Abstract
The conversion of Pseudomonas aeruginosa to the mucoid phenotype coincides with the establishment of chronic respiratory infections in cystic fibrosis (CF). A major pathway of conversion to mucoidy in clinical strains of P. aeruginosa is dependent upon activation of the alternative sigma factor AlgU (P. aeruginosa ςsgr;E). Here we initiated studies of AlgU-dependent global expression patterns in P. aeruginosa in order to assess whether additional genes, other than those involved in the production of the mucoid exopolysaccharide alginate, are turned on during conversion to mucoidy. Using genomic information and the consensus AlgU promoter sequence, we identified 35 potential AlgU (ςsgr;E) promoter sites on the P. aeruginosa chromosome. Each candidate promoter was individually tested by reverse transcription and mRNA 5′-end mapping using RNA isolated from algU+ and algU::Tcr mutant cells. A total of 10 new AlgU-dependent promoters were identified, and the corresponding mRNA start sites were mapped. Two of the 10 newly identified AlgU promoters were upstream of predicted lipoprotein genes. Since bacterial lipoproteins have been implicated as inducers of inflammatory pathways, we tested whether lipopeptides corresponding to the products of the newly identified AlgU-dependent lipoprotein genes, lptA and lptB, had proinflammatory activity. In human peripheral blood monocyte-derived macrophages the peptides caused production of interleukin-8, a proinflammatory chemokine typically present at excessively high levels in the CF lung. Our studies show how genomic information can be used to uncover on a global scale the genes controlled by a given ςsgr; factor (collectively termed here sigmulon) using conventional molecular tools. In addition, our data suggest the existence of a previously unknown connection between conversion to mucoidy and expression of lipoproteins with potential proinflammatory activity. This link may be of significance for infections and inflammatory processes in CF.
Cystic fibrosis (CF) is the most common lethal inheritable disease in Caucasians (60). The primary contributors to the high morbidity and mortality in CF are the chronic respiratory infections caused by bacterial pathogens (59). The predominant CF pathogen is Pseudomonas aeruginosa, and over 90% of CF patients eventually become colonized with this organism (20). A classical feature of P. aeruginosa strains infecting CF patients is that they mutate into the mucoid, exopolysaccharide alginate-overproducing form, in a process referred to as conversion to the mucoid phenotype (23). This conversion is concomitant with the establishment of chronic bacterial colonization (28, 42). The emergence of mucoid strains also correlates with a poor clinical prognosis (23, 28, 42). The exact mechanisms leading to the worsening of disease coinciding with the conversion to mucoidy in P. aeruginosa are not fully understood, but are believed to stem from excessive inflammation (4, 27, 29) and associated irreversible lung tissue damage.
At the genetic level, the conversion to mucoidy in P. aeruginosa occurs via mutations in a cluster of genes encoding the alternative sigma factor AlgU (35), also known as AlgT (16, 21), and an array of AlgU regulators: MucA, MucB, MucC, and MucD (5, 7, 36, 37). The mutations causing mucoidy in CF isolates most frequently occur in the mucA gene (8, 37). These mutations release AlgU from the inhibitory activities of MucA (49, 53). AlgU is the P. aeruginosa ortholog of Escherichia coli and Salmonella ςsgr;E (63), an alternative sigma factor that directs transcription of genes in response to extreme stress conditions (24, 39, 48). Recently, it has been shown that AlgU can also direct transcription of the major heat shock sigma factor RpoH (50). As an alternative sigma factor, AlgU is likely to play a role in global gene expression, but the extent of its effects and the exact genes controlled, with the exception of the alginate-specific genes, are not known.
While alginate overproduction by mucoid strains of P. aeruginosa has an established role in pathogenesis (23), it alone cannot account for the inflammation and further clinical deterioration that correlate with the timing of the emergence of mucoid strains. One hypothesis, which takes into account the likelihood that AlgU directs transcription of more than just the alginate biosynthesis genes, includes the possibility of coexpression of toxic or proinflammatory products upon conversion to mucoidy. As a first step towards testing this hypothesis, we initiated global studies of AlgU dependent genes using the P. aeruginosa genomic sequence as a newly available resource (58).
From our previous studies (14, 15, 38, 52) and reports by others (18, 32), a tight consensus sequence [(−35)GAACTT-N16/17-(−10)TCtgA (invariable residues in capital letters)] for the AlgU and ςsgr;E promoters has been derived. Using this consensus sequence, we searched the P. aeruginosa genome for AlgU promoters and identified 35 potential sites. In an experimental follow-up, we carried out mRNA 5′-end mapping by reverse transcription and established AlgU dependence for a number of newly identified promoters. These analyses increased the number of characterized P. aeruginosa AlgU (ςsgr;E) promoters from 5 to 15. Our studies also indicate a previously unappreciated connection between the conversion to mucoidy and expression of Pseudomonas genes encoding lipoproteins with substantial proinflammatory activity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa PAO381 and its mucoid derivatives PAO578I (mucA22) and PAO578II (mucA22 sup-2) have been described previously (5, 22). The nonmucoid PAO6865 (algU::Tcr) strain is derived from PAO578II (6). For RNA isolation, strains were cultured with shaking at 37°C overnight in Luria-Bertani medium, and 1 ml of the overnight culture was used to inoculate 100 ml of Luria-Bertani medium with 0.3 M NaCl (PAO578II and PAO6865) or without the salt supplement (PAO381 and PAO578I) and grown for 4 h at 37°C.
Tissue culture cells and conditions.
Human peripheral blood monocytes were seeded onto glass cover slips and differentiated in RPMI medium with 5% human serum (Sigma, St. Louis, Mo.) to yield confluent monolayers of macrophages.
Genomic searches.
The PAO1 genomic sequence was obtained from the Pseudomonas Genome Project (www.pseudomonas.com) (58). Data were imported for analysis by MacVector sequence analysis software (version 6.0/7.0; Eastman Kodak Co.). A subsequence search corresponding to the AlgU consensus, GAACTT-N16/17-TCNNA, was carried out to determine potential AlgU promoter sites in the genome. Regions starting 50 bp and ending 1,000 bp downstream of the putative promoter sites were used in a global BlastX search against the National Center for Biotechnology Information database to examine potential open reading frames in the right orientation and position (candidate genes for regulation by the AlgU promoters). Additionally, information from the PseudoCAP annotation database was used (www.pseudomonas.com).
Primer design and DNA methods.
We used 16-mer primers (nine G/C and seven A/T) generated for each of the suspected recognition sites 500 bp upstream and downstream of the sites. These primers were used in a PCR to generate a 1-kb fragment from total genomic PAO1 DNA to serve as a sequencing template. A 22-mer primer was designed 60 bp downstream of each suspected site and oriented to extend back towards the putative promoter to generate a transcript using reverse transcriptase in primer extension analyses as well as to sequence the promoter region using a 33P sequencing kit (Amersham, Piscataway, N.J.). A second primer (E5 primer 2) was designed for the promoter E5 (lptB) which had its 3" end 65 bp from the AlgU-dependent mRNA start site.
RNA isolation and primer extension analysis.
RNA isolation and reverse transcription were carried out as described (26). After rapid cooling in an ethanol-dry ice bath, cells were washed on ice in 50 mM Tris (pH 7.5) and lysed in sodium dodecyl sulfate. Total RNA was isolated by centrifugation over a 5.7 M CsCl cushion overnight. The pellet was dried, resuspended in diethyl pyrocarbonate-treated water, and chloroform extracted. RNA was stored in ethanol at −20°C until just prior to use. Following centrifugation, RNA was resuspended in water, and a spectrophotometry reading at an optical density of 260 was taken to determine yield.
Primers were end labeled by polynucleotide kinase with [γ32-P]ATP for 1 h at 37°C. The reaction was stopped with 0.5 M EDTA, diluted with Tris-EDTA, and heat inactivated by incubation at 65°C for 5 min. Labeled primers were annealed to 15 μg of total cellular RNA in hybridization buffer (0.5 M KCl, 0.24 M Tris-HCl [pH 8.3]) by dissociating at 95°C for 1 min, followed immediately by annealing at 55°C for 2 min and stabilization on ice for 15 min. Primers were extended using Superscript II (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions and loaded adjacent to a sequencing ladder which utilized the same primer (44).
Peptide design and synthesis.
Leader sequences denoting lipopeptide modification (45) were observed for two ORFs downstream of AlgU-dependent promoters. Two peptides, LPTA(6) Pam3Cys-DKKEE-OH and LPTB(6) Pam3Cys-DSQTN-OH, consisting of the palmitylated cysteine (Pam3Cys) after the cleavage site plus five amino acids from the amino terminus of each lipoprotein, were synthesized (Bio-Synthesis, Inc., Lewisville, Tex.). The proinflammatory synthetic bacterial lipopeptide Pam3Cys-SKKKK-OH was also synthesized (1, 25).
Cytokine assays.
Cells were incubated with lipopolysaccharide (LPS) (1 μg/ml), human tumor necrosis factor alpha (12.5 ng/ml), or bacterial lipopeptides (10 μg/ml) or given no stimulation for 24 h. Cell culture supernatants were removed, and any particulate matter was cleared by centrifugation. Supernatants were assayed at a 100-fold dilution for secreted interleukin-8 (IL-8) according to the manufacturer's instructions (Quantikine; R&D Systems).
RESULTS
Genomic analysis of the P. aeruginosa PAO1 chromosome for sequences representing potential AlgU (ςsgr;E) promoters.
The P. aeruginosa PAO1 genome (58) was subjected to a search for sequences corresponding to the AlgU (ςsgr;E) promoter consensus (14, 18, 32, 38) using computer-assisted subsequence match analysis. The subsequence used to search the database was GAACTT-N16/17-TCNNA, where N is any base and 16 or 17 is the spacing between the −10 and the −35 regions. This search resulted in 35 sites situated around the chromosome (designated A1 to A6, B1 to B9, C1 to C9, D1 to D4, and E1 to E7, based on ≥1-Mb segments [A to F] used to partition the genomic sequence) that represented potential AlgU promoters (Table 1).
TABLE 1.
Predicted AlgU (ςsgr;E) promoter sequences in the P. aeruginosa genomea
| Designation | Sequence | Spacing (bp) | Position | Orientation |
|---|---|---|---|---|
| Consensus | --GAACTT-----16/17-----TCtgA-5/6-+1 | |||
| A1 | TTGAACTTGTTCCGGGTCCGGCGCTCTTAAAGCGAGCC | 16 | 1947915 | + |
| A2 | AAGAACTTTGAGGGCAAGTCGAAGTTCAAAACGTGGCT | 17 | 1920705 | + |
| A3 | TGGAACTTCACGCCAGCGCAAATGTTCAAAGGGCTACA | 17 | 1735129 | − |
| A4 | AGGAACTTCGACATCAGCAGCGCCCTCGGACCGACCCG | 17 | 1310177 | + |
| A5 | TTGAACTTGGTCATCGCGAGCGTCCTCAGAACGGCCAC | 17 | 1169833 | − |
| A6 | CGGAACTTTCCTCCGCGCCGTGGCTCTGAACAGCCGAC | 16 | 1119661 | − |
| B1 | CGGAACTTGCGCTCGACGTCGTACTCGCAGGCGAAGCC | 16 | 962434 | − |
| B2 | TCGAACTTAGGCGCAGAATGTCGGGTCCCACGGACACA | 17 | 935412 | + |
| B3 | TCGAACTTTATCTTTTGATAGTTCTCGAAAATGAACCT | 16 | 900426 | + |
| B4 | TTGAACTTGCCCGGGCCGTAGCGCTCGTACAGCTCGGC | 16 | 861036 | + |
| B5 | TGGAACTTTCTTAGACGCATCGGTTCCAAAGCAGGATG | 16 | 831031 | + |
| B6 | GAGAACTTGTAGCCGCGACGGTATTCGAACTTGTCCAC | 16 | 634932 | + |
| B7 | CCGAACTTGGCGCCCATGGCGTCGTCGGACAGGTAGGT | 16 | 431038 | + |
| B8 | AGGAACTTATACACCCGCTTGCAGTCAGATATCCGAGT | 16 | 420610 | + |
| B9 | CTGAACTTGTCCGGCTGCGTCGTCCTCCCATGTAGACA | 17 | 73409 | + |
| C1 | CTGAACTTTCCCGTTTTGCCGACAGTCAGAACACACGA | 17 | 6183692 | − |
| C2 | TGGAACTTTCCTTGCGCGCCTTGCATCGCATACTCAAG | 17 | 5954923 | + |
| C3 | GGGAACTTTCTTAAGTAGAGGCGGTCGTAGACCGAATG | 16 | 5835256 | − |
| C4 | GCGAACTTCACCGCCTTGCCGGACTCGCACCAACTTCC | 16 | 5738203 | + |
| C5 | TCGAACTTGGCGAGACGGGAGAAATCGTACTTGCTGGC | 16 | 5624107 | + |
| C6 | GAGAACTTCGCCGAATCGAAATACTTCGGATCCGGCCA | 17 | 5577383 | + |
| C7 | CCGAACTTTGCGAGGAAAAACCGATTCTAACCAAGCCA | 17 | 5302202 | + |
| C8 | TCGAACTTGCCGCGCAACCCGGTCTCGAAGCTCTGGCT | 16 | 5290874 | − |
| C9 | GTGAACTTTGCCACAAAACGCATATCTGAATCCATTGA | 16 | 5289088 | + |
| D1 | CAGAACTTTCCCAAGTCTGGACGGTTCCAAGCGGATTG | 17 | 5125987 | − |
| D2 | AGGAACTTGAGATCGTGCAGGGTGCTCCAATATTTCCC | 17 | 4433393 | − |
| D3 | GCGAACTTGTCGCAGTCGTGCCGTTCCAAGCAGCCAGC | 16 | 4371837 | − |
| D4 | TGGAACTTGGTGGTTTTTGCCCAGTCCTAGGCAAGGCA | 16 | 4276049 | − |
| E1 | CGGAACTTCCCTCGCAGAGAAAACATCCTATCACCGCG | 17 | 3962424 | + |
| E2 | TCGAACTTCTTGCCCGCCAGCGCATCGGAATCGTGACC | 16 | 3931084 | + |
| E3 | AAGAACTTTCCGGGCGGCACCCAGTCCCAACCAGCACG | 16 | 3808920 | + |
| E4 | TGGAACTTGCAAAGGAGCACCTGCCTCGAAAGCCTCCG | 17 | 3682884 | + |
| E5 | TTGAACTTATCCGCGCGCACCTGTTCCTATTGCCCATA | 16 | 3650579 | − |
| E6 | AGGAACTTGCCAAGACTGCCATGCTCTGAATTGATCCG | 16 | 3218496 | − |
| E7 | TGGAACTTCTGGCGGGGCGATAGCTCCCATTGAGCCGC | 17 | 3153481 | + |
Potential AlgU promoter sequences were identified using MacVector subsequence search with the AlgU (ςsgr;E) promoter consensus sequence shown and the Pseudomonas Genome Project database (www.pseudomonas.com). Spacing between the −35 and −10 regions was 16 or 17 bp, as indicated. Nucleotide positions and orientation are indicated relative to the P. aeruginosa PAO1 genomic sequence (58).
Regions including 50 bp upstream and 1,000 bp downstream of the potential AlgU promoters were examined for the presence of previously annotated genes or subjected to global searches to detect potential open reading frames downstream of the candidate promoter sites (Table 2). In addition to the algD, algU, and rpoH loci, previously shown to have AlgU-dependent promoters (13, 39, 50), several of the putative promoter sites were found upstream of genes whose products might aid bacterial adaptation to changing environmental conditions (Table 2). (i) oprF encodes porin F, the major outer membrane protein of P. aeruginosa (61). The levels of OprF have recently been shown to increase during periods of stress in an AlgU (AlgT)-dependent manner (34). (ii) The osmC gene encodes an E. coli outer membrane protein that is transcriptionally induced during hyperosmotic stress (9). (iii) The lptB gene encodes a predicted peptidyl-proly cis/trans isomerase (PPIase), most likely functioning in protein folding. (iv) The slyB gene product shows homology to the proposed porin SlyB of Salmonella enterica serovar Typhimurium (33). (v) The betT gene is in the bet locus, involved in synthesis of the osmoprotectant glycine betaine from choline (19). (vi) The dksA gene encodes a suppressor that offsets mutations in heat shock genes such as dnaK, dnaJ, and grpE (2). (vii) The phuR gene encodes a hemin receptor and is controlled by the iron-sensitive regulator Fur (40). The remaining 24 sites (with the exception of TalB [56], a transaldolase that could be involved in generating the alginate precursor fructose-6-phosphate from sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate [64]) had no annotated P. aeruginosa genes within 1-kb regions downstream of the putative AlgU promoters.
TABLE 2.
Genes downstream of the predicted AlgU (ςsgr;E) promotersa
| Promoter class | Designation | Downstream gene | Function or positionb | Distance (bp) to initiation codonc |
|---|---|---|---|---|
| Mapped mRNA 5" ends | A2 | oprF | Porin F | 438 |
| A3 | lptA | Predicted lipoprotein | 35 | |
| A6 | asmA6 | Orf (1119618-1118120) | 12 | |
| B2 | asmB2 | Orf (935427-935975[+]) preceding bolA | 15 | |
| B5 | algU | Sigma factor | 237 | |
| B8 | rpoH | Sigma factor | 41 | |
| B9 | osmC | Osmolarity-induced protein (E. coli) | 56 | |
| C1 | asmC1 | Orf (6183338-6182907 [−]) | 254 | |
| D2 | asmD2 | Orf (4433169-4432594 [−]) | 224 | |
| D3 | asmD3 | Orf (4371747-4371286 [−]) | 90 | |
| D4 | slyB | Proposed porin (Salmonella) | 60 | |
| E1 | algD | GDP mannose dehydrogenase | 368 | |
| E5 | lptB | Predicted lipoprotein/PPIased | 82 | |
| Putative | C2 | betT | Choline transporter (E. coli) | 223 |
| C7 | dksA | Suppressor of dnaK defects (E. coli) | 279 | |
| C9 | phuR | Hemin receptor | 97 | |
| E6 | Proposed glycosylase | 46 | ||
| E7 | talB | Transaldolase B (E. coli) | 69 |
Genes were determined by sequence identity or homology of predicted gene products. The genes designated asm (for AlgU sigmulon members) are previously unannotated open reading frames (Orfs).
Previously identified genes in P. aeruginosa or homologs in other organisms. The position and orientation (+ or −) of Orfs corresponding to asmA6, asmB2, asmC1, asmD2, and asmD3 are given as the nucleotide numbers of the P. aeruginosa PAO1 chromosomal sequence.
Distance from the mapped mRNA start site (or from the predicted mRNA start site for putative promoters) to the translational start.
Contains conserved domains of FKBP-like peptidyl-prolyl cis/trans-isomerases (PPIases), including conserved catalytic residues.
Mapping of the AlgU promoter upstream of the oprF gene.
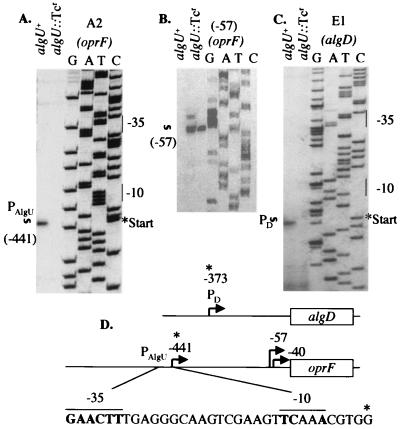
Recently, porin F levels have been shown to increase in mucoid, mucA mutant P. aeruginosa strains (35). This finding supported our observation that site A2 was located upstream of the oprF gene, suggesting that this could indeed be an AlgU-dependent promoter. To test this possibility, we carried out mapping of the mRNA 5′ end by reverse transcription. A primer was designed downstream of the predicted mRNA start site. The same primer also served to generate the corresponding sequencing ladder. Primer extension analysis of the oprF promoter (Fig. 1A) revealed the presence of a transcript initiating 5 nucleotides downstream from the −10 region of the predicted AlgU-dependent promoter. The oprF transcript was dependent on AlgU, as it was lost in the algU knockout strain (Fig. 1A). As an additional control, we examined the AlgU-independent promoter of oprF, with the mRNA 5′ end at −57 from the translational start (see Fig. 1D). The band corresponding to this promoter was not absent, and its intensity was only 10% lower in the algU::Tcr samples (Fig. 1B). Identical relationships (presence of the transcript in the algU+ strain and absence of the corresponding band in algU::Tcr mutants) were observed with the previously mapped algD promoter (Fig. 1C). These analyses demonstrate that an AlgU-dependent promoter exists upstream of oprF and most likely contributes to oprF expression.
FIG. 1.
Mapping of the AlgU-dependent oprF promoter. (A) Primer extension mapping of the mRNA 5′ end corresponding to the AlgU promoter of oprF. Total RNA was isolated from the algU+ mucA22 mucoid strain PAO578II and from its nonmucoid algU knockout derivative (algU::Tcr; PAO6865) grown under conditions promoting the mucoid phenotype (Materials and Methods). Primer extension products were run adjacent to the sequencing ladder generated with the same primer used for reverse transcription. Vertical bars indicate the −35/−10 regions of the AlgU-dependent promoter of oprF. The nucleotide corresponding to the mRNA 5" end is designated PAlgU with an asterisk. (B) Control for panel A, showing the presence of the oprF AlgU-independent promoter at −57 upstream of the initiation codon. In this particular gel, the intensity of the band in the algU lane was 17% stronger than in the algU::Tcr mutant. (C) Primer extension of the previously mapped AlgU-dependent promoter PD of algD, included here as a control (13). All procedures and designations as in panel A. (D) Maps showing the relative positions of the AlgU-dependent promoters upstream of the algD and oprF genes. A previously mapped oprF promoter, corresponding to the mRNA start site at −40, is dependent on the alternative sigma factor SigX (11). The promoter at −57 is regulated by an unknown sigma factor (17). All numbering is relative to the initiation codon.
Transcriptional analysis of the predicted AlgU (ςsgr;E) promoters.
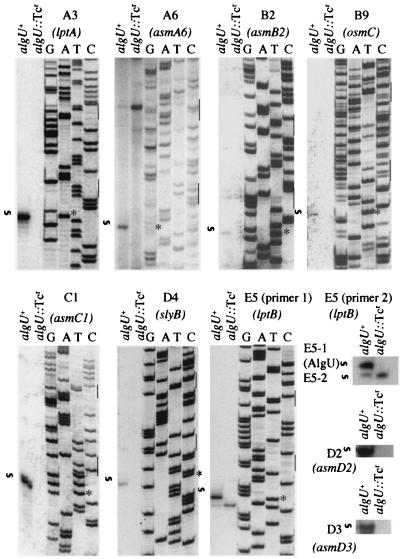
Having demonstrated that one of the putative AlgU promoters is indeed an AlgU-dependent transcriptionally active site, we tested all other potential AlgU promoters predicted from our genomic search. Nine additional AlgU promoters (for a total of 10 new AlgU promoters, including the oprF AlgU promoter) were identified, and the corresponding mRNA 5′ ends were mapped (Fig. 2) using the same approach as for oprF AlgU promoter mapping. In all 10 cases, the band corresponding to the transcriptional start site was downstream of the predicted −35/−10 AlgU promoter regions, with the mRNA 5" end coinciding with a nucleotide located 4 to 6 bp downstream of the canonical −10 AlgU promoter sequence (Fig. 2 and Table 3). In all cases, the transcript was completely absent in the algU mutant strain, with one exception (Fig. 2, E5).
FIG. 2.
Primer extension analysis of the newly identified AlgU-dependent promoters. Promoter mapping was carried out as described for Fig. 1. Designations are given as in Tables 1 and 2. The mRNA start site(s) has been mapped to the nucleotides shown in Table 3. The start site for E5 (primer 1) was also mapped using an additional oligonucleotide (E5 primer 2), to demonstrate that the lower band (AlgU independent) was not an artifact. The sequencing ladders for D2 and D3 are not shown.
TABLE 3.
Mapped AlgU (ςsgr;E) promoters in P. aeruginosa and enteric bacteria
| Bacteria | Designationa | Sequenceb |
|---|---|---|
| P. aeruginosa | A2 oprF | AAGAACTTTGAGGGCAAGTCGAAGTTCAAAACGTGG |
| A3 lptA | TGGAACTTCACGCCAGCGCAAATGTTCAAAGGGCT | |
| A6 asmA6 | CGGAACTTTCCTCCGCGCCGTGGCTCTGAACAGCC | |
| B2 asmB2 | TCGAACTTAGGCGCAGAATGTCGGGTCCCACGGACA | |
| B9 osmC | CTGAACTTGTCCGGCTGCGTCGTCCTCCCATGTAGA | |
| C1 asmC1 | CTGAACTTTCCCGTTTTGCCGACAGTCAGAACACA | |
| D2 asmD2 | AGGAACTTGAGATCGTGCAGGGTGCTCCAATATTTC | |
| D3 asmD3 | GCGAACTTGTCGCAGTCGTGCCGTTCCAAGCAGCC | |
| D4 slyB | TGGAACTTGGTGGTTTTTGCCCAGTCCTAGGCAAGG | |
| E5 lptB | TTGAACTTATCCGCGCGCACCTGTTCCTATTGCCCA | |
| Pa algU P1 | GAGAACTTTTGCAAGAAGCCCGAGTCTATCTTGGCA | |
| Pa algU P3 | TGGAACTTTCTTAGACGCATCGGTTCCAAAGCAGGA | |
| Pa algR | GGGCACTTTTCGGGCCTAAAGCGAGTCTCAGCGTCG | |
| Pa algD | CGGAACTTCCCTCGCAGAGAAAACATCCTATCACCG | |
| Pa rpoH | AGGAACTTATACACCCGCTTGCAGTCAGATATCCGA | |
| Consensus | GAACTT-----16/17 bp-----TCcaA 5/6 bp + 1 | |
| Enterobacteriaceae | Ec rpoH P3 | TTGAACTTGTGGATAAAATCACGGTCTGATAAAACA |
| Ec htrA | CGGAACTTCAGGCTATAAAACGAATCTGAAGAACA | |
| Ec rpoE | CGGAACTTTACAAAAACGAGACACTCTAACCCTTTG | |
| St rpoE | CGGAACTTTACGAAACATAGACACTCTAACCTGTTG | |
| St htrA | CGGAACTTCGCGTTATAAAATGAATCTGACGTACAC | |
| St slyB | ATGACCTTAACTGCATAAATGCCATATAATTTAGCTA | |
| Consensusb | GAACTT------16 bp------TCTRA 5/6 bp + 1 |
Pa, P. aeruginosa; Ec, E. coli; St, S. enterica serovar Typhimurium. The ςsgr;E promoter of Salmonella slyB was not previously recognized, although the mRNA start site has been mapped (34).
Nucleotides that match those in the consensus sequence are shown in boldface type; less-conserved nucleotides are shown in lowercase type. R, purine (A or G).
In the case of E5, the strong AlgU-dependent top band disappeared, but a second, downstream band, which was weak in the algU+ sample, intensified in the algU::Tcr mutant (Fig. 2, E5, primer 1). The second band was not a reverse transcription artifact, as the same pattern was observed using a different primer (Fig. 2, E5, primer 2). Since P. aeruginosa has close to 20 sigma factors from the same class as AlgU, we interpret this observation as a possible recognition of the AlgU promoter E5 by another AlgU homolog. The presence of this slightly intensified second band corresponding to this transcript also demonstrates that the amounts of RNA loaded were comparable in the algU+ and algU::Tcr lanes in all samples.
For the AlgU promoter sites positively identified by reverse transcription in this work but without previously acknowledged or annotated genes downstream of the promoter sites, in four of five cases we could identify an open reading frame downstream of the mRNA 5" end. The corresponding putative genes, designated here as AlgU sigmulon members, have been named asmA6, asmB2, asmC1, asmD2, and asmD3 (Table 2). The distances of these potential genes from the transcriptional start sites ranged from 12 to 254 nucleotides, which is well within the range of the typical AlgU promoter-coding sequence distance (Table 2).
The increased expression of the previously characterized AlgU-dependent alginate-specific genes is dependent on inactivation of mucA, a negative regulator of AlgU (37). We next examined how many of the newly identified promoters were affected by the mucA22 mutation. For this analysis, the nonmucoid mucA+ strain PAO381 and its constitutively mucoid mucA22 derivative (strain PAO578I) were used. The majority of the promoters showed elevated expression levels (ranging from 1.2- to 8.5-fold) in the mucA mutant relative to the mucA+ parent (Fig. 3). These findings indicate that the majority of the AlgU promoters are activated upon the loss of MucA inhibitory activity on AlgU in mucA22 mutants. Nevertheless, basal expression levels were seen in mucA+ strains with most of the AlgU-dependent promoters.
FIG. 3.
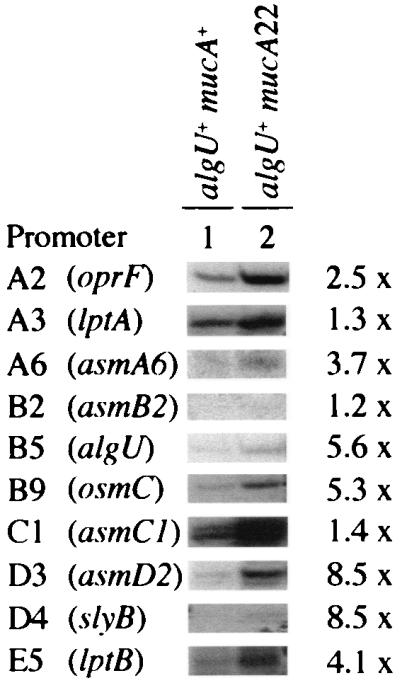
Inactivation of MucA, a negative regulator of AlgU, enhances the expression of the majority of the newly identified AlgU-dependent promoters. Total RNA was isolated from algU+ mucA+ strain PAO381 (lane 1) and its mucoid mucA22 derivative, PAO578I (lane 2), and subjected to primer extension analysis as described for Fig. 1 and 2. Fold induction (determined by densitometry) relative to that in the mucA+ background is indicated to the right of each set.
One of the initially detected transcripts, B2 (Fig. 2), did not show a signal (Fig. 3) in the parental mucA+ (PAO381) strain and showed only a weak signal in the mucA22 mutant strain (PAO578I). Since the strains PAO578II (used in the initial screen) and PAO578I differ in their environmental requirements for the expression of the mucoid phenotype due to the presence of an additional sup-2 mutation in PAO578II (51), we compared all three strains (PAO381, PAO578I, and PAO578II) grown under conditions promoting maximal alginate production (Materials and Methods). As shown in Fig. 4, the B2 promoter signal was reproducibly detected in PAO578II. These observations confirm the notion that the expression of at least some of the AlgU-dependent promoters depends on additional factors even in mucA mutants (51). Consequently, we cannot exclude the possibility that at least some of the 25 putative sites showing negative transcriptional results represent AlgU-dependent promoters but remain silent unless additional regulators are activated or environmental requirements are met.
FIG. 4.
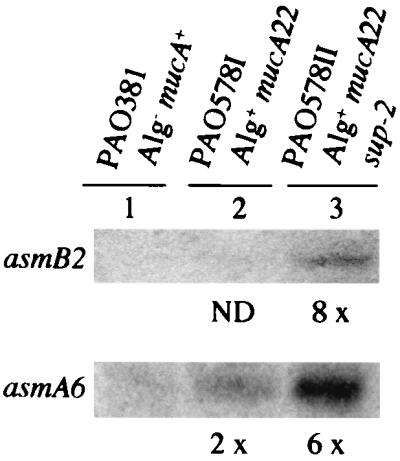
Expression of some AlgU-dependent promoters is dependent on strain type and environmental conditions. The B2 promoter was tested for activity by reverse transcription in the nonmucoid parent strain PAO381 (algU+ mucA+), its constitutively mucoid derivative PAO578I (algU+ mucA22), and strain PAO578II (algU+ mucA22 sup-2; derived from PAO578I), which requires additional environmental stimuli for expression of the mucoid phenotype (see Materials and Methods). A6 is included for comparison. Fold induction levels relative to PAO381 (mucA+) are indicated below the lanes. ND, not detectable.
AlgU controls lipoprotein genes: implications for inflammatory processes in CF.
A destructive, hyperinflammatory response is one of the hallmarks of CF (3, 47). Detailed analyses of open reading frames downstream of the newly identified AlgU-directed promoters revealed that 2 of the 10 characterized AlgU-dependent genes encoded products with putative lipoprotein leader sequences (Fig. 5A). Given the recent implication of bacterial lipoproteins in Toll-like receptor-mediated inflammation (1, 10), we examined whether lipoproteins controlled by AlgU might have proinflammatory activity.
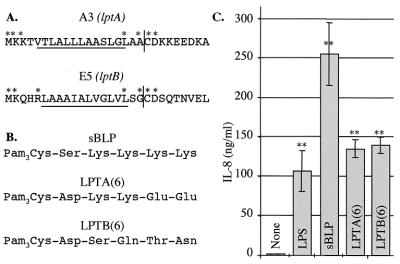
FIG. 5.
Lipopeptides corresponding to the amino termini of the newly identified AlgU-dependent lipoprotein-encoding genes induce IL-8 secretion by human macrophages. (A) Leader peptide sequences corresponding to the predicted products of lptA and lptB. Asterisks indicate residues required for processing. The underlined portion demarcates the hydrophobic residues within the leader peptide. The leader peptide is cleaved (vertical line) just prior to the cysteine residue which is modified by palmitylation (45). (B) Sequence of synthesized lipopeptides corresponding to the first six amino acids of mature LptA [LPTA(6)] and LptB [LPTB(6)]. sBLP, standard synthetic bacterial lipopeptide (1, 25). (C) Human macrophages derived from peripheral blood monocytes were incubated for 24 h with medium alone (bar labeled None), LPS (1 μg/ml), or bacterial lipopeptides (10 μg/ml), as indicated. Supernatants were assayed for IL-8 production by enzyme-linked immunosorbent assay. ∗∗, P < 0.001 compared to uninduced control (None).
Figure 5A displays the amino-terminal sequence of the predicted P. aeruginosa lptA and lptB gene products, highlighting (i) the residues critical for lipopeptide sequence modification, (ii) the leader sequences containing typical cleavage sites, and (iii) the lipid-modified cysteine residues. To address the possibility that LptA and LptB may have proinflammatory activity, we synthesized palmitylated lipopeptides corresponding to the first 6 amino acids of the predicted mature LptA and LptB (Fig. 5B). The synthesized lipopeptides were assayed for their ability to induce IL-8 secretion by primary human macrophages derived from peripheral blood monocytes (Fig. 5C). Upon stimulation with lipopeptides LPTA(6) and LPTB(6), used in standard concentrations usually applied in such experiments (1, 10) albeit exceeding that of LPS, significant amounts of IL-8 were detected (P < 0.001). The peptides corresponding to the LptA and LptB N termini caused IL-8 secretion equal in potency to that of LPS and comparable to the standard peptide simulating lipoprotein action (1, 25). These findings suggest that at least some of the genes coexpressed with the alginate system during conversion to mucoidy may play a role in inflammatory processes in CF.
DISCUSSION
Using the newly available P. aeruginosa genomic sequence information and conventional molecular biology techniques, we have identified 10 new P. aeruginosa promoters dependent on the alternative sigma factor AlgU. Of the 35 candidate sites containing the AlgU (P. aeruginosa ςsgr;E) consensus sequence, one-third were confirmed by mRNA 5"-end mapping and additionally demonstrated to depend on AlgU for transcription. The present work has tripled the number of known and mapped P. aeruginosa AlgU promoters from the previously defined 5 to a current total of 15. The confirmed promoters did firm up the AlgU (ςsgr;E) promoter consensus further, as shown in Table 3.
In the process of comparing AlgU-controlled genes and corresponding homologs in S. enterica serovar Typhimurium and E. coli, we also recognized a previously unappreciated ςsgr;E promoter in front of the Salmonella slyB gene encoding an outer membrane porin (33), which coincides with the reported slyB mRNA 5" end (Table 3). Thus, the current study also helped find an additional ςsgr;E promoter in another organism.
Follow-up studies presented here have uncovered a previously unknown link of conversion to the mucoid phenotype in P. aeruginosa with the induction of potential proinflammatory factors. We identified two genes, lptA and lptB, that encode putative lipoproteins, as being controlled by AlgU. The promoter activity of lptA and lptB is induced in mucA mutant cells. The synthetic lipopeptides corresponding to the N termini of the mature, processed LptA and LptB caused IL-8 production in primary human macrophages derived from peripheral blood monocytes. This observation expands the potential impact of conversion to mucoidy in P. aeruginosa on pathogenesis issues. This phenomenon has been ascribed in the past primarily, if not exclusively, to the production of alginate, with the following proposed roles for the mucoid coating: (i) overall inhibition of P. aeruginosa clearance from the lungs (8, 62), possibly related to the proposed inhibition of opsonic (54) and nonopsonic phagocytosis (30, 41); (ii) potential radical scavenging function of alginate in reducing the impact of reactive oxygen intermediates and hypochlorite generated by immune defense cells (31, 55); and (iii) inhibition of leukocyte function, including chemotaxis, complement activation, and oxidative burst (26, 43, 57). The new observations presented here provide an expanded view of the conversion to mucoidy in P. aeruginosa by linking it with the inflammatory response in the host. Coinduction of lipoproteins that have proinflammatory potential may be a mechanism by which conversion to mucoidy could contribute to the excessive inflammatory response typically observed in the infected CF lung.
Not all of the predicted P. aeruginosa AlgU promoters identified with the sequence consensus search were experimentally confirmed. The presence of a band for the promoter designated B2 under varied conditions raises the possibility that some of the potential AlgU-dependent promoters that remain unconfirmed may be functional only under optimized conditions. In the case of phuR, the predicted AlgU promoter overlaps the two Fur boxes, a region shown to be under tight repression by the Fur protein (40). Conditions of low iron could relieve the negative regulation by Fur and allow promoter recognition by AlgU.
In addition to the potential increase in actual promoter number resulting from the possibility of specific expression requirements, the number of AlgU promoters in P. aeruginosa could be even greater, considering some known and well-defined AlgU promoters that depart from the consensus by one residue. Notable examples are the promoters for algR, which has a substitution in the −35 region, and the P1 promoter for algU, which has a substitution in the final position of the −10 region. Neither of these promoters was detected by a subsequence search with the original criteria. Allowing for additional substitutions individually (e.g., −35 GCACTT [algR] and −10 TCTAT [algU P1]; underlined residues indicate departures from the consensus) revealed 63 additional sites on the P. aeruginosa chromosome.
LptA and LptB were examined in this work in the context of their lipoprotein structure, in line with our original idea that proinflammatory factors may be coinduced with alginate production in mucA mutant mucoid cells. However, at least one of these putative gene products most likely plays a role in protein folding. LptB shows strong homology to FKBP-like peptidyl-prolyl cis/trans isomerases, which catalyze the interconversion of the proline peptide bonds between the cis and trans isomers (46). This proposed function of LptB is in keeping with the nature of the majority of genes controlled by AlgU (i.e., stress response or protein folding) identified here and elsewhere (14, 18, 32, 38). The corresponding ςsgr;E-controlled genes in enterics include another peptidyl-prolyl isomerase (fkpA) (12).
The role of LptA is more obscure because it encodes a short polypeptide of 78 residues and has no homology to any known sequence. It is possible that LptA participates in some processes similar to what seems to be the common theme for AlgU-regulated genes, i.e., general defense against stress. It may also be that LptA (or both LptA and LptB) is more narrowly associated with alginate production in the extracytoplasmic spaces, as it is coinduced with alginate production. Future inactivation of lptA and lptB genes will address this possibility.
Unlike the case of LptB, there are very few a priori clues regarding the possible function of LptA, apart form the potential role in alginate-specific processes discussed above. At present, we do not exclude the possibility that LptA, and perhaps some other molecules of short amino acid stretches with perfect lipoprotein processing and modification signals, is made as a bacterial product that acts primarily to cause uncontrolled inflammatory processes in the infected host. Should this be the case, we propose the possibility that such products could be a novel class of bacterial toxins directed to stimulate host pattern recognition receptors.
Our current data are consistent with the idea that LptA and LptB can act as proinflammatory agents. Based on the data presented here, the activation of the AlgU sigmulon may have broader implications for the host with CF in that at least some factors coinduced with alginate overproduction may act to exacerbate inflammation and lung disease in CF.
Acknowledgments
We thank Michal H. Mudd for technical expertise and help with this study and Lisa Tatterson and Jens Poschet for discussions.
This work was supported by NIH grant AI31139.
REFERENCES
- 1.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 2.Bass, S., Q. Gu, and A. Christen. 1996. Multicopy suppressors of Prc mutant Escherichia coli include two HtrA (DegP) protease homologs (HhoAB), DksA, and a truncated RlpA. J. Bacteriol. 178:1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birrer, P., N. G. McElvaney, A. Rudeberg, C. W. Sommer, S. Liechti-Gallati, R. Kraemer, R. Hubbard, and R. G. Crystal. 1994. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 150:207-213. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hillard, J. B. Hillard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, J. C., J. Martinez-Salazar, M. J. Schurr, M. H. Mudd, H. Yu, and V. Deretic. 1996. Two distinct loci affecting conversion to mucoidy in Pseudomonas aeruginosa in cystic fibrosis encode homologs of the serine protease HtrA. J. Bacteriol. 178:511-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher, J. C., M. J. Schurr, and V. Deretic. 2000. Dual regulation of mucoidy in Pseudomonas aeruginosa and sigma factor antagonism. Mol. Microbiol. 36:341-351. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, J. C., M. J. Schurr, H. Yu, D. W. Rowen, and V. Deretic. 1997. Pseudomonas aeruginosa in cystic fibrosis: role of mucC in the regulation of alginate production and stress sensitivity. Microbiology 143:3473-3480. [DOI] [PubMed] [Google Scholar]
- 8.Boucher, J. C., H. Yu, M. H. Mudd, and V. Deretic. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect. Immun. 65:3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouvier, J., S. Gordia, G. Kampmann, R. Lange, R. Hengge-Aronis, and C. Gutierrez. 1998. Interplay between global regulators of Escherichia coli: effect of RpoS, Lrp and H-NS on transcription of the gene osmC. Mol. Microbiol. 28:971-980. [DOI] [PubMed] [Google Scholar]
- 10.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 11.Brinkman, F. S., G. Schoofs, R. E. Hancock, and R. De Mot. 1999. Influence of a putative ECF sigma factor on expression of the major outer membrane protein, OprF, in Pseudomonas aeruginosa and Pseudomonas fluorescens. J. Bacteriol. 181:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danese, P. N., and T. J. Silhavy. 1997. The sigma(E) and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 13.Deretic, V., J. F. Gill, and A. M. Chakrabarty. 1987. Pseudomonas aeruginosa infection in cystic fibrosis: nucleotide sequence and transcriptional regulation of the algD gene. Nucleic Acids Res. 15:4567-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deretic, V., M. J. Schurr, J. C. Boucher, and D. W. Martin. 1994. Conversion of Pseudomonas aeruginosa to mucoidy in cystic fibrosis: environmental stress and regulation of bacterial virulence by alternative sigma factors. J. Bacteriol. 176:2773-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deretic, V., M. J. Schurr, and H. Yu. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 3:351-356. [DOI] [PubMed] [Google Scholar]
- 16.DeVries, C. A., and D. E. Ohman. 1994. Mucoid-to-nonmucoid conversion in alginate-producing Pseudomonas aeruginosa often results from spontaneous mutations in algT, encoding a putative alternate sigma factor, and shows evidence for autoregulation. J. Bacteriol. 176:6677-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duchene, M., A. Schweizer, F. Lottspeich, G. Krauss, M. Marget, K. Vogel, B. U. von Specht, and H. Domdey. 1988. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J. Bacteriol. 170:155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson, J. W., and C. A. Gross. 1989. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 3:1462-1471. [DOI] [PubMed] [Google Scholar]
- 19.Eshoo, M. W. 1988. lac fusion analysis of the bet genes of Escherichia coli: regulation by osmolarity, temperature, oxygen, choline, and glycine betaine. J. Bacteriol. 170:5208-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzSimmons, S. C. 1993. The changing epidemiology of cystic fibrosis. J. Pediatr. 122:1-9. [DOI] [PubMed] [Google Scholar]
- 21.Flynn, J. L., and D. E. Ohman. 1988. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J. Bacteriol. 170:1452-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fyfe, J. A., and J. R. Govan. 1980. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved in control. J. Gen. Microbiol. 119:443-450. [DOI] [PubMed] [Google Scholar]
- 23.Govan, J. R. W., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiratsu, K., M. Amemura, H. Nashimoto, H. Shinagawa, and K. Makino. 1995. The rpoE gene of Escherichia coli, which encodes sigma E, is essential for bacterial growth at high temperature. J. Bacteriol. 177:2918-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann, P., S. Heinle, U. F. Schade, H. Loppnow, A. J. Ulmer, H. D. Flad, G. Jung, and W. G. Bessler. 1988. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology 177:158-170. [DOI] [PubMed] [Google Scholar]
- 26.Jensen, E. T., A. Kharazmi, K. Lam, J. W. Costerton, and N. Hoiby. 1990. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect. Immun. 58:2383-2385. [DOI] [PMC free article] [PubMed]
- 27.Khan, T. Z., J. S. Wagener, T. Bost, J. Martinez, F. J. Accurso, and D. W. Riches. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151:1075-1082. [DOI] [PubMed] [Google Scholar]
- 28.Koch, C., and N. Hoiby. 1993. Pathogenesis of cystic fibrosis. Lancet 341:1065-1069. [DOI] [PubMed] [Google Scholar]
- 29.Konstan, M. W., P. J. Byard, C. L. Hoppel, and P. B. Davis. 1995. Effect of high-dose ibuprofen in patients with cystic fibrosis. N. Engl. J. Med. 332:848-854. [DOI] [PubMed] [Google Scholar]
- 30.Krieg, D. P., R. J. Helmke, V. F. German, and J. A. Mangos. 1988. Resistance of mucoid Pseudomonas aeruginosa to nonopsonic phagocytosis by alveolar macrophages in vitro. Infect. Immun. 56:3173-3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Learn, D. B., E. P. Brestel, and S. Seetharama. 1987. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect. Immun. 55:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipinska, B., S. Sharma, and C. Georgopoulos. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 16:10053-10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig, A., C. Tengel, S. Bauer, A. Bubert, R. Benz, H. J. Mollenkopf, and W. Goebel. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Gen. Genet. 249:474-486. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra, S., L. A. Silo-Suh, K. Mathee, and D. E. Ohman. 2000. Proteome analysis of the effect of mucoid conversion on global protein expression in Pseudomonas aeruginosa strain PAO1 shows induction of the disulfide bond isomerase, DsbA. J. Bacteriol. 182:6999-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, D. W., B. W. Holloway, and V. Deretic. 1993. Characterization of a locus determining the mucoid status of Pseudomonas aeruginosa: AlgU shows sequence similarities with a Bacillus sigma factor. J. Bacteriol. 175:1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin, D. W., M. J. Schurr, M. H. Mudd, and V. Deretic. 1993. Differentiation of Pseudomonas aeruginosa into the alginate-producing form: inactivation of mucB causes conversion to mucoidy. Mol. Microbiol. 9:497-506. [DOI] [PubMed] [Google Scholar]
- 37.Martin, D. W., M. J. Schurr, M. H. Mudd, J. R. W. Govan, B. W. Holloway, and V. Deretic. 1993. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 90:8377-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin, D. W., M. J. Schurr, H. Yu, and V. Deretic. 1994. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to sigma E and stress response. J. Bacteriol. 176:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Missiakas, D., and S. Raina. 1998. The extracytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 40.Ochsner, U. A., Z. Johnson, and M. L. Vasil. 2000. Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185-198. [DOI] [PubMed] [Google Scholar]
- 41.Oliver, A. M., and D. M. Weir. 1983. Inhibition of bacterial binding to mouse macrophages by Pseudomonas alginate. J. Clin. Lab. Immunol. 10:221-224. [PubMed] [Google Scholar]
- 42.Pedersen, S. S. 1992. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS 100(Suppl. 28):1-79. [PubMed] [Google Scholar]
- 43.Pedersen, S. S., A. Kharazmi, F. Espersen, and N. Hoiby. 1990. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect. Immun. 58:3363-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poschet, J. F., A. M. Firoved, and V. Deretic. 2001. Conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Methods Enzymol. 336:65-76. [DOI] [PubMed] [Google Scholar]
- 45.Pugsley, A. P. 1993. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahfeld, J. U., K. P. Rucknagel, G. Stoller, S. M. Horne, A. Schierhorn, K. D. Young, and G. Fischer. 1996. Isolation and amino acid sequence of a new 22-kDa FKBP-like peptidyl-prolyl cis/trans-isomerase of Escherichia coli. Similarity to Mip-like proteins of pathogenic bacteria. J. Biol. Chem. 271:22130-22138. [DOI] [PubMed] [Google Scholar]
- 47.Richman-Eisenstat, J. B., P. G. Jorens, C. A. Hebert, I. Ueki, and J. A. Nadel. 1993. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am. J. Physiol. 264:L413-L418. [DOI] [PubMed] [Google Scholar]
- 48.Rouviere, P. E., A. De Las Penas, J. Mecsas, C. Z. Lu, K. E. Rudd, and C. A. Gross. 1995. rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J. 14:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowen, D. W., and V. Deretic. 2000. Membrane-to-cytosol redistribution of ECF sigma factor AlgU and conversion to mucoidy in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Mol. Microbiol. 36:314-327. [DOI] [PubMed] [Google Scholar]
- 50.Schurr, M. J., and V. Deretic. 1997. Microbial pathogenesis in cystic fibrosis: co-ordinate regulation of heat-shock response and conversion to mucoidy in Pseudomonas aeruginosa. Mol. Microbiol. 24:411-420. [DOI] [PubMed] [Google Scholar]
- 51.Schurr, M. J., D. W. Martin, M. H. Mudd, and V. Deretic. 1994. Gene cluster controlling conversion to alginate-overproducing phenotype in Pseudomonas aeruginosa: functional analysis in a heterologous host and role in the instability of mucoidy. J. Bacteriol. 176:3375-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schurr, M. J., H. Yu, J. C. Boucher, N. S. Hibler, and V. Deretic. 1995. Multiple promoters and induction by heat shock of the gene encoding the alternative sigma factor AlgU (sigma E) which controls mucoidy in cystic fibrosis isolates of Pseudomonas aeruginosa. J. Bacteriol. 177:5670-5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schurr, M. J., H. Yu, J. M. Martinez-Salazar, J. C. Boucher, and V. Deretic. 1996. Control of AlgU, a member of the sigma E-like family of stress sigma factors, by the negative regulators MucA and MucB and Pseudomonas aeruginosa conversion to mucoidy in cystic fibrosis. J. Bacteriol. 178:4997-5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson, J. A., S. E. Smith, and R. T. Dean. 1988. Alginate inhibition of the uptake of Pseudomonas aeruginosa by macrophages. J. Gen. Microbiol. 134:29-36. [DOI] [PubMed] [Google Scholar]
- 55.Simpson, J. A., S. E. Smith, and R. T. Dean. 1989. Scavenging by alginate of free radicals released by macrophages. Free Radic. Biol. Med. 6:347-353. [DOI] [PubMed] [Google Scholar]
- 56.Sprenger, G. A., U. Schorken, G. Sprenger, and H. Sahm. 1995. Transaldolase B of Escherichia coli K-12: cloning of its gene, talB, and characterization of the enzyme from recombinant strains. J. Bacteriol. 177:5930-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stiver, H. G., K. Zachidniak, and D. P. Speert. 1988. Inhibition of polymorphonuclear leukocyte chemotaxis by the mucoid exopolysaccharide of Pseudomonas aeruginosa. Clin. Investig. Med. 11:247-252. [PubMed] [Google Scholar]
- 58.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 59.Tatterson, L. E., J. F. Poschet, A. Firoved, J. Skidmore, and V. Deretic. 2001. CFTR and Pseudomonas infections in cystic fibrosis. Front. Biosci. 6:D890-D897. [DOI] [PubMed] [Google Scholar]
- 60.Welsh, M. J., L.-C. Tsui, T. F. Boat, and A. L. Beaudet. 1995. Cystic fibrosis, p. 3799-3876. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle (ed.), The metabolic and molecular basis of inherited disease, vol. III. McGraw-Hill, Inc., New York, N.Y. [Google Scholar]
- 61.Woodruff, W. A., and R. E. Hancock. 1988. Construction and characterization of Pseudomonas aeruginosa protein F-deficient mutants after in vitro and in vivo insertion mutagenesis of the cloned gene. J. Bacteriol. 170:2592-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, H., M. Hanes, C. E. Chrisp, J. C. Boucher, and V. Deretic. 1998. Microbial pathogenesis in cystic fibrosis: pulmonary clearance of mucoid Pseudomonas aeruginosa and inflammation in a mouse model of repeated respiratory challenge. Infect. Immun. 66:280-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu, H., M. J. Schurr, and V. Deretic. 1995. Functional equivalence of Escherichia coli sigma E and Pseudomonas aeruginosa AlgU: E. coli rpoE restores mucoidy and reduces sensitivity to reactive oxygen intermediates in algU mutants of P. aeruginosa. J. Bacteriol. 177:3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, G., and M. E. Winkler. 1994. An Escherichia coli K-12 tktA tktB mutant deficient in transketolase activity requires pyridoxine (vitamin B6) as well as the aromatic amino acids and vitamins for growth. J. Bacteriol. 176:6134-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]