Abstract
As pollinators decline globally, competition for their services is expected to intensify, and this antagonism may be most severe where the number of plant species is the greatest. Using meta-analysis and comparative phylogenetic analysis, we provide a global-scale test of whether reproduction becomes more limited by pollen receipt (pollen limitation) as the number of coexisting plant species increases. As predicted, we find a significant positive relationship between pollen limitation and species richness. In addition, this pattern is particularly strong for species that are obligately outcrossing and for trees relative to herbs or shrubs. We suggest that plants occurring in species-rich communities may be more prone to pollen limitation because of interspecific competition for pollinators. As a consequence, plants in biodiversity hotspots may have a higher risk of extinction and/or experience increased selection pressure to specialize on certain pollinators or diversify into different phenological niches. The combination of higher pollen limitation and habitat destruction represents a dual risk to tropical plant species that has not been previously identified.
Keywords: extinction, latitudinal gradients, speciation, competition, pollen delivery
Wild plant species in biodiversity hotspots are an important world resource for the products and ecosystem services they provide, including medicine, food, nutrient cycling, and alternative resources for pollinators of domesticated crops (1). Maintaining plant biodiversity in hotspots is a critical challenge for conservation biologists because individual species may have reduced mean fitness in species-rich communities because of increased interspecific competition (2–4). In flowering plants, the presence of coflowering species can reduce pollination success at a local scale because of reduced visitation of generalist pollinators to the focal species, decreased delivery of conspecific pollen, or stigma interference by heterospecific pollen (5–8). However, whether the number of competing species within broad geographic regions influences global patterns in pollination biology has not yet been examined.
Adaptations for effective pollination are widely accepted to have contributed to the tremendous radiation of angiosperms (9, 10). Pollen limitation may decrease as plants evolve traits that reduce reliance on pollinators (e.g., self-compatible breeding systems and vegetative reproduction), attract more specialized pollinators that deliver less heterospecific pollen, or reduce competition for pollinators (e.g., shifts in flowering time) (11). Studies have found that diversity in flowering phenologies and pollinator fauna is indeed higher in species-rich areas (12, 13). If plants in species-rich regions are more often pollen limited than those in species-depauperate areas, they may experience relatively strong natural selection favoring traits that reduce pollen limitation, making current biodiversity hotspots an even more valuable global resource as centers for future adaptation and, potentially, speciation.
We evaluate the potential effect of regional species diversity on the magnitude of pollen limitation by investigating a number of mitigating factors. First, we examine whether the number of observed pollinating taxa affected the relationship between pollen limitation and species richness. Second, we investigate whether this relationship varies with traits that affect a species' dependence on pollinator abundance and behavior. In particular, we expect that self-incompatible species may rely more on pollinators for pollen transfer than self-compatible species and, therefore, may be more vulnerable to pollen limitation as a result of competition to attract effective pollinators (14). Third, we investigate the effect of growth form because, in populations of trees, the mean distance between individuals in species-rich regions may exceed the mean interplant foraging distances of most pollinator species (15), whereas in sympatric herbs and shrubs, interplant spacing may still be well within the range of pollinator foraging distances.
A strong positive relationship between regional species richness and pollen limitation may imply that the number and identity of a plant species' neighbors determines the direction and intensity of selection on many attributes of plant species and plant-animal relationships such as floral specialization, floral phenology, breeding systems, and demography (16). Such global patterns in pollination success are interesting in their own right, but species richness is undoubtedly confounded by several factors that make it difficult to identify the primary cause of increased pollen limitation. First, it is possible that plant traits that confer higher magnitudes of pollen limitation are more prevalent in species-rich regions (e.g., if self-incompatible species experience greater pollen limitation and also disproportionately occupy species-rich areas). Second, a few well studied clades in species-rich regions (e.g., tropical Orchidaceae) may bias the apparent relationship between pollen limitation and regional species richness. Although some species' attributes, such as presence in forested or open habitats, may not be phenotypic in the conventional sense, related species may still be more similar in these traits, and using each of these related species as an independent data point in nonphylogenetic (or cross-species) analyses is a form of pseudoreplication (17). To conservatively reduce these sources of bias upon our observed pattern (12), we perform phylogenetically independent contrasts between sister taxa that differ in our trait(s) of interest (pollen limitation and regional species richness).
Results
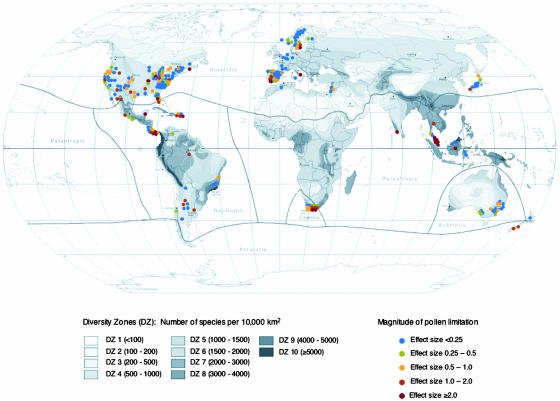
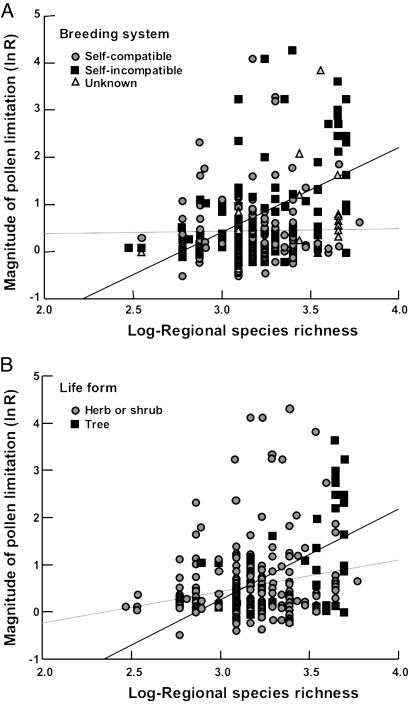
Our data set was comprised of pollen limitation studies from all continents throughout the world (except Antarctica), ranging from 60°N to 40°S in latitude (Fig. 1), with sampling well distributed among all major angiosperm orders (Fig. 2). Despite the relatively low number of pollen supplementation studies conducted in areas of either very low or very high diversity (Fig. 1), we find a strong positive relationship between regional species richness and the magnitude of pollen limitation (Fig. 3 A and B). This global-scale pattern is consistent with the hypothesis that competition among coflowering species for pollinators reduces plant reproductive success. In addition, the sensitivity of the relationship to the mating system and life history of the focal species is consistent with the hypothesis that the intensity of interspecific competition drives global patterns of pollen limitation. First, we find that the slope of the relationship between pollen limitation and species richness is significantly greater in self-incompatible than in self-compatible plants (Fig. 3A). Second, we find that the slope of the relationship is significantly steeper and more positive in trees than in herbs or shrubs (Fig. 3B). However, a higher proportion of tree species than herb/shrub species are self-incompatible, and a large portion of the effect of growth form may be due to the indirect effect of selfing ability. Nevertheless, we find no significant interaction between the effects of growth form and breeding system on pollen limitation (F1,221 = 3.23; P = 0.08).
Fig. 1.
Summary of the meta-analysis of fruit-set effect sizes of pollen-supplementation experiments conducted on 241 species in different biodiversity zones of the world. The biodiversity map was modified from ref. 18 (available upon request). Species richness is likely underestimated to a greater degree in areas of higher species richness (i.e., tropical regions), and these regions are the same areas where the fewest pollen supplementation studies have been conducted. We predict that further data will strengthen the patterns observed in this study.
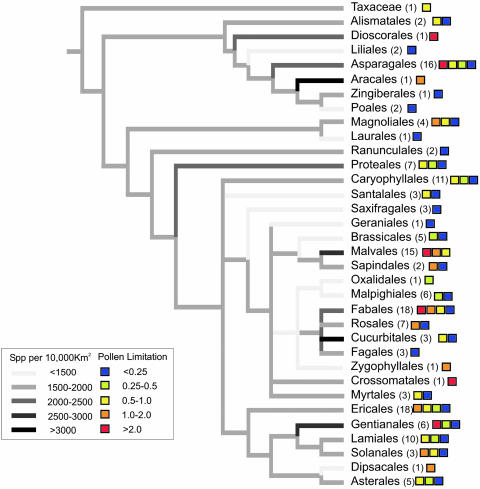
Fig. 2.
Summary of the magnitude of pollen limitation of 166 species standardized for the response variable (all effect sizes measured as fruit set) and the level of treatment (fruit set compared between hand-pollinated and natural-pollinated flowers/inflorescences) mapped onto the phylogeny of angiosperm orders. Gray shading (branches) indicates mean community species richness of locations where experiments were performed for all species in the order, whereas colors (tips) indicate the range of effect size(s) of pollen limitation of species in that order. Numbers in parentheses are the number of species in each angiosperm order in the data set, allowing some visualization of the phylogenetic signal of pollen limitation and species richness (e.g., all 15 studied species of Malvales display rather larger amounts of pollen limitation and reside in species-rich areas, whereas the three species of Saxifragales are not pollen-limited and live in relatively species-poor areas). Familial relationships within the phylogenetic tree were generated from the maximally resolved tree of seed plants within Phylomatic. We used the branch length adjuster (“bladj” option) within phylocom to estimate missing subfamilial and/or subordinal branch lengths from the ages of nodes on the tree (estimated by using fossil dates; ref. 19). Because contrasts can be conducted only at dichotomous nodes in the tree, generic relationships within families were further resolved where possible (phylogeny available upon request), allowing for a total of 128 phylogenetic contrasts in both the regional species richness and magnitude of pollen limitation.
Fig. 3.
The relationship among species between pollen limitation (flower- and plant-level studies combined) and log-regional species richness (F1,239 = 25.82; P < 0.0001) changes dramatically depending on breeding system (A) and growth form (B). (A) The positive relationship is apparent only for self-incompatible (SI) species (F1,103 = 31.14; P < 0.0001), and it does not characterize the relationship among self-compatible (SC) species (F1,117 = 0.03; P = 0.853); these slopes are significantly different (F1,220 = 10.06; P = 0.0001). (B) Although both trees and shrubs/herbs show a significant positive relationship (F1,38 = 11.94; P = 0.0014 for trees and F1,199 = 7.42; P = 0.0007 for shrubs/herbs), the slope is steeper for trees (F1,237 = 5.17; P = 0.02). Dividing the data into four classes, we find the patterns significant in both SI trees (F1,30 = 14.72, P = 0.0006) and SI herb/shrubs (F1,73 = 7.19, P = 0.0091) but not in SC trees (F1,8 = 1.30, P = 0.29) nor SC herbs/shrubs (F1,109 = 0.23, P = 0.63) (data not shown).
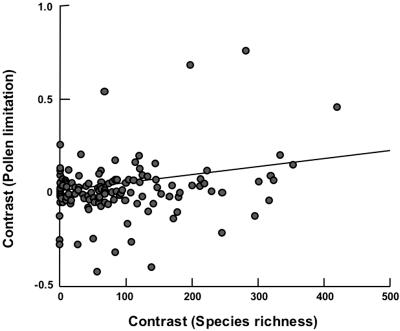
Using our constructed phylogeny, we found a strong phylogenetic signal for both pollen limitation and species richness; closely related species experience similar levels of pollen limitation and also live in areas with similar regional species richness (both traits: P = 0.0001, randomization test). However, pollen limitation is positively correlated with the regional species richness of the area in which the study was conducted, even when we apply a more conservative test that takes phylogenetic relationships into account (Fig. 4). Furthermore, lower latitudes (i.e., tropical regions) may have more canopy cover, and, given that regional species-richness decreases with increasing latitude in our data set (r239 = –0.76; P < 0.0001), it is possible that the increased pollen limitation could be due to lower pollination success in shaded habitats [i.e., if shading decreases pollinator activity (20) and if only the species in the shaded understory could be easily tested for pollen limitation] or to some other factor limited to tropical ecosystems. However, when we performed separate analyses for pollen limitation studies conducted in forested vs. open habitats, the slopes of the relationship between pollen limitation and regional species richness are indistinguishable (F1,144 = 0.75; P = 0.39). Furthermore, a positive relationship between pollen limitation and regional species-richness is still apparent, even when the effect of latitude is removed with residual regression (F1,239 = 3.98; P = 0.047).
Fig. 4.
Standardized phylogenetic independent contrasts between sister groups descended from the 128 nodes in the hypothesized phylogenetic tree (see Fig. 2). Correlation analysis indicates that increases between sister groups in pollen limitation, measured as the effect size of fruit set, are correlated with increases between sister groups in community species richness (number of heterospecifics in the area) (r = 0.27; P = 0.002). Along the x axis are the positivized contrasts in regional species richness [for instance, the absolute value of the difference between the average regional species richness of Magnoliales (Fig. 2) and the average species richness of its sister group, Laurales], and along the y axis are the corresponding pollen limitation contrasts (continuing with the same example, the difference between the average pollen limitation of Magnoliales and the average pollen limitation of Laurales). The figure shows the slope of the relationship forced through the origin (17).
We find evidence that plants located in more species-rich regions use fewer pollinating species (F1,161 = 3.79; P = 0.05). However, there is no difference in the relationship between pollen limitation and regional species richness for plant species pollinated by few (<5) or many (>5) pollinator species (F1,157 = 0.07; P = 0.79), suggesting that even species having relatively specialized pollinators still suffer when in competition for visits. Furthermore, although low sample size of wind-pollinated species (n = 7) precludes rigorous statistical analysis, we find that the slopes of the pollen-limitation vs. species-richness regressions for wind- and animal-pollinated species are virtually the same (data not shown). Although this pattern must be verified with more empirical evidence, we speculate that wind- and animal-pollinated plants experience similarly inefficient pollen delivery when in close proximity to other species because of increased delivery of heterospecific pollen.
Discussion
Global-scale analysis reveals that the magnitude of pollen limitation exhibited by a focal species is positively correlated with the regional species richness of the study area, even when phylogenetic relationships are taken into account. Self-incompatible species, which should be especially dependent on pollinator service, and tree species, which may suffer reduced pollen delivery due to large interplant spacing, display a particularly striking broad-scale pattern: Species with the highest numbers of competitors experience the lowest pollination success. Finally, by ruling out the contributions of canopy cover extent (20), or any other unknown factor limited to tropical latitudes, being the primary cause of our pattern, our suggestion of a causal relationship between species richness and pollen limitation is substantiated.
Surprisingly, our data suggest that adaptations to reduce pollen limitation (i.e., specializing on different pollinators or phenological niches) in competitive environments are insufficient. Plants located in species-rich regions use fewer pollinating species as predicted by recent models (21), but, surprisingly, species with fewer pollinators display the same relationship between pollen limitation and regional species richness as do species with many pollinators. This finding suggests that even species that may have evolved traits to increase pollination success still suffer due to competition for pollinator service. Plant species may also evolve flowering phenologies that differ from those of their coflowering competitors, thus increasing their conspecific pollen delivery (11–13). Although there is greater potential for phenological diversity in tropical (species-rich) areas due to the potentially long growing season (10), if numerous coexisting clades are diversifying in phenology, there may still be higher competition for pollination in species-rich relative to depauperate floras, regardless of flowering time.
If the relationship between pollen limitation and species-richness has existed over evolutionary time scales, pollen limitation and competition for pollinators may have been a driver of speciation in angiosperms. Species-rich plant regions may become even more species-rich because of biodiversity positive feedback (22). In other words, the pollen limitation caused by increased interspecific competition may be heterogeneous within a plant species' range. Such a spatial mosaic in competition could promote or intensify selection for different traits in different areas, thus promoting speciation (23, 24) and further increasing the species-richness of the region (25). Pollen limitation dynamics could thus potentially explain how the species richness in island archipelagos largely determines the subsequent rate of diversification of colonizing angiosperms (25). This possibility illustrates how community complexity could also contribute to a latitudinal gradient in angiosperm species richness and is deserving of more in-depth research.
It is imperative that comparative studies, such as this one, do not confuse correlation with causation. Although our results are consistent with the interpretation that species richness increases pollen limitation via increased competition for pollinators (or increased heterospecific pollen delivery), this pattern may be due to recent increases in habitat destruction and concomitant decreased pollinator service. In this case, pollen limitation may lead to increased extinction risk (26) and, therefore, threaten the maintenance of biodiversity. If the magnitude of pollen limitation is simply a reflection of the present diversity, abundance, and stability of pollinator communities (27), then our data suggest that biodiversity hotspots are experiencing greater declines in pollinator abundance and diversity than are less species-rich areas. In this case, we may expect that a decline in the viability of plant populations and species in biodiversity hotspots will soon follow. Following this line of reasoning, our analysis suggests that disturbance of pollinator communities puts tropical trees at the highest risk of extinction. Because pollen limitation in wind-pollinated species will not reflect pollinator abundance but may still increase with heterospecific pollen delivery, future comparisons of sympatric wind- and animal-pollinated species along gradients of species richness and habitat disturbance may discriminate between the hypotheses of pollinator declines versus increased competition.
Thus, our findings suggest a need for further study along two major avenues: (i) Plants in species-rich areas may be more pollen-limited than plants in species-poor regions as a result of the former having experienced greater recent reductions in pollinator abundance and diversity (15). In this case, maintaining plant diversity in species-rich areas will require special efforts to identify and to conserve their pollinators (26, 28) to prevent losing the vital resources that these plants provide for food and medicine. (ii) Community-level processes may drive plant speciation via competition for pollinator services and/or reduction of heterospecific pollen transfer, thereby contributing to latitudinal gradients in biodiversity. Distinguishing whether increased pollen limitation with increased regional species-richness represents a natural pattern that has contributed to the high species diversity in hotspots, or a pattern caused by recent severe declines in pollinator abundances (28, 29) in these hotspots should remain an important endeavor for plant population biologists.
Methods
We quantified the magnitude of pollen limitation (the reduction in reproductive success due to inadequate pollen receipt) from 1,013 published pollen supplementation experiments. An examination of our pollen limitation data set revealed that there is little consistency in how pollen limitation is measured (14): Many studies report fruit set (fruits/flowers) of plants given supplemental pollen compared to control plants receiving ambient pollen loads, whereas others report some metric of seed production (seeds/flower, seeds/fruit, or seed/plant). Because the metrics used influences the reported magnitude of pollen limitation, we standardized our data set, reducing it to the 482 estimates based on fruit set. The magnitude of pollen limitation was then calculated as the log response ratio (ln R),
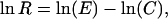 |
[1] |
where E indicates the mean fruit set of plants (or flowers, inflorescences) in the supplement treatment and C indicates the mean fruit set of control plants (14). A value of 0 reflects no difference in fruit set between plants in the supplement and control treatments, a positive value indicates higher fruit set in the supplement treatment, and a negative value indicates higher fruit set in the control treatment. Furthermore, pollen limitation of some species has been examined in a number of different years and by more than one researcher (e.g., there are >10 separate estimates of fruit set for Trillium grandiflorum; ref. 30), whereas other plant species have been the subject of a single study. Therefore, for each unique plant species (n = 241), we calculated the weighted average magnitude of pollen limitation for the species. The weight for each measure of pollen limitation was the reciprocal of its sampling variance (31).
We obtained an estimate of regional plant species richness for each location by estimating the average number of vascular plant species coexisting with the focal plant species by using estimates of species richness of vascular plants from biomaps (18). Species-richness values were log-transformed before analysis to reduce heteroscedasticity. Because studies have found that even traits of a geographical nature (17, 32) show similarities between closely related species and, thus, benefit from a phylogenetic approach to data analysis, we examined our question in this way as well. An hypothesized phylogenetic tree for the 166 plant species for which we have data standardized for methodology (see below) was obtained by using phylomatic (33) at www.phylodiversity.net/phylomatic.
For each published experiment, we documented the life form of the plant species studied (tree, shrub, or herb) and the breeding system (e.g., self-incompatible and self-compatible) as reported or ascertained experimentally in the publication (i.e., by excluding pollinators and applying only self-pollen). When breeding system information was not reported alongside the pollen supplementation experiment, we searched the literature for this information. Finally, many researchers both observed pollinators visiting flowers and performed supplemental pollination experiments. When pollinator observations were reported, we documented the number of pollinating species observed. We divided these studies into two broad categories that reflect the level of specialization in pollination: plants species pollinated by five or fewer pollinator species and those pollinated by >5 species.
In the majority of publications, the authors described features of the surrounding habitat in which the plant resides (i.e., location and some description of habitat quality). Our data set includes only 29 entries in which the study was conducted in a highly modified environment (e.g., monoculture) where the regional species richness may poorly reflect the local species richness. Because the removal of these entries strengthened rather than weakened our findings, we retained these entries in the data set used in the analysis reported below.
The level at which the pollination treatment is applied (i.e., to all flowers on the plant or to a portion of the flowers on the plant) can affect the magnitude of treatment response (34). When supplemental pollen is applied to a portion of the flowers on the plant (i.e., flower-level treatment), resource reallocation to treated flowers may inflate the estimated magnitude of pollen limitation (34). We distinguished between studies conducted at the flower vs. whole plant level in our data set. In the nonphylogenetic analysis, we used least-squares regression of the weighted average magnitude of pollen limitation (in both the flower- and plant-level studies combined; n = 241) on the species richness of an area; an analysis of covariance to test for heterogeneity of slopes between distinct classes of species (e.g., trees vs. nontrees, self-compatible vs. self-incompatible, open vs. closed habitat); and a two-way ANOVA to test whether there was a significant interaction between the effects of growth form and breeding system on pollen limitation. To test whether the effect of regional species richness on pollen limitation was independent of latitude, we used residual regression (i.e., the residuals of a regression of latitude on species richness were then regressed upon pollen limitation). We find qualitatively similar positive relationships between the magnitude of pollen limitation and regional species richness when we conduct analyses with all data points and when we conduct analyses separately for flower-level and plant-level studies. In the phylogenetic analysis, we include only data from flower-level studies (n = 166). Flower-level studies were most prevalent in our data set and, therefore, allowed for more contrasts. We did not combine flower- and plant-level studies in the phylogenetic-independent contrasts to avoid comparing sister groups that were potentially not standardized for measure of pollen limitation.
We examined whether a phylogenetic signal was present for pollen limitation and regional species richness, i.e., whether closely related clades are more similar with respect to the values of these attributes than one would expect by chance (Fig. 2). A statistically significant phylogenetic signal was detected for both attributes (P = 0.0001, randomization test). For such traits, relationships observed in a nonphylogenetic analysis (which treats species as independent data points) may be biased because of strong phenotypic similarities among closely related taxa. Therefore, a more appropriate and conservative way to examine patterns between pollen limitation and species richness is to seek evidence for correlated changes in these attributes between sister taxa (i.e., phylogenetic independent contrasts). In this approach, one asks whether evolutionary divergences between sister clades in one attribute of interest (e.g., the magnitude of pollen limitation) are associated with differences between sister clades in a second attribute (regional species richness). The analysis of phylogenetic signal and phylogenetic independent contrasts was performed by using the software application phylocom (www.phylodiversity.net/phylocom).
Acknowledgments
We thank J. Byrnes, J. Chase, B. Crespi, J. Damuth, E. Elle, D. Schluter, S. Otto, R. Sargent, S. Vamosi, an anonymous reviewer, and the participants of the “Beyond Hand Pollination” from the National Science Foundation's National Center for Ecological Analysis and Synthesis (NCEAS) working group for their comments and contributions to the development of ideas in this study. This work was supported by NCEAS.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ricketts, T. H. (2004) Conserv. Biol. 18, 1262–1271. [Google Scholar]
- 2.Levine, J. M. & Rees, M. (2002) Am. Nat. 160, 452–467. [DOI] [PubMed] [Google Scholar]
- 3.Levins, R. & Culver, D. (1969) Proc. Natl. Acad. Sci. USA 62, 1246–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer, T. M., Stanton, M. L. & Young, T. P. (2003) Am. Nat. 162, Suppl. 4, S63–S79. [DOI] [PubMed] [Google Scholar]
- 5.Bell, J. M., Karron, J. D. & Mitchell, R. J. (2005) Ecology 86, 762–771. [Google Scholar]
- 6.Campbell, D. R. & Motten, A. F. (1985) Ecology 66, 554–563. [Google Scholar]
- 7.Levin, D. A. & Anderson, W. W. (1970) Am. Nat. 104, 455–467. [Google Scholar]
- 8.Chittka, L. & Schurkens, S. (2001) Nature 411, 653. [DOI] [PubMed] [Google Scholar]
- 9.Stebbins, G. L. (1981) Biosciences 31, 573–577. [Google Scholar]
- 10.Bolmgren, K., Eriksson, O. & Linder, H. P. (2003) Evolution 57, 2001–2011. [DOI] [PubMed] [Google Scholar]
- 11.Rathcke, B. J. & Lacey, E. P. (1985) Ann. Rev. Ecol. Syst. 16, 179–214. [Google Scholar]
- 12.Mosquin, T. (1971) Oikos 22, 398–402. [Google Scholar]
- 13.Ashton, P. S., Givnish, T. J. & Appanah, S. (1988) Am. Nat. 132, 44–66. [Google Scholar]
- 14.Knight, T. M., Steets, J. A., Vamosi, J. C., Mazer, S. J., Burd, M., Campbell, D. R., Dudash, M., Johnston, M., Mitchell, R. J. & Ashman, T.-L. (2005) Ann. Rev. Ecol. Syst. 36, 467–497. [Google Scholar]
- 15.Johnson, S. D., Neal, P. R., Craig, I. P. & Edwards, T. J. (2004) Biol. Conserv. 120, 31–39. [Google Scholar]
- 16.Thompson, J. N. (2005) The Geographic Mosaic of Coevolution (Univ. of Chicago Press, Chicago).
- 17.Garland, T., Harvey, P. H. & Ives, A. R. (1992) Syst. Biol. 41, 18–32. [Google Scholar]
- 18.Barthlott, W., Bledinger, N., Braun, G., Feig, F., Kier, G. & Mutke, J. (1999) Acta Bot. Fenn. 162, 103–110. [Google Scholar]
- 19.Wikstrom, N., Savolainen, V. & Chase, M. W. (2001) Proc. Roy. Soc. London B 268, 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera, C. M. (1995) Ecology 76, 218–228. [Google Scholar]
- 21.Sargent, R. D. & Otto, S. P. (2006) Am. Nat. 167, 67–80. [DOI] [PubMed] [Google Scholar]
- 22.Crespi, B. J. (2004) Trends Ecol. Eval. 19, 627–632. [DOI] [PubMed] [Google Scholar]
- 23.Hansen, T. F., Armbruster, W. S. & Antonsen, L. (2000) Am. Nat. 156, S17–S34. [DOI] [PubMed] [Google Scholar]
- 24.Thompson, J. N. (1999) Am. Nat. 153, S92–S93. [Google Scholar]
- 25.Emerson, B. C. & Kolm, N. (2005) Nature 434, 1015–1017. [DOI] [PubMed] [Google Scholar]
- 26.Wilcock, C. & Neiland, R. (2002) Trends Plant Sci. 7, 270–277. [DOI] [PubMed] [Google Scholar]
- 27.Kearns, C. A. (1998) Ann. Rev. Ecol. Syst. 29, 83–112. [Google Scholar]
- 28.Bond, W. J. (1994) Philos. Trans. Roy. Soc. London B 344, 83–90. [Google Scholar]
- 29.Oleson, J. M. & Jordano, P. (2002) Ecology 83, 2416–2424. [Google Scholar]
- 30.Knight, T. M. (2003) Oecologia 442, 557–563. [DOI] [PubMed] [Google Scholar]
- 31.Gurevitch, J., Curtis, P. & Jones, M. H. (2001) Adv. Ecol. Res. 32, 199–247. [Google Scholar]
- 32.Davies, T. J., Savolainen, V., Chase, M. W., Goldblatt, P. & Barraclough, T. G. (2005) Am. Nat. 166, 418–425. [DOI] [PubMed] [Google Scholar]
- 33.Webb, C. O. & Donoghue, M. J. (2005) Mol. Ecol. Notes 5, 181. [Google Scholar]
- 34.Knight, T. M., Steets, J. A. & Ashman, T.-L. (2006) Am. J. Bot. 92, 270–276. [DOI] [PubMed] [Google Scholar]