Chilton, and Y. Dessaux. 1998. The cryptic plasmid of Agrobacterium tumefaciens cointegrates with the Ti plasmid and cooperates for opine degradation. Mol. Plant-Microbe Interact. 11:583-591. [Google Scholar]
- 52.Vaudequin-Dransart, V., A. Petit, C. Poncet, C. Ponsonnet, X. Nesme, J. B. Jones, H. Bouzar, W. S. Chilton, and Y. Dessaux. 1995. Novel Ti plasmids in Agrobacterium strains isolated from fig tree and chrysanthemum tumors and their opinelike molecules. Mol. Plant-Microbe Interact. 8:311-321. [DOI] [PubMed] [Google Scholar]
- 53.Vereecke, D., S. Burssens, C. Simón-Mateo, D. Inzé, M. Van Montagu, K. Goethals, and M. Jaziri. 2000. The Rhodococcus fascians-plant interaction: morphological traits and biotechnological applications. Planta 210:241-251. [DOI] [PubMed] [Google Scholar]
- 54.Vereecke, D., R. Villarroel, M. Van Montagu, and J. Desomer. 1994. Cloning and sequence analysis of the gene encoding isocitrate lyase from Rhodococcus fascians. Gene 145:109-114. [DOI] [PubMed] [Google Scholar]
- 55.Wendisch, V. F., M. Spies, D. J. Reinscheid, S. Schnicke, H. Sahm, and B. J. Eikmanns. 1997. Regulation of acetate metabolism in Corynebacterium glutamicum: transcriptional control of the isocitrate lyase and malate synthase genes. Arch. Microbiol. 168:262-269. [DOI] [PubMed] [Google Scholar]
- 56.Wilson, R. B., and S. R. Maloy. 1987. Isolation and characterization of Salmonella typhimurium glyoxylate shunt mutants. J. Bacteriol. 169:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright, F., and M. J. Bibb. 1992. Codon usage in the G+C-rich Streptomyces genome. Gene 113:55-65. [DOI] [PubMed] [Google Scholar]
- 58.Yamada, T. 1993. The role of auxin in plant-disease development. Annu. Rev. Phytopathol. 31:253-273. [DOI] [PubMed] [Google Scholar]
- 59.Zukowski, M. M., D. F. Gaffney, D. Speck, M. Kauffmann, A. Findeli, A. Wisecup, and J.-P. Lecocq. 1983. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc. Natl. Acad. Sci. USA 80:1101-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]