Abstract
Cell-bound C-signal guides the building of a fruiting body and triggers the differentiation of myxospores. Earlier work has shown that transcription of the csgA gene, which encodes the C-signal, is directed by four genes of the act operon. To see how expression of the genes encoding components of the aggregation and sporulation processes depends on C-signaling, mutants with loss-of-function mutations in each of the act genes were investigated. These mutations were found to have no effect on genes that are normally expressed up to 3 h into development and are C-signal independent. Neither the time of first expression nor the rate of expression increase was changed in actA, actB, actC, or actD mutant strains. Also, there was no effect on A-signal production, which normally starts before 3 h. By contrast, the null act mutants have striking defects in C-signal production. These mutations changed the expression of four gene reporters that are related to aggregation and sporulation and are expressed at 6 h or later in development. The actA and actB null mutations substantially decreased the expression of all these reporters. The other act null mutations caused either premature expression to wild-type levels (actC) or delayed expression (actD), which ultimately rose to wild-type levels. The pattern of effects on these reporters shows how the C-signal differentially regulates the steps that together build a fruiting body and differentiate spores within it.
Myxococcus xanthus cells build a fruiting body by coordinating their movements to form a hemispherical mound. Then they sporulate within that mound. The C-signal, encoded by the csgA gene, causes cells to modify their movement behavior, which leads them to aggregate (11, 14, 20). This cell-bound signal also induces sporulation within the fruiting body (12). It is proposed that sporulation is restricted to the fruiting body because C-signaling depends on end-to-end contacts between cells, since side-by-side contacts are unable to transmit that signal (15, 28). The cell arrangement within a nascent fruiting body is such that there are many end-to-end contacts between the cells while there are few end-to-end contacts between peripheral cells (12). The ability of C-signal to guide aggregation and later to induce sporulation is a consequence of the continuous rise in specific activity of CsgA protein in a developing culture (9). Sporulation is confined to the fruiting body because C-signal-dependent sporulation genes have a higher C-signal specific activity threshold than do genes that control aggregation (14, 20). The rise in CsgA specific activity ensures proper ordering of aggregation and sporulation. That rise is produced by the products of four genes of the act operon, which regulate the timing and the level of csgA expression (9). Null mutations in act genes change both the time and intensity patterns of the C-signal production. actB encodes a sigma-54 activator protein (8). This activator gene, which led to the operon, also names it. The actA and actB genes regulate the maximum level of the CsgA protein, and deletions of these genes scale down the instantaneous levels while retaining the normal time pattern. The ΔactC and ΩactD mutants change the time pattern of CsgA production, yet they both achieve the same maximum level as the wild type does, at either an earlier (actC) or later (actD) time. Null mutations in any of the act genes decrease sporulation, indicating that both the time pattern and the maximum level of C-signal are important for maximum sporulation.
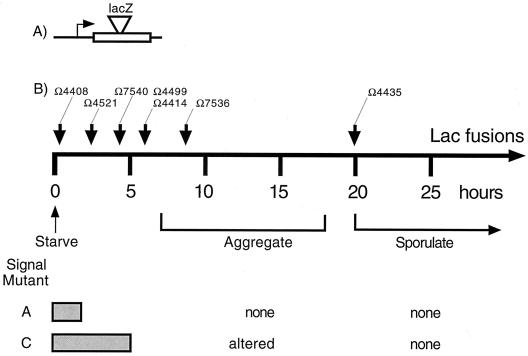
The transcriptional program for fruiting-body development, is controlled by five signals (5, 10). Program dependence on the A- and C-signals is illustrated by the timely expression of a series of gene reporters generated by the properly oriented insertion of the Tn5lac element (16) (Fig. 1A). Each developmentally regulated promoter that has been fused to a promoterless lacZ gene has characteristic signal requirements (16). A-signal acts early (at 2 h) and C-signal acts later (about 5 h) in the program, as indicated in Fig. 1. Reporters that are poorly expressed in an asgB (A-signal-deficient) mutant relative to the wild type or in a csgA null mutant are A-signal- and C-signal-dependent genes, respectively (16, 19). Figure 1 summarizes the facts that A-signal-deficient mutants can express the Ω4408 reporter on time but fail to express any of the later reporters shown, fail to aggregate, and fail to sporulate. C-signal deficient (csgA) mutants express several early reporters, including Ω4408, Ω4521, and Ω7540; they fail to express the later reporters shown in Fig. 1B, are aggregation defective, and fail to sporulate (16). Using act mutants which specifically alter the intensity or timing of C-signal production, it is possible to test the in vivo responses of a series of developmentally regulated genes to the amount of the C-signal they receive. Those responses show how the C-signal controls the time of developmental gene expression.
FIG. 1.
(A) General structure of the lacZ reporter fusions constructed with Tn5lac. The horizontal line represents a segment of the M. xanthus chromosome. The box represents a transcription unit into which Tn5lac (wedge) has inserted. (B) Reporters (Ω numbers) are shown above arrows that indicate the time of gene expression during starvation-induced development (16). The dependence of each reporter on the A- and C-signals is shown below the time line by gray bars to indicate genes that are expressed normally in an asg (A-signal defective) or a csg (C-signal defective) mutant. Some of the insertions inactivate a known developmental function: Ω4408, sdeK; Ω4521, spi; Ω7540, fruA; Ω4414, dev; Ω7536, a spore shape function.
MATERIALS AND METHODS
Introduction of reporter gene fusions.
Myxophages Mx4 ts8 ts27 hrm (3) and Mx8 clp2 ts3 (23) were used to transduce previously characterized reporter mutant alleles (16) into the described act operon mutant strains to create double mutants. The transposons were selected by their drug resistance, Tetr, Kmr, or Bler (for bleomycin resistance) (4), which can be selected in M. xanthus with the antibiotic zeocin. The structure of the transposon insertions in each recombinant strain was confirmed by Southern blot hybridization. Restriction sites in the vicinity of these reporters have been previously mapped, providing fingerprints that identify each reporter (18). The double-mutant strains are described in Table 1.
TABLE 1.
M. xanthus strains used in this studya
| Insertion mutation (reference) | Strain designationb
|
||||
|---|---|---|---|---|---|
| act+ | ΔactA | ΔactB | ΔactC | ΩactD | |
| None | DK1622 | DK10605 | DK10603 | DK10604 | DK10601 (9) |
| pLAG2::actB (8) | DK7837c | ||||
| Tn5lac Ω4499 (16) | DK4499 | DK10606 | DK10607 | DK10608 | DK10633 |
| Tn5-132::csgA (16) | DK5208 | DK10609 | DK10610 | DK10611 | |
| fruA::Tn5lac Ω7540 (6) | DK11063 | DK10615 | DK10616 | DK10617 | DK10635 |
| Tn5lac Ω4435 (18) | DK5204 | DK10618 | DK10619 | DK10620 | DK10636 |
| Tn5lac Ω7536 (22) | DK10524 | DK10621 | DK10622 | DK10623 | DK10637 |
| Tn5lac Ω4414 (18) | DK5279 | DK10624 | DK10625 | DK10626 | DK10638 |
| Tn5lac Ω4408, sdeK (18) | DK4300 | DK10627 | DK10628 | DK10629 | DK10639 |
| Tn5lac Ω4521, spi (18) | DK4521 | DK10630 | DK10631 | DK10632 | DK10640 |
Not fitting within the format of this table, strain DK7853 is an asgA mutant (8) used for A-signal assays.
The table gives the strain number whose genotype is defined by its column, showing its allele of act, and row, showing its plasmid or Tn5 insertion. For example, DK10606 has an in-frame deletion in actA and the Tn5lac Ω4499 insertion.
DK7837 has a plasmid insertion in its otherwise normal actB gene (8).
Bioassay of A- and C-signals.
To determine the amounts of A- and C-signals made by the mutants, the strain in question was induced to develop on TPM agar (8) in coculture with either DK7853 (asgA) or DK5208 (csgA) as described previously (8).
Developmental β-galactosidase assays.
To measure promoter expression in terms of β-galactosidase produced by these and mutant derivatives of the Tn5lac promoter fusion strains, 1.5 × 108 cells were placed in submerged cultures in 400 μl of A50 buffer (10 mM morpholinepropanesulfonic acid [MOPS] buffer [pH 7.2], 1 mM CaCl2, 4 mM MgCl2, 50 mM NaCl) in Parafilm-sealed 24-well polystyrene tissue culture plates. The cultures were allowed to develop at 32°C and then, at the indicated time, were frozen. After all the samples had been taken for each reporter, they were processed alongside each other. First, each sample was thawed and briefly sonicated. Then the liquid volume in each well was brought to 1 ml, and the cultures were treated for 3 min in a cup sonicator (Vibra Cell; Sonics and Material, Inc.). Debris was removed by centrifugation, and the β-galactosidase activity in the supernatant fluid was determined. In 96-well polystyrene flat-bottom microplates with 50 μl of sample and 50 μl of a buffer (consisting of 120 mM Na2HPO4, 80 mM NaH2PO4, 20 mM KCl, 2 mM MgSO4, and 100 mM β-mercaptoethanol), the hydrolysis of o-nitrophenyl-β-d-galactopyranoside (2 mg/ml) was measured after 3 h of incubation. For hydrolysis, the plates were incubated at 37°C before the reaction was stopped by adding 100 μl of 1 M Na2CO3 solution after the yellow color had developed. The optical density at 420 nm was recorded in a SPECTRAmax250 96-well plate reader with the program Soft Max Pro version 1.2.0. The total protein from the samples was measured using the Bradford assay with the samples in 96-well plates and with immunoglobulin G (IgG) as the protein standard. β-Galactosidase activity was expressed as nanomoles of orthonitrophenol (ONP) produced per minute (Miller units) per milligram of total protein as described previously (18).
RESULTS
Control of developmental gene expression.
To clarify the role that act genes play in the program of developmental gene expression, a set of otherwise isogenic strains were constructed with each act mutation and a series of transcriptional reporters. Reporter alleles, which carry transcriptional fusions to lacZ that are marked by Tetr, Kmr, or Bler, were selected with the appropriate antibiotic. Reporters were transduced into appropriate drug-sensitive, in-frame deletion mutant strains of the act operon (9). These transductants constitute an isogenic set of mutant reporter strains that differ only by the reporter and act mutation that they contain (Table 1).
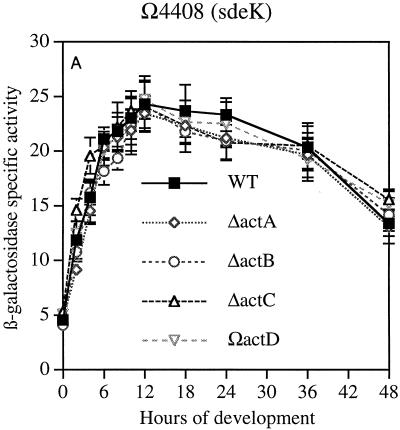
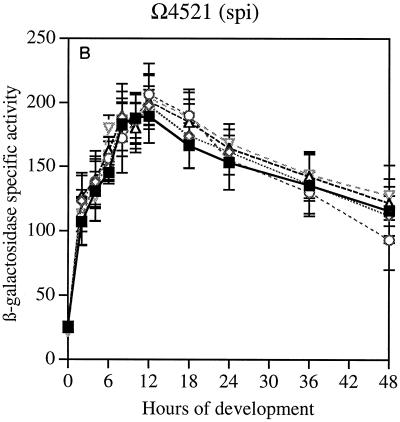
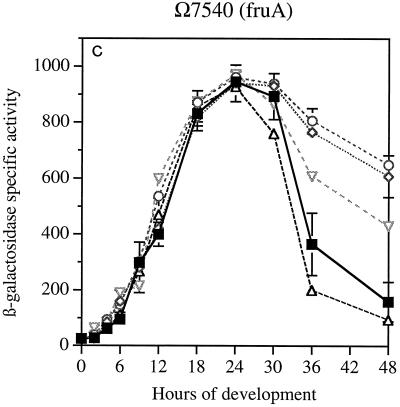
Gene expression was first measured in reporter strains for genes that are expressed early in development. The sdeK gene (Ω4408), which encodes a histidine protein kinase, is expressed at 1 h after initiation by starvation and is essential for development (7, 17, 27). The spi gene (Ω4521), which is expressed at 2 h (13, 19), is not essential for development, although it is strongly regulated by development. The fruA gene (Ω7540) is expressed at 3 to 6 h of development and encodes a response regulator which is also essential for development (6, 24). Previous work has shown that spi (19) and fruA (6) are A-signal dependent, and that sdeK (Ω4408) is A-signal independent (19).
The data in Fig. 2A show that neither the time at which sdeK expression begins nor its rate of increase once started is changed by null mutations in any of the actA, actB, actC, or actD genes. The ΔactA and ΔactB mutations decrease CsgA levels fourfold, and the ΔactC and ΩactD mutations advance or retard the timing of CsgA expression by several hours (9). Similarly, the expression of spi (Fig. 2B) was unaffected by null mutations in any of the four act genes. Expression of fruA was unaffected during the first 24 h (Fig. 2C). However, there were significant differences in the decay of reporter expression after 24 h among the mutants; this was not seen with the other reporters. The β-galactosidase levels detected during the first 24 h in strains containing these transcriptional fusions agree with the direct measurement of transcription of fruA mRNA, which showed that when present in wild-type or ΔactB backgrounds, the mRNA levels were practically the same (Table 2). Expression of these genes is unaffected by csgA null mutations (6, 16); accordingly they are csgA independent, and, as shown here, their expression during the first 24 h is unaffected by act mutations. However, the pattern of fruA expression after 24 h, which differs systematically among the act mutants, suggests a dependence of fruA decay on csgA.
FIG. 2.
Reporter β-galactosidase specific activity as a function of time after starvation-induced development in submerged culture. Tn5lac promoter fusions (Ω numbers) that carry act mutations are described in Table 1. (A) sdeK::lacZ Ω4408. (B) spi::lacZ Ω4521. (C) fruA::lacZ Ω7540. Specific activity is expressed as nanomoles of ONP produced per minute per milligram of total protein (Miller units). The symbols apply to all three panels. WT, wild type.
TABLE 2.
mRNA levelsa
| Time (h) | % Hybridization
|
||||||
|---|---|---|---|---|---|---|---|
|
fruA probe
|
devTRS probe
|
csgA probe
|
|||||
| act+ | ΔactB | act+ | ΔactB | act+ | ΔactB | ||
| 0 | 6 | 5 | 20 | 6 | 28 | 23 | |
| 8 | 100 | 87 | 100 | 39 | 100 | 41 | |
| 24 | 60 | 65 | 3 | 2 | 47 | 35 | |
mRNA was measured relative to the level of act+ (wild type) in Western slot blots. fruA mRNA was probed with a 123-bp fragment, AatII-KpnI, of the fruA gene (6). devTRS mRNA was probed with a 2.8-kbp HindIII-SalI fragment including most of devT and all of devR and devS (9). csgA mRNA was probed as described previously (9).
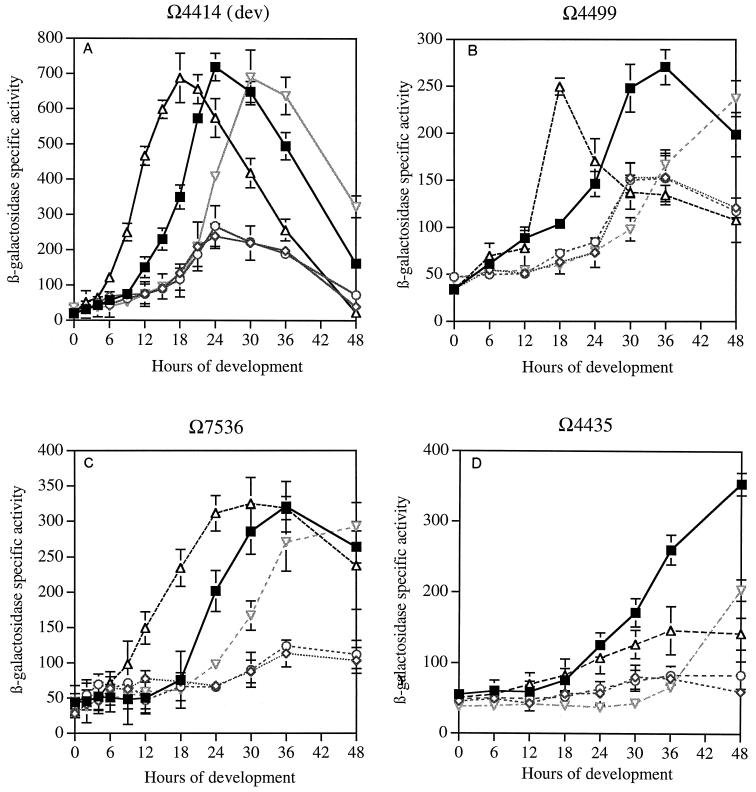
A second set of experiments examined genes that are expressed 6 h or later in development and are expected from prior work to be partially or absolutely dependent on C-signaling (16) (Fig. 1). The dev operon is expressed at 6 h (30) and is C-signal dependent (16), and its products play an important role in sporulation (12, 30). The dev reporter (Ω4414) fuses a properly oriented lacZ gene to the transcription start of the dev operon and interrupts devR, creating a polar devR devS double mutant (A. G. Garza, B. Julien, and D. Kaiser, unpublished results). Expression of the Ω4499 reporter is detectable at 6 h. It is known to require an intermediate level of C-factor for its expression based on addition of partially purified C-factor to cells (14). The Ω7536 operon is expressed at 8 h and is known to be involved directly in changing the cell shape into a spherical spore (22). Finally, the Ω4435 reporter is expressed at 20 h, the time of sporulation (14), although the insertion mutant has no obvious sporulation defect (17).
The ΔactA and ΔactB mutants express dev at much lower maximum levels, but the β-galactosidase level in the mutants rises and falls in parallel with those in an act+ Ω4414 strain (Fig. 3A). Measurements of dev mRNA, presented in Table 2, show that at 8 h, the level of hybridization in the mutant is less than half that in the wild type. Measurement of csgA mRNA at 8 h in the same experiment shows a similar dependence on actB. These data imply that the observed changes of expression of both dev and csgA due to deletion of actB are at the transcriptional level. Both Ω4414 β-galactosidase specific activities and mRNA levels by hybridization observed here parallel the fourfold-lower peak CsgA protein levels in the ΔactA and ΔactB mutants (9). Loss of actC leads to expression of β-galactosidase 6 h earlier than in the wild type (Fig. 3A). (The initial rise is 6 h earlier and the peak occurs at 18 h, whereas the wild-type strain reaches its peak at 24 h.) This time of expression agrees well with the reported precocious C-factor production by 6 h in theΔactC mutant (9). Inactivation of actD in the ΩactD mutant delays β-galactosidase production approximately 6 h, also in agreement with the reported delay in C-signal production in the ΩactD mutant (9).
FIG. 3.
C-signal-dependent developmental β-galactosidase expression. Measurements are as described in the legend to Fig. 2. (A) dev::lacZ (Tn5lac Ω4414). (B). Tn5lac Ω4499. (C) Tn5lac Ω7536. (D) Tn5lac Ω4435. Symbols are as in Fig. 2.
The Ω4499 reporter shows a qualitatively similar pattern to that of the Ω4414 reporter in the ΔactA and ΔactB mutants, although the maximum β-galactosidase activities were lower (Fig. 3B). β-Galactosidase expression in the ΔactC mutant also occurred earlier than in the wild type, by 18 h peak to peak. The ΩactD mutation delays the rise of Ω4499 expression to eventual wild-type levels by 18 h. Expression of the Ω7536 sporulation reporter in each of the four mutant strains is qualitatively similar to that of the Ω4499 andΩ4414 reporters. The expression profiles for Ω7536 are similarly perturbed in the mutants: ΔactA and ΔactB were depressed, ΔactC was 8 h earlier than the wild type, and ΩactD was 8 h later than the wild type. With respect to both timing and level, the response of expression of β-galactosidase from the Ω4499, Ω4414, and Ω7536 reporters parallels production of CsgA protein in the act mutant strains without reporters.
Finally, the Ω4435 sporulation reporter shows a general expression pattern for the wild type and act mutants (Fig. 3D) that is more than 10 h later than that for Ω4414, Ω7536, or Ω4499. The ΔactA and ΔactB mutants allow only a small amount of Ω4435 expression, too little to distinguish its time course. The ΔactC mutant of Ω4435 breaks an otherwise general pattern among C-signal-dependent reporters. While the other reporters show precocious expression for ΔactC, Ω4435 expression in the ΔactC mutant begins to follow the wild-type course and then, at about one-third maximum, levels off. It is as if another factor that appears only at about 20 h is needed, in addition to high C-signal, for Ω4435 expression. The ΩactD mutant shows an expression delayed 17 h relative to the wild type, like the other reporters.
Production of A- and C-signals.
The absence of any change by the four act mutants in the expression of three early reporters, two of which, spi and fruA, are A-signal dependent, suggests that A-signal production is unaffected by act. To examine A-signal production more directly, A-factor was measured by a cell-mixing bioassay. An asg mutant can be rescued to produce spores if a strain capable of A-factor production is allowed to develop along with the asg mutant (19). The number of spores produced by the asg mutant quantifies A-factor activity. Production of A-factor is known to require starvation and the expression of a number of developmentally regulated genes, including Ω4408 (7). A-factor production requires AsgA, a histidine protein autokinase (21); the putative transcription factor AsgB (25); and AsgC, an rpoD homolog (26). All three proteins are expected to be present in the act mutants. Table 3 quantifies the capacity of null mutants for each of the act genes to produce A-factor. These assays confirm by comparison with wild-type cells (DK1622) that all four act mutants produce normal levels of A-factor as measured by the sporulation of the AsgA strain.
TABLE 3.
A-factor biological activity produced by act mutantsa
| Strain 1 | Strain 2 | Relative no. of spores (%) of:
|
|||
|---|---|---|---|---|---|
| Strain 1 after 3 days | Strain 2 after 3 days | Strain 1 after 5 days | Strain 2 after 5 days | ||
| DK1622 | DK7853 | 100 | 100 | 100 | 100 |
| ΔactA | DK7853 | <10−4b | 113 | <10−4 | 106 |
| ΔactB | DK7853 | <10−4 | 139 | <10−4 | 100 |
| ΔactC | DK7853 | 56 | 100 | 93 | 92 |
| ΩactD | DK7853 | 50 | 119 | 79 | 100 |
Cultures of strain 1 and strain 2 were mixed, allowed to develop for the time shown, and then harvested; the viable heat- and sonication-resistant spores were counted, as described in reference 8. Strain 1 and strain 2, differing in antibiotic resistance, were counted separately in each experiment. The data in columns 3, 4, 5, and 6 show the relative sporulation frequencies measured as percentages of spores formed by DK1622, the wild type, alone, which were determined alongside the strain 1-strain 2 mixture in each experiment. The absolute numbers of spores for DK7853, which is an asgA mutant, when mixed with DK1622 were 5.7 × 105 and 8.7 × 105 spores after 3 and 5 days, respectively.
No spores were detected by plating. If there had been one colony per plate, the frequency would have been 10−4.
Contrasting with normal A-signaling by act mutants, the strong and regular effects of act mutations on expression of C-signal-dependent reporters Ω4414, Ω4499, Ω7536, and Ω4435 imply changes in C-signaling. The transmission of biologically active C-signal was quantified by the rescue of csgA mutant sporulation (Table 4). The table documents a substantial loss of C-signal biological activity in the ΔactA and ΔactB mutants. The ΔactC and ΩactD mutants show only about one-quarter the wild-type level of sporulation in the cell mixture with a csgA mutant strain. These bioassay data for C-signal activity in the various mutants show the same rank order as for the total amounts of CsgA protein produced during development. CsgA protein levels were measured by quantitative Western blot analyses, and the data are shown in the last column of Table 4. In sum, the different defects in C-signal production evident in the effects of the four act mutations on gene expression exactly parallel their CsgA protein levels.
TABLE 4.
C-signal biological activity produced by act mutantsa
| Strain 1 | Strain 2 | Relative no. of spores (%) of:
|
||||
|---|---|---|---|---|---|---|
| Strain 1 after 3 days | Strain 2 after 3 days | Strain 1 after 5 days | Strain 2 after 5 days | Strain 1 (total Csg protein) | ||
| DK1622 | DK5208 | 100 | 100 | 100 | 100 | 100 |
| ΔactA | DK5208 | <10−4b | 6 | <10−4 | 8 | 31 |
| ΔactB | DK5208 | <10−4 | 6 | <10−4 | 8 | 35 |
| ΔactC | DK5208 | 29 | 28 | 28 | 28 | 47 |
| ΩactD | DK5208 | 20 | 17 | 30 | 29 | 51 |
Cultures of strain 1 and strain 2 were mixed, allowed to develop for the time shown, and then harvested; viable heat- and sonication-resistant spores were counted, as described in reference 8. Spores of strain 1 and strain 2, differing in antibiotic resistance, were counted separately in each experiment. The data in columns 3, 4, 5, and 6 show the relative sporulation frequencies measured as percentages of spores formed by DK1622, the wild type, alone, which were determined alongside the strain 1-strain 2 mixture in each experiment. The last column in the table (column 7) shows the total CsgA protein, as measured in Western blots and integrated from 0 to 24 h. The absolute numbers of DK5208 (a csgA mutant) spores, when mixed with DK1622, were 5.3 × 105 and 10.3 × 105 after 3 and 5 days, respectively. The entire experiment was carried out twice with similar results. The table shows numbers from one of those experiments.
No colonies were detected. If there had been one colony per plate, the frequency would have been 10−4.
DISCUSSION
The responses of individual developmentally regulated genes to C-signal levels in act mutants show a very regular pattern. First, there were no alterations in the expression of sdeK, spi, or fruA during the first 24 h of development in any of the act mutant strains. This absence of effect is explained by the fact that these genes are C-signal independent, although they are dependent on A-signal or E-signal or starvation (5, 6, 19). There is a suggestion in Fig. 2C that C-signal is responsible for a decay of fruA expression after 24 h. The greatest decay was observed with the wild type and the ΔactC mutant. The other act mutants showed less decay than did the wild type in proportion to the amount of C-signal they expressed at 24 h. For substantiation, this suggestion calls for additional experiments.
Second, the act mutations have strong effects on all the C-signal-dependent reporters tested, i.e., Ω4414, Ω7536, Ω4499, and Ω4435. Third, the effects of ΔactA or of ΔactB on all these C-signal-dependent reporters are basically the same—lowering of the expression level without a change in the time of rise or fall of expression. The effects of ΔactC on all C-signal-dependent reporters is to advance β-galactosidase expression without changing the peak level. Here there is one exception: ΔactC did not bring about premature expression of the Ω4435 reporter. This suggests that expression of Ω4435 requires an additional factor that is not made until 20 h. Finally, the effects of a null mutation in actD (ΩactD) on all C-signal-dependent reporters is to delay β-galactosidase expression, again without changing the peak value. These regularities in reporter expression parallel qualitatively and in rank order earlier observations to the effect that elimination of each of the act genes generates a specific time pattern for CsgA protein levels (9). The similarity of the β-galactosidase expression and the CsgA protein patterns implies that the C-signal-dependent reporters are responding in proportion to the number of CsgA molecules per C-signal donor cell. These data support the idea that aggregation and sporulation have different thresholds for CsgA specific activity because these processes depend on different sets of developmentally regulated genes.
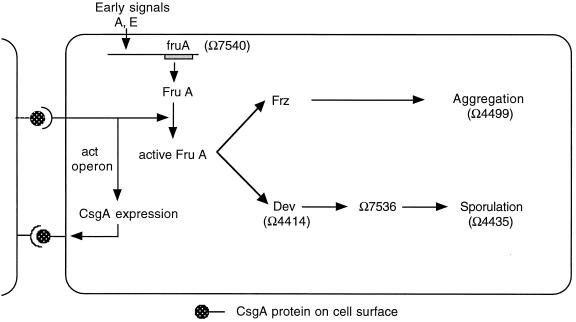
The specific activity of C-signal has been shown to rise progressively during normal development (9). The rise is explained by a positive feedback loop produced by the act operon products, which enhances csgA gene expression, as shown in a model of the C-signal response circuit diagram in Fig. 4. The aggregation and sporulation branches of the C-signaling pathway in Fig. 4 require different intensities of C-signaling (14, 20, 29). Consequently, fruiting-body development proceeds from early preparatory stages such as rippling, with low C-signal specific activity (9), to aggregation, with intermediate levels, to sporulation, with high levels. According to this scheme, the developmental phenotype of each act mutant is related to the altered progression of C-signaling in the mutant. The act gene mutations limit the level or alter the timing of transcription of csgA. CsgA protein levels rise above their 0-h level in the ΔactA and ΔactB mutants but never above one-quarter those of the wild type (9). This level is sufficient to signal proteins of the frz pathway, which then change cell behavior (reversal frequency, stop time, and speed) in such a way that cells stream into aggregates (11). However, this level is apparently not high enough to induce sporulation, inasmuch as these mutants form only 10−6 the wild-type number of spores, even though they construct mounds (9). The ΔactC or ΩactD mutants synthesize peak wild-type levels of CsgA protein on an abnormal time schedule. As a result, they synthesize less total C-signal (by sporulation rescue) and less total CsgA protein (by integrating CsgA Western blot data over time). They aggregate and sporulate. However, they make fewer spores than the wild-type cells do because the time-integrated level of CsgA protein is lower than in the wild type and C-signal activity is lower than in the wild type (Table 4).
FIG. 4.
The time of expression of a C-signal-dependent gene depends on its position within the C-signal transduction pathway and on its threshold C-signal specific activity (6, 12). Two cells are shown signaling to each other; both have the same signal transduction circuit, but for clarity the circuit is shown only in the cell on the right.
These parallels suggest that during development of wild-type cells, the time at which each C-signal-dependent gene is expressed is determined by the time at which the specific activity of C-signal has risen to a level that is appropriate for it. The experiments reported here show how those developmentally regulated genes fit into the C-signal response pathway, as reflected by their placement in Fig. 4. The abnormal time pattern of csgA expression produced by ΔactC and ΩactD mutants reveals in a new way that the expression of fruA is C-signal independent. Although C-signal does not affect fruA expression during the first 24 h, it does modify FruA protein posttranslationally, most probably by phosphorylation (6). By activating FruA protein, C-signaling regulates both aggregation and sporulation. The studies with act mutants show that Ω4499 is activated by moderate C-signal levels such as those available at 6-7 h. Reporters Ω4414 and Ω7536 require a higher level of C-signaling for their activation. Such levels are only achieved inside a nascent fruiting body due to multiple rounds of C-signaling and positive feedback through act that occur there. As a consequence, these reporters are associated with sporulation (12).
Another aspect of act gene function and the C-signaling pathway is revealed in these experiments. Without exception, ΔactA mutants have the same β-galactosidase expression profiles as ΔactB mutants do. The ActB protein has the sequence of a transcription activator of the NTRC protein class (8), and the ActA protein appears to be a compound response regulator most closely related to pleD of Caulobacter crescentus (1) and to celR2 of Rhizobium leguminosarum (2). Equivalent expression profiles (this work) and equivalent CsgA protein levels (9) suggest that the actA and actB proteins are serial elements in a signal transduction pathway that responds to the reception of C-signal. ActA might detect C-signal directly or indirectly.
All the experimental results presented here agree in suggesting that the specific activity of C-signal is the principal factor limiting development between 6 and 20 h. C-signal limitation during the late phase of development is clearly shown by premature aggregation of the ΔactC mutant and the premature expression of the C-signal-dependent reporters, with the possible exception of the ΔactC mutant of Ω4435. Delayed reporter expression in the ΩactD mutant reflects the same point. The effects of act mutations on reporter expression are thus explained by the C-signaling pathway with thresholds (Fig. 4). These reporters show how the genes of the aggregation and sporulation pathways participate in a C-signal-controlled progression to build the fruiting body.
Acknowledgments
An antiserum specific to CsgA protein was the kind gift of T. Kruse, S. Lobendanz, N. Berthelsen, and L. Sogaard-Andersen, Odense University.
This investigation was supported by U.S. Public Health Service grant GM 23441 to D.K. from the National Institute of General Medical Sciences.
REFERENCES
- 1.Aldridge, P., and U. Jenal. 1999. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 32:379-391. [DOI] [PubMed] [Google Scholar]
- 2.Ausmees, N., H. Jonsson, S. Hoglund, H. Ljunggren, and M. Lindberg. 1999. Structural and putative regulatory genes involved in cellulose synthesis in Rhizobium leguminosarum bv. trifolii. Microbiology 145:1253-1262. [DOI] [PubMed] [Google Scholar]
- 3.Campos, J., J. Geisselsoder, and D. Zusman. 1978. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:167-178. [DOI] [PubMed] [Google Scholar]
- 4.Collis, C., and R. Hall. 1985. Identification of a Tn5 determinant conferring resistance to phleomycins, bleomycins, and tallysomycins. Plasmid 14:143-151. [DOI] [PubMed] [Google Scholar]
- 5.Downard, J. 1993. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J. Bacteriol. 175:7762-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellehauge, E., M. Norregaard-Madsen, and L. Søgaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal coordination of intercellular signals in M. xanthus development. Mol. Microbiol. 30:807-813. [DOI] [PubMed] [Google Scholar]
- 7.Garza, A. G., J. S. Pollack, B. Z. Harris, A. Lee, I. M. Keseler, E. F. Licking, and M. Singer. 1998. SdeK is required for early fruiting-body development in Myxococcus xanthus. J. Bacteriol. 180:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorski, L., T. Gronewold, and D. Kaiser. 2000. A sigma-54 activator protein necessary for spore differentiation within the fruiting body of Myxococcus xanthus. J. Bacteriol. 182:2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gronewold, T. M. A., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for M. xanthus development. Mol. Microbiol. 40:744-756. [DOI] [PubMed] [Google Scholar]
- 10.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 11.Jelsbak, L., and L. Søgaard-Andersen. 1999. The cell-surface associated C-signal induces behavioral changes in individual M. xanthus cells during fruiting body morphogenesis. Proc. Natl. Acad. Sci. USA 96:5031-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 97:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan, H. B., A. Kuspa, and D. Kaiser. 1991. Suppressors that permit A signal-independent developmental gene expression in Myxococcus xanthus. J. Bacteriol. 173:1460-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, S. K., and D. Kaiser. 1990. Cell alignment required in differentiation of Myxococcus xanthus. Science 249:926-928. [DOI] [PubMed] [Google Scholar]
- 16.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 17.Kroos, L., A. Kuspa, and D. Kaiser. 1990. Defects in fruiting body development caused by Tn5lac insertions in M. xanthus. J. Bacteriol. 172:484-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 19.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 20.Li, S., B. U. Lee, and L. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., and L. Plamann. 1996. Purification and phosphorylation of Myxococcus xanthus AsgA protein. J. Bacteriol. 178:289-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licking, E., L. Gorski, and D. Kaiser. 2000. A common step for changing the cell shape in fruiting-body and starvation-independent sporulation of Myxococcus xanthus. J. Bacteriol. 182:3553-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin, S., E. Sodergren, T. Masuda, and D. Kaiser. 1978. Systematic isolation of transducing phages for Myxococcus xanthus. Virology 88:44-53. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 25.Plamann, L., J. M. Davis, B. Cantwell, and J. Mayor. 1994. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J. Bacteriol. 176:2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plamann, L., and H. B. Kaplan. 1999. Cell-density sensing during early development in Myxococcus xanthus, p. 67-82. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria, ASM Press, Washington, D.C.
- 27.Pollack, J. S., and M. Singer. 2001. SdeK, a histidine kinase required for Myxococcus xanthus development. J. Bacteriol. 183:3589-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sager, B., and D. Kaiser. 1994. Intercellular C-signaling and the traveling waves of Myxococcus. Genes Dev. 8:2793-2804. [DOI] [PubMed] [Google Scholar]
- 29.Søgaard-Andersen, L., F. Slack, H. Kimsey, and D. Kaiser. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10:740-754. [DOI] [PubMed] [Google Scholar]
- 30.Thœny-Meyer, L., and D. Kaiser. 1993. devRS, an autoregulated and essential genetic locus for fruiting-body development in Myxococcus xanthus. J. Bacteriol. 175:7450-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]