Abstract
We have characterized the yyaA gene of Bacillus subtilis, located near the origin of chromosome replication (oriC). Its protein product is similar to the Spo0J protein, which belongs to the ParB family of chromosome- and plasmid-partitioning proteins. Insertional inactivation of the yyaA gene had no apparent effect on chromosome organization and partitioning during vegetative growth or sporulation. Subcellular localization of YyaA by immunofluorescence microscopy indicated that it colocalizes with the nucleoid, and gel retardation studies confirmed that YyaA binds relatively nonspecifically to DNA. Overexpression of yyaA caused a sporulation defect characterized by the formation of multiple septa within the cell. This phenotype indicates that YyaA may have a regulatory role at the onset of sporulation.
Chromosome segregation ensures the spatial separation of sister chromosomes into daughter cells prior to cell division. The mechanism that governs chromosome partitioning ensures that chromosome loss happens very infrequently (<0.03% of newborn cells) (18).
Molecular genetics methods have led to the identification of some genes implicated in Bacillus subtilis chromosome segregation. spo0J (also called spo0jb) is located in the origin region of the chromosome (21, 36), and its gene product is a member of the ParB family of DNA-binding proteins involved in chromosome and plasmid partitioning (53). The Spo0J protein forms discrete assemblies closely associated with the oriC region (15, 28, 31), and a series of Spo0J-binding sites (parS sites) have been identified in the origin-proximal 20% of the chromosome (30). Spo0J foci duplicate early in the replication cycle and move rapidly apart, providing strong evidence for an active mechanism of chromosome segregation (15, 28, 48).
The product of the gene directly upstream of spo0J, soj (also called spo0JA), is similar to members of the ParA family of ATPases (53). Soj has been found to inhibit sporulation by dissociating transcription initiation complexes formed by phosphorylated Spo0A, a key regulator of sporulation initiation, and RNA polymerase and to directly associate with promoter regions of sporulation genes (7, 44). This inhibitor-like activity of Soj is antagonized by Spo0J (21). The role of Soj in chromosome partitioning is less clear, as a null mutation has only a minor effect on segregation (21). However, Soj was shown to be required, in conjunction with Spo0J, to stabilize an otherwise unstable plasmid bearing a Spo0J-binding site (30). Soj, localized in irregular nucleoid-associated patches, appears to be involved in the condensation of Spo0J foci and thus probably is involved in the organization of the origin region, at least during sporulation (32).
ParA and ParB homologues are present in a wide range of bacteria (though not in Escherichia coli and Haemophilus influenzae), and in Caulobacter crescentus the ParB-encoding gene is essential and apparently needed for chromosome segregation (35). Thus, it is likely that the basic mechanism by which ParB proteins function is conserved. However, though a B. subtilis spo0J null mutation increases chromosome loss by 100-fold (21), it is not lethal, indicating that other mechanisms contribute to efficient chromosome segregation in this organism.
B. subtilis differs from organisms such as Pseudomonas putida and C. crescentus in having a second parB-like gene, yyaA, also located in the origin region (35, 36). YyaA and Spo0J are 36% identical, and the close proximity of the encoding genes suggests that they arose by gene duplication (36). With the expectation that chromosome segregation is essential (and because spo0J null mutants are viable), it seemed possible that YyaA might be another component of the chromosome segregation system.
Here we describe the initial characterization of the yyaA gene and its protein product. Unexpectedly, a yyaA null mutation had no obvious effect on chromosome segregation or nucleoid organization. However, overexpression of the yyaA gene drastically perturbed sporulation, leading to the formation of multiple sporulation septa in the cell as judged by electron microscopy. The latter result suggests a role for YyaA in the regulation of septum formation in sporulation.
MATERIALS AND METHODS
Bacterial strains.
The strains and plasmids used in this work are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or characteristics | Construction, source, or reference |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| SG38 | trpC2 amyE | 11 |
| JH642 | trpc2 phe-1 | Laboratory stock |
| SG38::pPS1326 | trpC2 amyE Ω(amyE::gpr"-"lacZ cat) | 56 |
| 2008 | trpC2 amyE Ω(amyE::gpr"-"lacZ cat) pRM113 | SG38::pPS1326 transformed to erythromycin resistance with pRM113 |
| 2009 | trpC2 amyE Ω(amyE::gpr"-"lacZ cat) pHT315 | SG38::pPS1326 transformed to erythromycin resistance with pHT315 |
| 2023 | trpC2 amyE yyaA::ermC | SG38 transformed to erythromycin resistance with pSG1443 |
| 2048 | trpC2 amyE pRM113 (multicopy plasmid) | SG38 transformed to erythromycin resistance with pRM113 |
| 2049 | trpC2 amyE pHT315 (multicopy plasmid) | SG38 transformed to erythromycin resistance with pHT315 |
| ts-134 | trpC2 thyA thyB dnaB-134(Ts) | 33 |
| JH21083 | trpC2 phe-1 amyE Ω(amyE::yyaA"-lacZ cat) | JH642 transformed to chloramphenicol resistance with pMB115 |
| JH21084 | trpC2 phe-1 spo0A12 amyE Ω(amyE::yyaA"-lacZ cat) | JH642 spo0A12 transformed to chloramphenicol resistance with pMB115 |
| JH21085 | trpC2 phe-1 spo0H81 amyE Ω(amyE::yyaA"-lacZ cat) | JH642 spo0H81 transformed to chloramphenicol resistance with pMB115 |
| JH21093 | trpC2 phe-1 abrB::Tn917erm amyE Ω(amyE::yyaA"-lacZ cat) | JH642 abrB::Tn 917 transformed to chloramphenicol resistance with pMB115 |
| JH21094 | trpC2 phe-1 amyE Ω(amyE::spoIIA"-lacZ cat) pHT315 | JH642 amyE::spoIIA"-lacZ transformed to erythromycin resistance with pHT315 |
| JH21095 | trpC2 phe-1 amyE Ω(amyE::spoIIA"-lacZ cat) pRM113 | JH642 amyE::spoIIA"-lacZ transformed to erythromycin resistance with pRM113 |
| JH21096 | trpC2 phe-1 amyE Ω(amyE::spoIIA"-lacZ cat) pHT315 | JH642 amyE::spoIIG"-lacZ transformed to erythromycin resistance with pHT315 |
| JH21097 | trpC2 phe-1 amyE Ω(amyE::spoIIG"-lacZ cat) pRM113 | JH642 amyE::spoIIG"-lacZ transformed to erythromycin resistance with pRM113 |
| E. coli | ||
| DH5α | F−endA1 hsdR17 supE44 thi-1 λ−recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 φ80d lacZΔM15 | Gibco BRL |
| BL21(DE3)/pLysS | B F−dcm ompT hsdS(rB− mB−) gal λ(DE3) [pLysS] Camr | Promega |
| Plasmids | ||
| pET-3a | T7 expression vector | 51 |
| pET16b | T7 expression vector | Novagen |
| pJM116 | lacZ transcriptional fusion | 38 |
| pHT315 | Replicative vector (Ermr) | 3 |
| pBS19 | Replicative vector (Cmr) | 39 |
| pRM113 | pHT315 containing yyaA | This study |
| pSG250 | bla ermC | 9 |
| pSG1442 | pET-3a containing yyaA | This study |
| pSG1443 | ermC cassette from pSG250 inserted into SstI site of pSG1442 | This study |
| pMB2 | pET16b containing yyaA | This study |
| pMB115 | pJM116 containing yyaA promoter fused to lacZ | This study |
General methods.
B. subtilis strains were transformed by the method described by Anagnostopoulos and Spizizen (1), as modified by Jenkinson (22). Transformants were selected on Oxoid nutrient agar containing, as necessary, kanamycin (5 μg ml−1), spectinomycin (50 μg/ml−1), erythromycin (1 μg ml−1 for ermC-carrying strains or 25 μg ml−1 for pHT315-containing strains), or chloramphenicol (5 μg ml−1). Sporulation was induced by growth in Schaeffer's sporulation medium (47) or in a hydrolyzed casein medium, followed by resuspension in a starvation medium (SM) (37, 49). S medium was as described by Karamata and Gross (23).
DNA manipulations and E. coli transformations were carried out as described by Sambrook et al. (46). All cloning was done in E. coli DH5α (Gibco BRL).
β-Galactosidase activity of cultures grown in Schaeffer's sporulation medium were assayed as described by Ferrari et al. (12), and the ONPG (o-nitrophenyl-β-d-galactopyranoside) units were calculated as described by Miller (34). For strains induced to sporulate by the resuspension method, β-galactosidase activity was measured by the method of Errington and Mandelstam (11). One methylumbelliferyl-β-d-galactopyranoside (MUG) unit of β-galactosidase catalyzes the production of 1 nmol of 4-methylumbelliferone per min under the standard reaction conditions. (One MUG unit represents about 10 ONPG units.)
Alkaline phosphatase (APase) was assayed as described by Errington and Mandelstam (10). One unit of enzyme hydrolyzes 1 nmol of p-nitrophenyl phosphate per min under standard reaction conditions.
Construction of a yyaA null mutant.
The yyaA gene was amplified by PCR from strain SG38 using primers 6778 (5"-GTAGGCATATGAAGCATTC-3") and 6779 (5"-GAATTGATCAACAAGCTCAAAG-3"), introducing NdeI and BclI sites, respectively. The NdeI-BclI fragment was ligated to NdeI- and BamHI-digested pET-3a to generate pSG1442. A yyaA disruption was made by insertion of the ermC resistance cassette of pSG250 into the unique SstI site within plasmid pSG1442. The ermC gene was excised as an SstI fragment and ligated into SstI-digested pSG1442 to give pSG1443. pSG1443 was transformed into B. subtilis with selection for erythromycin resistance. Transformants were screened by Southern blotting and PCR amplification to identify recombinants which carried an inactivated yyaA gene arising from a double-crossover event (data not shown). One recombinant obtained (strain 2023) was chosen for further analysis.
Antibody production and Western blotting.
To purify YyaA for antibody production, pSG1442 was transformed into E. coli BL21(pLysS) (Promega) and the resultant strain was grown in 100 ml of 2× YT (46) containing 100 μg of ampicillin/ml at 37°C. When the culture reached an optical density at 600 nm of 0.5, IPTG (isopropyl-β-d-thiogalactopyranoside) was added (final concentration, 0.5 mM). The induced cells were grown for a further 2 h before harvesting. The culture pellet was resuspended in 10 ml Tris-EDTA and disrupted by sonication. The resulting preparation was clarified by centrifugation at 20,000 × g. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (4×) was added to the collected supernatant, and the mixture was heated to 95°C for 2 min. The extract was separated by SDS-12% PAGE, and the proteins were visualized by shaking with ice-cold KCl (0.25 M) for 5 min, followed by washing with cold water. YyaA was obtained by elution of a slice of gel containing the overproduced protein, followed by acetone precipitation. The precipitate was resuspended in 1× phosphate-buffered saline and used for rabbit injection. A polyclonal antiserum was raised by standard procedures (16) using a university service. The antibodies were affinity purified as described by Reznekov et al. (45), using MgCl2 as the eluant. The final purified antibody was used at dilutions of 1:1,000 for Western blot analysis. Western blotting was carried out essentially as described by Wu and Errington (55) using an enhanced chemiluminescence detection system (Amersham).
Immunofluorescence and light microscopy.
Cells were fixed, permeabilized, and stained for immunofluorescence microscopy (IF) as described previously (29, 42, 45). An affinity-purified anti-YyaA antibody was used at a concentration of 1:20. Images were grabbed, processed, and assembled as described previously (29). Cell morphology was analyzed by phase-contrast light microscopy after fixation with ethanol as described previously (17). The images obtained were processed with IPLab Spectrum, version 3.1, software (Signal Analyticals, Vienna, Va.), and final images were assembled in Adobe Photoshop, version 3.0.5.
Electron microscopy.
Samples (10 ml) from sporulating cultures of strains SG38, 2048, and 2049 were centrifuged at 4°C, resuspended in 1 ml of Kellenberger buffer (24) containing 4% glutaraldehyde, and fixed at 4°C for 2 h. The cells were pelleted and washed twice with cold Kellenburger buffer. Postfixation, processing, embedding, staining, and sectioning were performed as described by Illing and Errington (20). The sections were observed as described by Feucht et al. (13) in a Zeiss 912 Omega electron microscope (accelerating voltage, 80 kV), and images were collected with a charge-coupled device camera (1,024-by-1,024 chip; depth, 14 bits) and processed in Adobe Photoshop, version 3.0.5.
Inhibition of DNA replication.
DNA replication was inhibited in strain ts-134, which carried a temperature-sensitive dnaB mutation (33). The cells were grown in S medium at 30°C to an optical density of 0.2 and then shifted to 45°C and incubated for 1 h. Cells were fixed for IF as described above.
Protein expression and purification.
The yyaA gene was amplified by PCR from chromosomal DNA using oligonucleotides that introduced an NdeI site at the 5" end (5"-TGTAGGCATATGAAGCATTCATTCTCTCGT-3") and a BamHI site at the 3" end (5"-TTTTCAGGATCCCTTCTATTTTGGTATGCGAA-3"). The fragment obtained was cloned in the pET16b vector (Novagen), thereby creating an extension of 10 histidine codons at the 5" end of the yyaA gene. After expression was induced with 3 mM IPTG for 2 h at 37°C, the YyaA protein was purified from E. coli BL21(DE3) on a nickel affinity column according to the manufacturer's protocol (Ni-nitrilotriacetic acid NTA agarose; Qiagen). Protein concentration was measured by the Bradford-based Bio-Rad protein assay. The protein was stored in a buffer containing 50 mM Tris-HCl, pH 8.0, 50 mM KCl, 5 mM β-mercaptoethanol, and 5% glycerol.
Gel mobility shift assay.
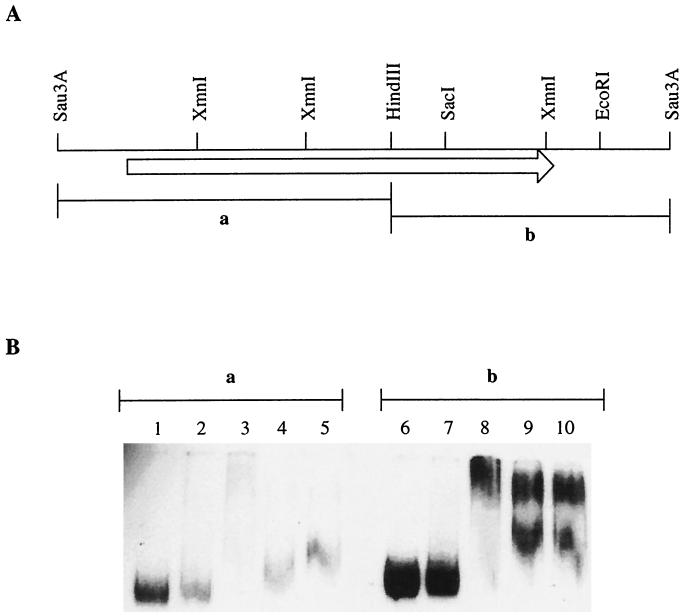
DNA fragments were generated by digestion of plasmid pRM113 with HindIII and BamHI. Fragment A (710 bp) contained the promoter region and the yyaA 5" half end, while fragment B (650 bp) contained the yyaA 3"-terminal portion and downstream region (see Fig. 4A). After digestion, the fragments were labeled at both ends with [α-32P]dATP using the Klenow enzyme. The DNA was fractionated on a 1% agarose gel, and gel slices containing the labeled fragments were excised and the DNA was recovered by spin elution (GenElute; Sigma) and precipitated with ethanol.
FIG. 4.
YyaA is a DNA-binding protein. (A) Restriction map of the yyaA locus. The lengths and positions of the fragments (a and b) used in the gel mobility shift assay are indicated. Note that the Sau3A sites shown are not unique. (B) The gel mobility shift assay was carried out as described in Materials and Methods. The [α-32P]dATP-labeled fragments (a and b; 500 pM) were mixed with YyaA protein at 0.84 (lanes 2 and 7) or 1.68 μM (lanes 3, 4, 5, 8, 9, and 10). Calf thymus DNA (1 μg) was added to lanes 4 and 9, while poly(dA-dT)-poly(dG-dC) (1 μg) was added to lanes 5 and 10. The reactions were carried out for 4 min at room temperature before loading on a 5% polyacrylamide gel.
Binding of YyaA to DNA fragments was carried out for 4 min at room temperature in 10-μl reaction volumes containing 10 mM HEPES, pH 8.0, 10 mM Mg acetate, 0.1 mM dithiothreitol, 80 mM K acetate, and 0.1 mg of bovine serum albumin/ml. Labeled DNA was used at approximately 500 pM per reaction, and the amounts of YyaA were 0.84 and 1.68 μM, respectively. Loading dye was added, and the sample was immediately applied to a prerun 5% polyacrylamide gel running at 7 V/cm in a Tris-acetate-EDTA buffer at room temperature. After electrophoresis, the gel was dried and exposed to Kodak X-Omat AR film.
Overexpression of yyaA.
SG38 was transformed with multicopy plasmids pRM113 (containing yyaA) and pHT315 (control) to give strains 2048 and 2049, respectively. Strains 2048 and 2049 were selected and maintained in the presence of erythromycin (25 μg ml−1). For sporulation experiments, these strains were grown in hydrolyzed casein medium supplemented with the appropriate antibiotic and resuspended in unsupplemented SM.
RESULTS
yyaA, a second parB-like gene in B. subtilis, has no apparent role in chromosome segregation.
Analysis of the sequence downstream of the yyaA gene revealed that it is separated from the soj-spo0J operon by a putative stem-loop structure for transcription termination. A second putative stem-loop structure (ΔG-17) separates yyaA from the gene located upstream, gidB. The deduced amino acid sequence of YyaA shows extensive homology to that of the Spo0J protein (35% identical residues and 21% conserved substitutions), which, in accordance with the close proximity of the corresponding encoding genes, suggests that they arose by gene duplication (36).
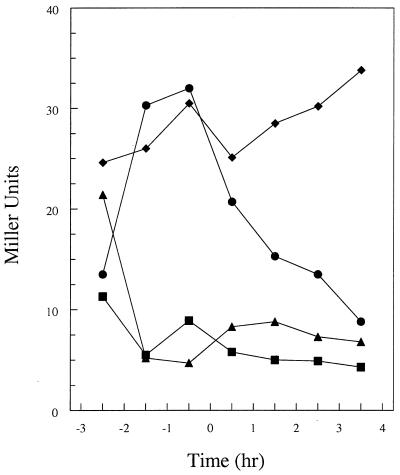
Transcriptional analysis by means of a β-galactosidase fusion inserted at the amyE locus revealed that a region including 220 bp upstream of the yyaA start codon contains the promoter determinants for yyaA transcription (data not shown). The analysis showed that yyaA was transcribed during vegetative growth, with a peak reached at about the time of transition to the sporulation phase, followed by a sharp decline in transcription (Fig. 1). Transcription was greatly reduced by a spo0A mutation but was increased by mutant abrB, suggesting that it is under negative control of transition state regulator AbrB (Fig. 1) (40). A spo0H mutation also severely affected the transcription of yyaA, consistent with the presence of putative −35 (GCAGGAAAA) and −10 (AGAAT) consensus sequences for ςsgr;sgr;H-containing RNA polymerase, just upstream of the gene. Western blot analysis (see also below) carried out on vegetative and sporulating cells of the wild-type strain confirmed the presence of the YyaA protein in vegetative cells and a slow decline in concentration during sporulation (Fig. 2, lanes 1 to 5).
FIG. 1.
β-Galactosidase analysis of transcription from the yyaA promoter fused to the lacZ gene of E. coli. Assays were carried out with the following strains: JH21083 (wild type; circles), JH21084 (spo0A mutant; triangles), JH21093 (abrB mutant; diamonds), and JH 21085 (spo0H mutant; squares). Cultures were grown in Schaeffer's sporulation medium. Time zero, time when the culture shifts from exponential growth to stationary phase.
FIG. 2.
Expression of the yyaA gene in vegetative and sporulating cells. The Western blot shows the detection of YyaA protein in strains SG38 (wild type; lane 1) and 2048 (overproducing YyaA; lane 6) during vegetative growth and during sporulation induced by the resuspension method (1, 2, 3, and 4 h after resuspension; lanes 2 to 5 and 6 to 10, respectively). Arrow, position of YyaA protein.
To examine the phenotypic effects of yyaA loss, we inactivated the gene by insertion of an ermC cassette (see Materials and Methods). The resulting strain, 2023, showed growth rates and sporulation efficiencies comparable to those of the parental strain, and no significant impairment of chromosome segregation was detected by DAPI (4",6"-diamidino-2-phenylindole) staining of chromosomal DNA (data not shown). To test the possibility that the function of the yyaA gene was partially redundant to that of spo0J and soj, knockout mutations of these genes were introduced into strain 2023. Nucleoid partitioning and sporulation efficiencies in the yyaA spo0J double mutant and yyaA soj spo0J triple mutant were not significantly different from those observed for the respective single and double mutants (data not shown) (21).
Localization of YyaA by IF.
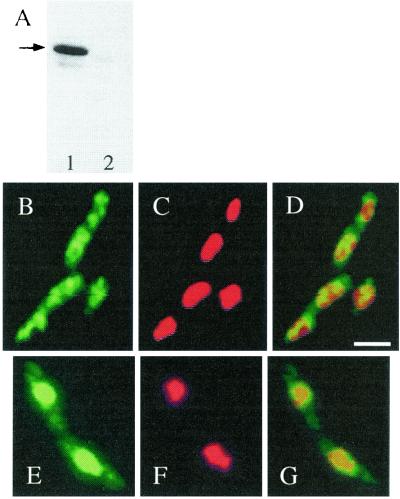
To carry out subcellular localization studies, an anti-YyaA polyclonal antiserum was affinity purified to obtain specific antibodies. This specificity was confirmed by Western blotting (Fig. 3A). A single band of mobility expected for the predicted YyaA protein (32.6 kDa) was detected in extracts of vegetative cells of B. subtilis but not in those of a yyaA null mutant. IF revealed that the YyaA protein was dispersed throughout the cytoplasm of vegetatively growing cells (Fig. 3B to D). No signal was detected in cells of the yyaA null mutant (data not shown). This signal was significantly different from the discrete oriC-associated foci formed by the related Spo0J protein (15, 31). Thus, if YyaA also binds to DNA, its binding sites must be more dispersed than those of Spo0J or it might have less sequence specificity. To test whether YyaA was actually associated with the DNA, strain ts-134, with a temperature-sensitive mutation in the dnaB gene, which is essential for the initiation of DNA replication, was used. At the nonpermissive temperature (45°C), ongoing rounds of replication can be completed but new rounds are not initiated in this mutant (33). The cells continue to grow, and completed nucleoids continue to segregate. In cells of ts-134 incubated in S medium at the permissive temperature (30°C), the distribution of the YyaA protein was similar to that in wild-type cells (data not shown). However, after a 1-h incubation at the nonpermissive temperature (45°C), the majority of the YyaA proteins clearly colocalized with the nucleoids positioned at or near midcell of the elongated cells (Fig. 3E to G).
FIG. 3.
Subcellular localization of YyaA in B. subtilis wild-type strain and a dnaB(Ts) mutant. (A) Western blot, performed after SDS-PAGE and electrotransfer, demonstrating the specificity of the anti-YyaA antiserum. Lane 1, wild-type strain (SG38); lane 2, yyaA null mutant (strain 2023). Arrow, position of YyaA protein. (B to F) Immunofluorescence micrographs showing the distributed localization of YyaA in growing wild-type cells (S medium) (B to D) and the colocalization of YyaA with the nucleoid in cells of strains ts-134 [bearing a dnaB(Ts) mutation] after shift to the nonpermissive temperature (45°C) for 1 h (E to G). The cells were stained for YyaA protein (green channel; B and E) and for DNA with DAPI (red channel; C and F); (D and G) merged images. Scale bar, 2 μm.
YyaA is a DNA-binding protein.
To test more directly whether the YyaA protein had DNA-binding capability and to examine its specificity, we used a gel mobility shift assay involving purified YyaA and two labeled DNA fragments generated by digestion of plasmid pRM113 (see Materials and Methods; Fig. 4A). Fragment A (710 bp) contained the yyaA promoter region and the 500 bp at the 5"-proximal end of the coding sequence, while fragment B (640 bp) contained the 350 bp at the 3"-proximal end and downstream sequences. As shown in Fig. 4B, YyaA was found to bind to both fragments in a concentration-dependent manner. The binding, however, was not specific, as it could be competed away with calf thymus DNA or poly(dA-dT)-poly(dG-dC) (Fig. 4B). This however does not negate the possibility that a specific binding site for YyaA exists as it does for Spo0J. The latter protein indeed shows a very similar retardation pattern on random B. subtilis chromosomal DNA fragments using salmon sperm DNA as a nonspecific competitor (G. B. Spiegelman, personal communication).
YyaA overproduction causes a sporulation defect.
New insights into the possible function of yyaA emerged when the gene was isolated by screening a multicopy plasmid library of the B. subtilis genome for genes inhibiting sporulation when overexpressed. The plasmid with yyaA emerged among the 0.1% or so of clones that appeared to cause an early sporulation defect (data not shown). Strain 2048 carrying yyaA multicopy plasmid pRM113 (causing the overproduction of the YyaA protein in vegetative and sporulating cells as judged by Western blot analysis; Fig. 2, lanes 6 to 10) showed a three- to fivefold reduction in sporulation compared with strain 2049 carrying insertless vector pHT315 (Table 2). Similar results were also obtained using Schaeffer's sporulation medium (data not shown).
TABLE 2.
Sporulation efficiency of strains carrying multicopy plasmidsa
| Strain | No. of spores (cells counted) | % of spores relative to the wild type |
|---|---|---|
| SG38 (wild type) | 235 (300) | 100 |
| 2048 (YyaA overproduced) | 28 (314) | 11 |
| 2049 (control) | 128 (329) | 50 |
Samples of sporulating cells of strains SG38, 2048, and 2049 were taken 7 h after resuspension and fixed with ethanol as described in Materials and Methods. The numbers of phase-bright spores in fields of cells viewed by phase-contrast microscopy were scored. There was no lysis of 2048 and 2049 cells detected at this stage.
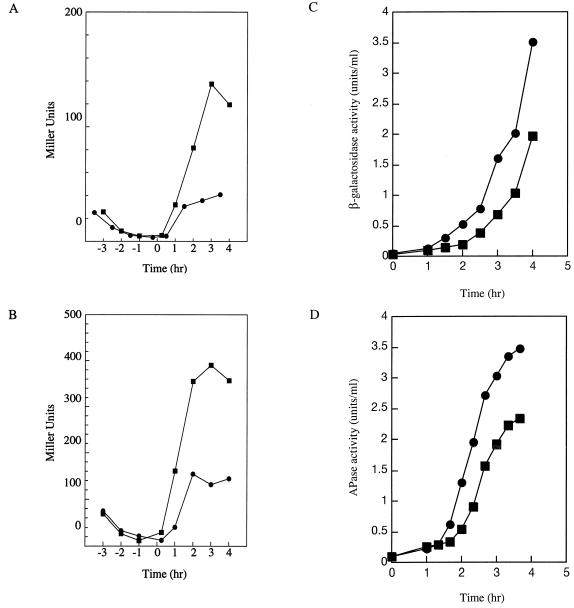
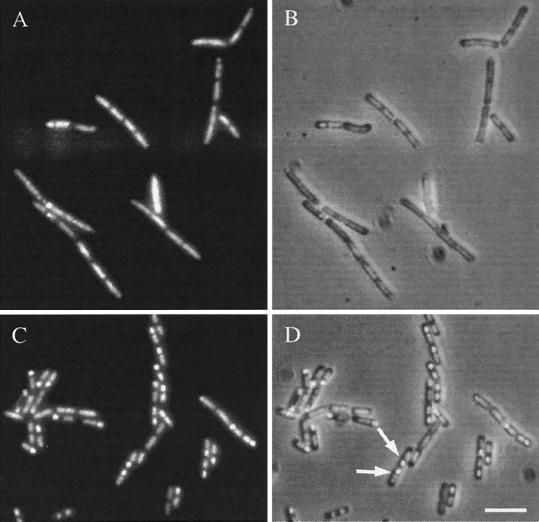
Overexpression of yyaA had no significant effect on vegetative growth. Cell division and chromosome segregation appeared to be normal in strain 2048 compared with those in control strain 2049 (data not shown). To determine at what point YyaA overproduction affected sporulation, pRM113 was introduced into strains carrying spo0A-lacZ and abrB-lacZ fusion constructs. β-Galactosidase assays indicated that transcription from both promoters was not affected by the overexpression of yyaA (data not shown), suggesting that the phosphorelay for sporulation initiation was activated normally (19, 50). We then analyzed the transcription of stage II genes spoIIA and spoIIG. In accordance with normal activation of the Spo0A transcription factor, the induction time and initial expression rates of the two reporters were not significantly affected by YyaA overproduction (Fig. 5A and B) (25, 54). However, at later time points both reporters were overexpressed (approximately threefold) compared with expression in control strains carrying pHT315 (Fig. 5A and B), suggesting that cells overproducing YyaA did not pass the preseptation stage of sporulation as in cell division mutants (26). Expression of later sporulation genes, activated by the compartment-specific sigma factors ςsgr;sgr;F and ςsgr;sgr;E was significantly curtailed. In strains overproducing YyaA, expression of a ςsgr;sgr;F-dependent reporter gene (gpr-lacZ) and APase activity, a convenient marker for ςsgr;sgr;E-dependent transcription, were reproducibly reduced to ∼60% compared with those in strains carrying pHT315 (Fig. 5C and D). The reduced activity of ςsgr;sgr;F and ςsgr;sgr;E could also be interpreted as the result of a partial block in septation or chromosome segregation (4, 5, 6, 8, 14, 26, 52). DNA staining of sporulating 2048 cells also indicated that sporulation was affected at an early stage (i.e., about the time when the asymmetric septum is normally formed; Fig. 6A and B). In cells of strain 2049, many prespore chromosomes were visible as brightly stained bodies at the cell poles, whereas the mother cell nucleoids were diffuse and slightly elongated (Fig. 6C and D). In contrast, cells of strain 2048 generally showed an abnormal chromosome distribution: many nucleoids appeared to remain dispersed throughout the cytoplasm, and typical brightly stained prespore chromosomes were absent in most cells (Fig. 6A and B).
FIG. 5.
Effects of YyaA overproduction on the activity of three reporter genes, spoIIA-lacZ (A), spoIIG-lacZ (B), and gpr-lacZ (C), and on the activity of APase (D) in strains overproducing YyaA (squares) and in control strains harboring the insertless multicopy plasmid (circles). Samples were taken at the indicated times after growth in Schaeffer's sporulation medium (A and B) or after resuspension in SM (C and D) and assayed for β-galactosidase and APase activity, respectively.
FIG. 6.
Sporulation defect in a strain overproducing YyaA. Shown are images of strains 2048 (overproducing YyaA; A and B) and 2049 (carrying pHT315; C and D). Samples of cells were taken 3 h after resuspension, fixed in ethanol, stained for DNA with DAPI, and subjected to phase-contrast and fluorescence microscopy as described in Materials and Methods. (A and C) DNA; (B and D) overlays of DNA and phase-contrast images. Arrows, examples of cells with apparently normal staining of prespore and mother cell DNA. Scale bar, 5 μm.
Cells overproducing YyaA form multiple sporulation septa.
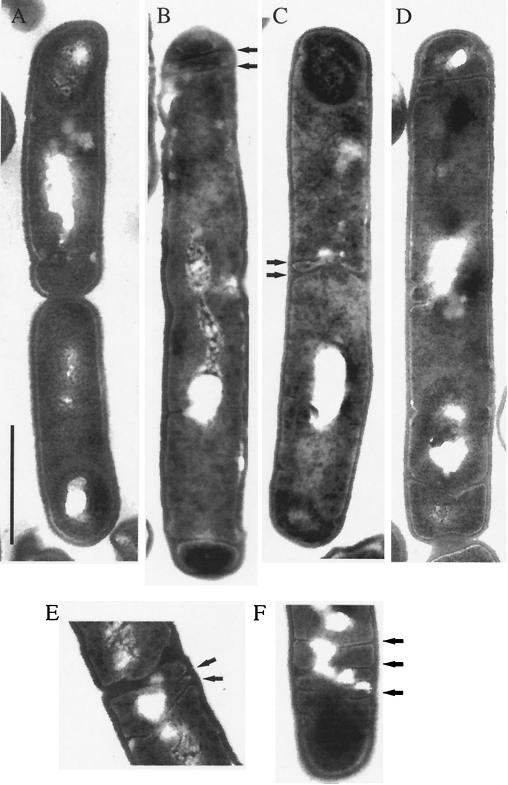
When samples from strain 2048 taken 3 h after the induction of sporulation were viewed by electron microscopy, it appeared that septum formation was dramatically impaired. At this stage of sporulation, many cells of strain 2049 contained engulfed prespores, visible as darker bodies close to one cell pole (Fig. 7A). Strikingly, a high proportion of strain 2048 cells bore multiple completed or invaginating septa, prevalent at the cell poles where the asymmetric division normally occurs (Fig. 7B, D, and F). However, septum formation was not restricted to the cell poles. Some pairs of cells were divided by septa that resembled thin sporulation septa (Fig. 7C and E). This indicated that these cells, when induced to sporulate, had failed to undergo a last round of vegetative division, and instead a septum containing unusually little peptidoglycan was formed. Other cells were approximately twice as long as wild-type cells, presumably because medial septation was completely (or partially) blocked (Fig. 7B and D). Although cells lying completely in the plane of the section were rare, of 131 such cells, 66 (50%) showed abnormal and/or multiple septa, 50 (38%) lacked septal structures, and 15 (11%) resembled sporulating wild-type cells, consistent with the reduced sporulation efficiency of 2048 (Table 2). The division phenotype of 2048 was not observed in control strain 2049 (harboring the insertless multicopy plasmid) and therefore was a consequence of the increased cellular levels of YyaA protein. In summary, the most prominent sporulation phenotype we observed when yyaA was overexpressed was the formation of multiple and misplaced sporulation-like septa.
FIG. 7.
Multiple and misplaced septa in YyaA-overproducing cells induced to sporulate. Shown are electron micrographs of strains 2049 (carrying pHT315; A) and 2048 (overproducing YyaA; B to F). Cell samples were taken 3 h after resuspension (t3), fixed, and prepared for electron microscopy as described in Materials and Methods. Whereas wild-type cells contained engulfed prespores at t3 (A), in strain 2048 (B to F) misplaced and multiple invaginating septa were visible, mainly at cell poles but also at other positions (arrows). Scale bar, 1 μm.
DISCUSSION
The yyaA gene is similar to spo0J, which is thought to be involved in chromosome organization and segregation in B. subtilis (15, 30, 31, 48). yyaA is located in the origin region of chromosomal replication, just upstream of the operon containing soj and spo0J. It is expressed during vegetative growth, and its promoter is possibly used by ςsgr;sgr;H-containing RNA polymerase. The range of genes controlled by ςsgr;sgr;H is not limited to competence and sporulation genes. The gene for fumarase (citG) and sporulation gene spoIIJ also depend on the spo0H gene product (2, 43). Accordingly, the possibility that yyaA may be involved in chromosome segregation was examined. However, no chromosome aberrations were observed in vegetatively growing or sporulating cells of the yyaA null mutant. yyaA does not appear to be partially redundant either, as the introduction of additional mutations in spo0J and/or soj revealed phenotypes that were not significantly different from those of the yyaA+ versions of these strains. Furthermore, the localization of Spo0J in the form of bipolar foci and of Soj in large nucleoid-associated patches was not perturbed in a yyaA null mutant, supporting the idea that YyaA does not function in concert with Spo0J and Soj (data not shown).
As for other ParB-like proteins, YyaA appears to be a DNA-binding protein. By IF using a polyclonal anti-YyaA antiserum, YyaA appeared to colocalize with the nucleoid. YyaA showed a punctate distribution throughout wild-type cells, but its distribution was somewhat reminiscent of that of the nucleoid, which is a relatively amorphous mass that almost fills the whole cytoplasm in living cells. A nucleoid association was supported by the results of experiments with a temperature-sensitive dnaB mutant. At the nonpermissive temperature this mutant forms elongated cells with a single central nucleoid and the majority of YyaA formed a large patch overlapping the DNA (Fig. 3). Unlike Spo0J, which binds to specific sites (parS) located in the origin-proximal 20% of the chromosome, YyaA has DNA-binding activity that might be nonspecific (though an underlying degree of sequence specificity cannot be excluded). This view was supported by the results of gel retardation assays, which confirmed that YyaA is a DNA-binding protein with no strong sequence preference and can bind not only to its own promoter region and coding sequence but also to unrelated competitor DNA. Thus, YyaA could have a general role in chromosome organization. However, as a yyaA null mutation resulted in no obvious defect in chromosome segregation and/or organization, the precise function of YyaA is not yet clear.
The effects of increased cellular levels of YyaA were also examined. They did not seem to impair chromosome segregation or septation in vegetative cells of B. subtilis. However, YyaA overproduction did affect sporulation, indicating that it might have a role in this process. The sporulation block was shown to be a relatively early one, as transfer of the prespore chromosome into the prespore was not detected at a significant frequency and ςsgr;sgr;F- and ςsgr;sgr;E-mediated transcription was significantly reduced. High-resolution (electron-microscopical) examination of cells induced to sporulate revealed a serious defect in the regulation of division frequency and positioning. In a large proportion of cells, septa that apparently contained little peptidoglycan and therefore resembled sporulation septa (20, 41) were misplaced and multiple signs of septum invagination were visible, predominantly at the poles of the cell. After resuspension in sporulation medium, cells that have passed a certain stage in the life cycle divide once more by binary fission before septation switches to one pole of the cell. This last vegetative division was also apparently impaired when yyaA was overexpressed. Either septation was blocked, resulting in cells roughly twice as long as wild-type sporulating cells, or a thin, sporulation-like septum was formed at midcell. Little is known about how the switch from the medial to the polar division is regulated during sporulation. Polar Z ring formation depends on transcription factor Spo0A (27), but the gene or genes that actually govern the site of FtsZ polymerization are not yet identified. If YyaA is involved in this process, we would expect its action to be modulated in the life cycle. However, the protein was only slowly depleted during sporulation, as judged by Western blot analysis (though yyaA expression was apparently repressed at the onset of differentiation). The overexpression phenotype could be a secondary effect of the unnaturally high cellular concentration of this DNA-binding protein. At high concentration, the protein may perturb sporulation-specific transcription of a regulator gene or it may directly affect the activity of a protein involved in positioning the asymmetric septum. The formation of multiple septa, possibly partitioning the chromosome into several different compartments, could be responsible for the defect in sporulation.
Localization and DNA-binding studies suggest that the Spo0J-like YyaA protein could be involved in nucleoid organization, but if so, its function is redundant. On the other hand, overexpression experiments hint at a different role for YyaA as a regulator protein, possibly controlling septum formation early in sporulation. Further work needs to be done to determine the exact function of yyaA, but, more importantly, other components of the B. subtilis segregation machinery remain to be identified.
Acknowledgments
This research was supported, in part, by grant 43/SF09210 from the BBSRC (to J.E.) and grants GM55594 and GM19416 from the National Institutes of General Medical Sciences, National Institutes of Health, USPHS (to M.P.). J.S. was the recipient of a Boehringer Ingelheim Fonds postgraduate fellowship. J.E. was the recipient of a BBSRC Senior Research Fellowship.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniewski, C., B. Savelli, and P. Stragier. 1990. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J. Bacteriol. 172:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108:115-119. [DOI] [PubMed] [Google Scholar]
- 4.Beall, B., and J. Lutkenhaus. 1989. Nucleotide sequence and insertional activation of a Bacillus subtilis gene that affects cell division, sporulation and temperature sensitivity. J. Bacteriol. 171:6821-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beall, B., and J. Lutkenhaus. 1991. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 5:447-455. [DOI] [PubMed] [Google Scholar]
- 6.Beall, B., and J. Lutkenhaus. 1992. Impaired cell division and sporulation of a Bacillus subtilis strain with the ftsA gene deleted. J. Bacteriol. 174:2398-2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cervin, M. A., G. B. Spiegelman, B. Raether, K. Ohlsen, M. Perego, and J. Hoch. 1998. A negative regulator linking chromosome segregation to development transcription in Bacillus subtilis. Mol. Microbiol. 29:85-95. [DOI] [PubMed] [Google Scholar]
- 8.Daniel, R. A., E. J. Harry, V. L. Katis, R. G. Wake, and J. Errington. 1998. Characterisation of the essential cell division gene ftsL (yllD) of Bacillus subtilis and its role in assembly of the division apparatus. Mol. Microbiol. 29:593-604. [DOI] [PubMed] [Google Scholar]
- 9.Errington, J., L. Appleby, R. A. Daniel, H. Goodfellow, S. R. Partridge, and M. D. Yudkin. 1992. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for ςsgr;sgr;G activity at an intermediate stage of sporulation. J. Gen. Microbiol. 138:2609-2618. [DOI] [PubMed] [Google Scholar]
- 10.Errington, J., and J. Mandelstam. 1983. Variety of sporulation phenotypes resulting from mutations in a single regulatory locus, spoIIA, in Bacillus subtilis. J. Gen. Microbiol. 129:2091-2101. [DOI] [PubMed] [Google Scholar]
- 11.Errington, J., and J. Mandelstam. 1986. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J. Gen. Microbiol. 132:2967-2976. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari, E., D. J. Henner, M. Perego, and J. A. Hoch. 1988. Transcription of Bacillus subtilis subtilisin and expression of subtilisin in sporulation mutants. J. Bacteriol. 170:289-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feucht, A., T. Magnin, M. Yudkin, and J. Errington. 1996. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 10:794-803. [DOI] [PubMed] [Google Scholar]
- 14.Feucht, A., R. A. Daniel, and J. Errington. 1999. Characterization of a morphological checkpoint coupling cell-specific transcription to septation in Bacillus subtilis. Mol. Microbiol. 33:1015-1026. [DOI] [PubMed] [Google Scholar]
- 15.Glaser, P., M. E. Sharpe, B. Raether, M. Perego, K. Ohlsen, and J. Errington. 1997. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 11:1160-1168. [DOI] [PubMed] [Google Scholar]
- 16.Harlow, E., and D. P. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Hauser, P. M., and J. Errington. 1995. Characterization of cell cycle events during the onset of sporulation in Bacillus subtilis. J. Bacteriol. 177:3923-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraga, S. 1992. Chromosome and plasmid partitioning in Escherichia coli. Annu. Rev. Biochem. 61:283-306. [DOI] [PubMed] [Google Scholar]
- 19.Hoch, J. A. 1993. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu. Rev. Microbiol. 47:441-465. [DOI] [PubMed] [Google Scholar]
- 20.Illing, N., and J. Errington. 1991. Genetic regulation of morphogenesis in Bacillus subtilis: roles of σE and σF in prespore engulfment. J. Bacteriol. 173:3159-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ireton, K., N. W. Gunther IV, and A. Grossman. 1994. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176:5320-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkinson, H. F. 1983. Altered arrangement of proteins in the spore coat of a germination mutant of Bacillus subtilis. J. Gen. Microbiol. 129:1945-1958. [DOI] [PubMed] [Google Scholar]
- 23.Karamata, D., and J. D. Gross. 1970. Isolation and genetic analysis of temperature sensitive mutants of Bacillus subtilis defective in DNA synthesis. Mol. Gen. Genet. 108:277-287. [DOI] [PubMed] [Google Scholar]
- 24.Kellenberger, E., A. Ryter, and J. Sechaud. 1958. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J. Biophys. Biochem. Cytol. 4:671-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenney, T. J., K. York, P. Youngman, and C. P. Moran, Jr. 1989. Genetic evidence that RNA polymerase associated with sigma-A factor uses a sporulation-specific promoter in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 86:9109-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin, P. A., and R. Losick. 1994. Characterization of a cell division gene from Bacillus subtilis that is required for vegetative and sporulation septum formation. J. Bacteriol. 176:1451-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin, P. A., and R. Losick. 1996. Transcription factor Spo0A switches the localisation of the cell division protein FtsZ from a medial to a bipolar pattern. Genes Dev. 10:478-488. [DOI] [PubMed] [Google Scholar]
- 28.Lewis, P. J., and J. Errington. 1997. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localisation with the Spo0J partitioning protein. Mol. Microbiol. 25:945-954. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, P. J., T. Magnin, and J. Errington. 1996. Compartmentalised distribution of the proteins controlling the prespore-specfic transcription factor ςsgr;sgr;F of Bacillus subtilis. Genes Cells 1:881-894. [DOI] [PubMed] [Google Scholar]
- 30.Lin, D. C.-H., and A. Grossman. 1998. Identification and characterisation of a bacterial chromosome partitioning site. Cell 92:675-685. [DOI] [PubMed] [Google Scholar]
- 31.Lin, D. C.-H., P. A. Levin, and A. D. Grossman. 1997. Bipolar localisation of a chromosome partitioning protein in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 94:4721-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marston, A. L., and J. Errington. 1999. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organisation and developmental regulation. Mol. Cell 4:673-682. [DOI] [PubMed] [Google Scholar]
- 33.Mendelson, N. H., and J. D. Gross. 1967. Characterization of a temperature-sensitive mutant of Bacillus subtilis defective in deoxyribonucleic acid replication. J. Bacteriol. 94:1603-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Mohl, D. A., and J. W. Gober. 1997. Cell cycle-dependent polar localisation of chromosome partitioning proteins in Caulobacter crescentus. Cell 90:951-957. [DOI] [PubMed] [Google Scholar]
- 36.Ogasawara, N., and H. Yoshikawa. 1992. Genes and their organisation in the replication origin region of the bacterial chromosome. Mol. Microbiol. 6:629-634. [DOI] [PubMed] [Google Scholar]
- 37.Partridge, S. R., and J. Errington. 1993. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 8:945-955. [DOI] [PubMed] [Google Scholar]
- 38.Perego, M. 1993. Integrational vectors for genetic manipulation in Bacillus subtilis, p. 615-624. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 39.Perego, M., and J. A. Hoch. 1987. Isolation and sequence of the spo0E gene; its role in initiation of sporulation in Bacillus subtilis. Mol. Microbiol. 1:125-132. [DOI] [PubMed] [Google Scholar]
- 40.Perego, M., G. B. Spiegelman, and J. A. Hoch. 1988. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis Mol. Microbiol. 2:689-699. [DOI] [PubMed] [Google Scholar]
- 41.Piggot, P. J. 1973. Mapping of asporogenous mutations in Bacillus subtilis: a minimum estimate of the number of sporulation operons. J. Bacteriol. 114:1241-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pogliano, K., E. Harry, and R. Losick. 1995. Visualisation of the subcellular location of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol. Microbiol. 18:459-470. [DOI] [PubMed] [Google Scholar]
- 43.Price, V. A., I. M. Feavers, and A. Moir. 1989. Role of sigma H in expression of the fumarase gene (citG) in vegetative cells of Bacillus subtilis 168. J. Bacteriol. 171:5933-5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quisel, J. D., D. C.-H. Lin, and A. D. Grossman. 1999. Control of development by altered localization of a transcription factor in SB. subtilis. Mol. Cell 4:665-672. [DOI] [PubMed] [Google Scholar]
- 45.Resnekov, O., S. Alper, and R. Losick. 1996. Subcellular localisation of proteins governing the proteolytic activation of a developmental transcription factor in Bacillus subtilis. Genes Cells 1:529-542. [DOI] [PubMed] [Google Scholar]
- 46.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Schaeffer, P., J. Miller, and J. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54:701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe, M. E., and J. Errington. 1998. A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol. Microbiol. 28:981-990. [DOI] [PubMed] [Google Scholar]
- 49.Sterlini, J. M., and J. Mandelstam. 1969. Commitment to sporulation in Bacillus subtilis and its relationship to the development of actinomycin resistance. Biochem. J. 113:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strauch, M. A., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The Spo0A protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 52.Thomaides, H. B., M. Freeman, M. El Karoui, and J. Errington. 2001. Division-site-selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev. 15:1662-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams, D. R., and C. M. Thomas. 1992. Active partitioning of bacterial plasmids. J. Gen. Microbiol. 138:1-16. [DOI] [PubMed] [Google Scholar]
- 54.Wu, J. J., M. G. Howard, and P. J. Piggot. 1989. Regulation of transcription of the Bacillus subtilis spoIIA locus. J. Bacteriol. 171:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 56.Wu, L. J., A. Feucht, and J. Errington. 1998. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev. 12:1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]