Abstract
Salmonella enterica serovar Typhimurium produces two types of filamentous appendages on its surface. Fimbriae mediate adherence to tissues and cells via receptor-specific interactions, and flagella are the organelles of motility. These appendages play a role in colonization and dissemination, respectively, from infected surfaces and may be important components of bacterial survival. Increased expression of FimZ in serovar Typhimurium resulted in bacteria which were hyperfimbriated but were nonmotile in soft agar. This lack of motility was associated with down regulation of the flhDC master flagellar operon. Therefore, FimZ represents a molecular connection between flagella and fimbrial formation in serovar Typhimurium, indicating that the synthesis of flagella and fimbriae are oppositely controlled.
Motile strains of the Enterobacteriaceae swim using flagella and adhere to mannose-containing glycoconjugates of eucaryotic cells by means of type 1 fimbriae. Both of these phenomena are consequences of the production of proteinaceous appendages on the cell surface. The genetic systems of Salmonella enterica serovar Typhimurium involved in flagellar and type 1 fimbrial production, assembly, and regulation have been independently described (4, 5). The expression of flagellar genes is regulated by a complex process involving numerous regulatory proteins that control the production of components necessary for correct assembly of the flagellum structure (4). The molecular biology of bacterial locomotion has been extensively investigated and involves a complex signal transduction pathway (1).
The type 1 fimbrial genes of S. enterica serovar Typhimurium have been described previously (5). The synthesis of these appendages occurs via the chaperone-usher pathway, and the assembly components of the system are related to those described for Escherichia coli fimbrial biogenesis (10, 15). However, the regulation of fim gene expression in S. enterica serovar Typhimurium is different to that described in other bacteria. Three genes, fimZ, fimY, and fimW, of the fim gene cluster have been shown to affect the expression of fimA, encoding the major fimbrial subunit (16-18). Both FimZ and FimY are necessary for the expression of fimA in vivo although FimZ alone can bind in vitro to the fimA promoter region (14, 18). A comparison of the amino acid sequence of FimZ indicates that it is a member of the family of response regulators (9). However, a contiguous gene encoding a sensor kinase is not located adjacent to fimZ since the gene product of fimY does not exhibit relatedness to this class of proteins (17).
In the studies described below, we present evidence that the expression of FimZ from a strong heterologous promoter results in a reduced ability of S. enterica serovar Typhimurium to move through soft agar (Swim). This effect on motility is independent of the presence of surface-associated fimbriae and not due to a physical interference with flagellum formation by fimbrial structures. Therefore, FimZ provides a molecular connection between the Fim and Swim phenotypes in serovar Typhimurium.
Strains and growth conditions.
The strains of S. enterica serovar Typhimurium used in this study are shown in Table 1. To test for the production of type 1 fimbriae, bacteria were cultured at 37°C for 48 h in static, liquid broth as previously described and examined for the presence of surface-associated fimbriae by hemagglutination and reactivity with specific antiserum (12). To determine the Swim phenotype, strains were inoculated into soft agar (1% tryptone, 0.7% NaCl, 0.35% agar) and incubated overnight at ambient temperature. When appropriate, tetracycline was included at a final concentration of 20 μg/ml. The ability to move through the agar was recorded using a Kodak DC120 digital camera system.
TABLE 1.
Strains and plasmids used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| Serovar Typhimurium LT2 | ||
| TT18796 | proAB47/F"128(pro lac) zzf-3833::Tn10dTc[del25](T-POP) | 13 |
| TH1077 | fliC5050::MudJ | 7 |
| TH4038 | fimZ2::Tn10dTc[del-25](T-POP) | This work |
| LB5010 | Wild-type LT2 derivative, fimbriate | 3 |
| LBH4 | fimH::Kmr of LB5010, nonfimbriate | 8 |
| LBH4(T-POP) | fimZ2::Tn10(T-POP) transductant of LBH4 | This work |
| TH714 | fljb5001::MudJ | 7 |
| TH4054 | flhC5456::MudJ | This work |
| TH4313 | DUP4102[(fliC5050)MudA(flhC5213)] | This work |
| Serovar Typhimurium SL1344(T-POP) | fimZ2::Tn10(T-POP) transductant of SL1344 | This work |
| Plasmids | ||
| pISF182 | pACYC184 carrying fimZ | 18 |
| pISF187 | Kmr cassette inserted into fimZ on pISF182 | 18 |
| pISF239 | fimZ-lacZ reporter fusion | 16 |
Isolation and characterization of the conditional fimz mutant S. enterica serovar Typhimurium fimz2::Tn10(T-POP).
The conditional fimZ mutant of S. enterica serovar Typhimurium LT2 was isolated following transposon mutagenesis using the Tn10dTc[del-25](T-POP) system that has previously been described to isolate conditional (tetracycline-dependent) phenotypes (13). The Tn10dTc[del-25] T-POP will transcribe from the tetA promoter within the T-POP transposon into adjacent chromosomal DNA. The mutant was isolated by random T-POP mutagenesis of a strain carrying the fliC5050::Mu d-lac transcriptional fusion selecting for T-POP encoded tetracycline resistance as described by Rappeleye and Roth (13). The T-POP insertion in fimZ was identified as an insertion that resulted in a Lac− phenotype in the presence of tetracycline and a Lac+ phenotype in the absence of tetracycline (Tc-induced inhibition of fliC transcription).
The precise site of the Tn10d(T-POP) insertion was determined by sequence analysis using a primer specific for the left and right ends of the T-POP element. PCR amplification with these primers under decreasing stringency resulted in the amplification of a DNA fragment that was shown by DNA sequence analysis to contain the fimZ gene sequence. This was confirmed by further DNA sequence analysis and revealed that the transposon had inserted within the fimY-fimZ intergenic region of the S. enterica serovar Typhimurium LT2 fim gene cluster at a site that is 265 and 63 nucleotides upstream of the previously determined fimZ transcription and translation initiation sites, respectively (14, 18). The Tn10dTc[del-25] T-POP has a deletion of the normal transcriptional terminator of the tetA transcript and, in the presence of tetracycline, will transcribe genes adjacent to the site of insertion from the tetA promoter. As previously reported (5, 17), all the fim genes downstream of fimZ are transcribed in the opposite orientation to that of fimZ. Therefore, in the presence of tetracycline the expression of fimZ is the only gene of the cluster that is directly affected by the transposon insertion.
Following growth in the presence of tetracycline S. enterica serovar Typhimurium fimZ2::Tn10(T-POP) was found to be strongly fimbriate and characterized by the ability to mediate mannose-sensitive agglutination of guinea pig erythrocytes, react with specific fimbrial antiserum, and adhere to eucaryotic cells (Table 2). A comparison of fimbrial expression by this strain to the parental LT2 isolate indicates that many more appendages are produced by the transposon mutant following growth in the presence of tetracycline. This hyperexpression of type 1 fimbriae has a phenotype identical to that previously described by our group following overproduction of FimZ in S. enterica serovar Typhimurium (17, 18). Because FimZ is a transcriptional activator of fimA (14), the hyperfimbriate phenotype is consistent with a relatively high level of expression of the FimZ polypeptide.
TABLE 2.
Expression of the Fim and Swim phenotype by serovar Typhimurium strains
| Strain | Presence (+) or absence (−) of Tet during incubation | Fima | Swimb | β-Galactosidase activity of fimZ-lacZ fusionc |
|---|---|---|---|---|
| TH4038 | + | +++ | − | 293 |
| TH4038 | − | − | + | 19 |
| LBH4(T-POP) | + | − | − | 179 |
| LBH4(T-POP) | − | − | + | 28 |
| SL1344(T-POP) | + | +++ | − | NDd |
| SL1344(T-POP) | − | − | − | ND |
Type 1 fimbriae were detected using specific antiserum and the ability to agglutinate guinea pig erythrocytes only in the absence of mannose (12).
Expansion through soft agar after incubation for 18 to 24 h (see Fig. 1).
β-Galactosidase activity of the fimZ-lacZ fusion reported as previously described (17).
ND, not determined.
In the absence of tetracycline, no hemagglutination was observed and type 1 fimbriae were not detected on the surface of S. enterica serovar Typhimurium fimZ2::Tn10(T-POP). The lack of fimbrial expression is similar to observations that have been made using a fimZ mutant of serovar Typhimurium (18). Disruption of fimZ transcription by its homologous promoter following insertion of the Tn10(T-POP) transposon would be expected to result in relatively poor expression of fimZ in the absence of tetracycline.
Fimz production affects the ability of S. enterica serovar Typhimurium fimz2::Tn10(T-POP) to move through soft agar.
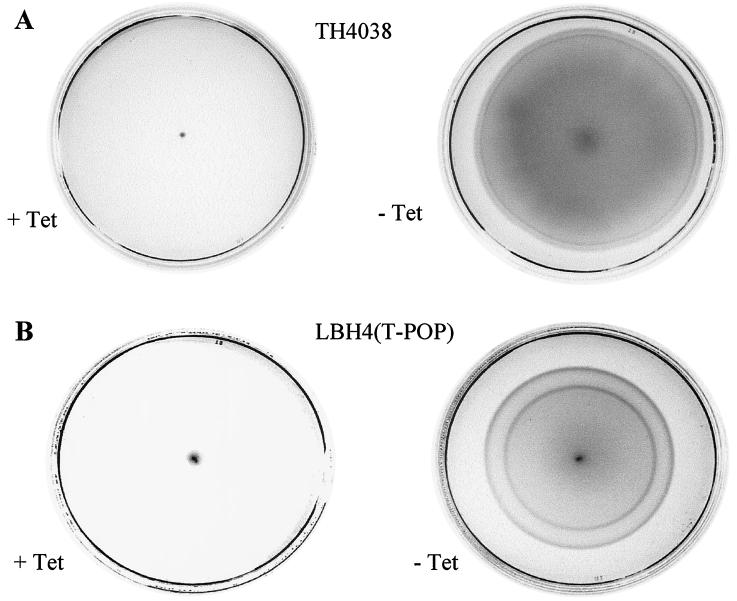
The ability of S. enterica serovar Typhimurium fimZ2::Tn10(T-POP) to move through soft agar is shown in Fig. 1A. In the presence of tetracycline, the bacteria remained localized to the point of the inoculum and were unable to swim, over an 18- to 24-h period, from this site. However, in the absence of tetracycline, the bacteria expanded from the inoculum and rapidly migrate through the agar.
FIG. 1.
(A) Movement of the fimbriate LT2 derivative (TH4038) through soft agar in the presence and absence of tetracycline. (B) Movement of the nonfimbriate fimH LT2 derivative (LBH4) through soft agar in the presence and absence of tetracycline. All cultures were incubated at ambient temperature for 24 h.
In order to demonstrate that the physical presence of fimbriae on the surface of the bacteria was not responsible for the lack of expansion through the agar in the presence of tetracycline, we examined the motility of a nonfimbriate mutant. S. enterica serovar Typhimurium LBH4 carries a mutation of the fimH gene and is nonfimbriate. The construction and characterization of this mutant has been described (8), and bacteria have never been observed to express type 1 fimbriae. Following transfer, by transduction, of the fimZ::Tn10(T-POP) allele to this strain, the ability of bacteria to migrate through soft agar was observed (Fig. 1B). As for S. enterica serovar Typhimurium fimZ2::Tn10(T-POP), the double mutant rapidly expanded from the site of the inoculum when grown in the absence of tetracycline but remained localized if grown in the presence of the antibiotic. As expected, this double mutant did not produce fimbriae under either condition. Consequently, the inability of the S. enterica serovar Typhimurium strains to rapidly expand through the soft agar in the presence of tetracycline cannot be explained by effects of fimbrial appendage formation on the surface of the bacteria. Also, a fimA mutant of the strain carrying fimZ::Tn10(T-POP) exhibited the same motility phenotype as S. enterica serovar Typhimurium LBH4. This mutant was constructed using the technique of Datsenko and Wanner (6) and carries a deletion of the fimA gene producing no FimA subunits. Therefore, the motility phenotype cannot be due to intracellular accumulation of high levels of FimA polypeptide in the presence of tetracycline.
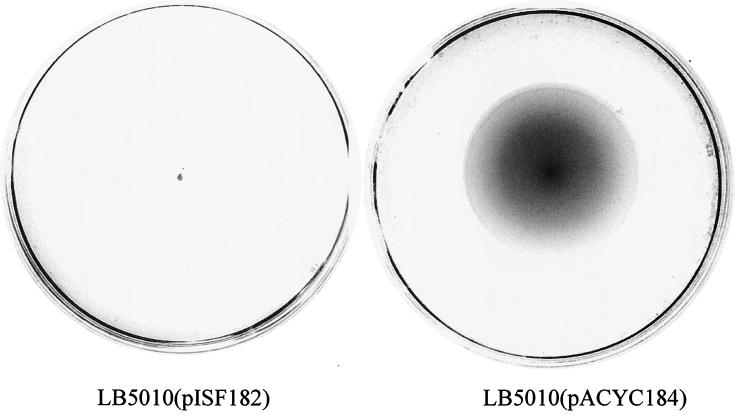
Since the differences associated with movement by S. enterica serovar Typhimurium through soft agar were observed due to the presence or absence of tetracycline, we determined whether the presence of the antibiotic itself was responsible for the difference in bacterial movement. S. enterica serovar Typhimurium LT(pISF182) is a transformant possessing a recombinant plasmid expressing the fimZ gene, and these transformants are strongly fimbriate due to the overexpression of both fimZ and fimY (17, 18). The presence of the plasmid confers chloramphenicol resistance on the bacteria that are tetracycline sensitive. S. enterica serovar Typhimurium LT2(pISF182) was strongly fimbriate and did not rapidly expand through soft agar (Fig. 2). Similarly, the nonfimbriate mutant, S. enterica serovar Typhimurium LBH4, transformed with pISF182 did not spread through the agar and is, as expected, nonfimbriate. Therefore, in all serovar Typhimurium LT2 derivatives examined, the lack of movement through soft agar was associated with overexpression of fimZ regardless of the antibiotic resistance phenotype. To confirm that FimZ production plays a role in altering bacterial movement through agar, S. enterica serovar Typhimurium LT2 transformants possessing plasmid pISF187 were examined. This plasmid is derived from pISF182 and carries a nonpolar mutation in fimZ (18). Bacteria carrying this plasmid were as motile as S. enterica serovar Typhimurium LT2.
FIG. 2.
Movement of LT2 (LB5010) transformants through soft agar. The plasmid pISF182 possesses a functional fimZ determinant and pACYC184 is the vector alone. Plates were incubated at ambient temperature for 18 h.
In order to determine whether increased expression of fimZ correlated with a decreased movement in a highly virulent strain of S. enterica serovar Typhimurium, the fimZ::Tn10(T-POP) allele was transferred, by transduction, to S. enterica serovar Typhimurium SL1344. This isolate is a mouse-virulent strain that has extensively been used to investigate serovar Typhimurium pathogenesis. An S. enterica serovar Typhimurium SL1344 transductant carrying the fimZ::Tn10(T-POP) mutation exhibited fimbriation and motility phenotypes in the presence or absence of tetracycline identical to those of the LT2 strain (Table 2). Therefore, the observed differences in movement were not strain specific but were found in two distinct isolates of S. enterica serovar Typhimurium when fimZ was overexpressed.
Expression of fimz by S. enterica serovar Typhimurium fimz2::Tn10(T-POP).
Since we have previously demonstrated that FimZ is an autoregulatory protein (18), a plasmid-based fimZ-lacZ reporter construct was used to detect the level of fimZ expression in serovar Typhimurium fimZ2::Tn10(T-POP). Table 2 shows the level of gene expression following growth in either the presence or absence of tetracycline. The level of fimZ-lacZ expression was 6 to 15 times greater following growth in the presence of tetracycline than following growth in its absence. Also, following growth in the presence of tetracycline, the level of flagellar antigen (Hi) expression, as detected by specific serum, was significantly decreased (Table 3).
TABLE 3.
Flagellum gene expression and antigen (Hi) production in serovar Typhimurium possessing fimZ2::T-POP allele
| Presence (+) or absence (−) of Tet | β-Galactosidase expression in:
|
Serum titer using anti-H(i)b | ||
|---|---|---|---|---|
| fliC-lacz | fljb-lacZ | flhC-lacZa | ||
| + | 110 | 8 | 20 | 80 |
| − | 1,100 | 620 | 200 | 640 |
The β-galactosidase levels were the same for flhC-lacZ in either a haploid strain (TH4054) that is disrupted for the flhC gene or in a tandem duplication strain (TH4313) carrying a functional flhDC operon in addition to the flhC-lacZ fusion.
Titer represents the reciprocal of the highest dilution of serum causing visible agglutination of a bacterial suspension.
Expression of flhdc by S. enterica serovar Typhimirium fimz2::T-POP.
fimZ2::T-POP was isolated as a strain with the tetracycline-induced Lac− phenotype of a strain carrying a fliC::lacZ fusion, demonstrating that the overexpression of fimZ resulted in decreased fliC transcription, consistent with decreased production of the FimH(i) flagellin. Expression of the two flagellin genes was measured using fliC-lac and fljb-lac transcriptional fusions in strains carrying the fimZ2::T-POP insertion. Induction of fimZ with tetracycline resulted in a 10-fold reduction in fliC transcription and a greater than 50-fold reduction in fljb transcription (Table 3). The fliC and fljb genes are transcribed from class 3 flagellar promoters. The flagellar regulon of serovar Typhimurium is a transcriptional hierarchy of three promoter classes (4). At the top of the transcriptional hierarchy is the class 1 flhDC operon. The FlhDC proteins are transcriptional activators for class 2 flagellar promoters. The fliA gene is transcribed from a class 2 promoter and encodes a flagellum-specific sigma factor, ςsgr;28, which specifically transcribes class 3 flagellum promoters, including the fliC promoter. In order to determine if the fimZ2::T-POP insertion exerted its regulatory effect at the level of class 1 flhDC promoter transcription, the expression of an flhDC-lac transcriptional fusion was measured in strains carrying the fimZ2::T-POP insertion in the presence and absence of tetracycline. The flhDC-lac fusion exhibited tetracycline-dependent repression of Lac expression in the presence of the fimZ2::T-POP insertion, indicating that the effect of fimZ overexpression on the flagellar regulon was at the level of transcription of the class 1 flhDC promoter (Table 3).
Summary and conclusion.
The ability of bacteria to adhere to solid surfaces under certain environmental conditions and also migrate to ecological niches favorable to growth are fundamental events in colonization and dissemination during bacterial survival. In the case of S. enterica serovar Typhimurium, these events would facilitate infection and transmission between hosts. Consequently, coordination between the machinery for production of both adherence factors and movement would facilitate coordination between these two systems. We have previously demonstrated that FimZ is a positive transcriptional activator of type 1 fimbrial expression in serovar Typhimurium and mediates fimA expression by binding to the fimA promoter region (18). In the studies described above, we have shown that a conditional (tetracycline-dependent) fimZ mutant of serovar Typhimurium is hyperfimbriate in the presence of tetracycline but nonfimbriate in its absence as expected. However, the overexpression of FimZ was also associated with a decrease in motility. This phenotype was not a result of large numbers of fimbriae expressed on the bacterial surface or due to the accumulation of FimA subunits intracellularly leading to a stress response, since the loss of movement was also demonstrated in nonfimbriate mutants. Overexpression of FimZ as a result of the presence of the fimZ gene carried on a multicopy plasmid also results in the inability of bacteria to migrate through the agar. The decrease in motility in the presence of increased amounts of FimZ was not due to the complete absence of flagella on the bacterial surface since electron microscopy indicated a few flagellum filaments on the surfaces of bacteria grown in the presence of tetracycline. However, under these conditions the amount of flagellar antigen produced was significantly reduced and could account for the decreased motility. In wild-type strains of serovar Typhimurium, the intracellular concentration of FimZ may be critical to producing opposite effects on flagellar and fimbrial gene expression. Consequently, relatively large amounts of FimZ are likely to favor type 1 fimbrial expression with decreasing flagellum production. Since we have previously demonstrated that FimZ binds to the fimA promoter region (18), we examined the flhDC promoter for nucleotide sequences similar to those found for fimZ. No regions of similarity were observed, and it is currently unknown whether FimZ binds directly to the flhDC promoter.
FimZ may represent a molecule that facilitates communication between type 1 fimbrial expression by the bacteria and the motility phenotype and could be part of a signal pathway facilitating coordination between localization by adherence and dissemination by locomotion. Several fimbrial gene clusters have been reported for S. enterica serovar Typhimurium, but their coordination with flagellar expression is unknown (2). Recently, the ability of a fimbrial gene product, MrpJ, to repress the flagellar regulon in Proteus mirabilis was reported (11). The molecular function of MrpJ in fimbrial expression is unknown, but it is believed to be part of the P. mirabilis mrp gene cluster. Our observations using S. enterica serovar Typhimurium demonstrate that a positive activator of fimA expression can down regulate flagellar gene expression. Therefore, communication at the molecular level between fimbrial and flagellar expression in enterobacteria may be a common phenomenon in motile organisms.
REFERENCES
- 1.Aizawa, S. I., C. S. Harwood, and R. J. Kadner. 2000. Signalling components in bacterial locomotion and sensory reception. J. Bacteriol. 182:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumler, A. J., A. J. Gilde, R. M. Tsolis, A. W. van der Velden, B. M. Ahmer, and F. Heffron. 1997. Contribution of horizontal gene transfer and deletion events to development of distinctive patterns of fimbrial operons during evolution of Salmonella serotypes. J. Bacteriol. 179:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullas, L. R., and J.-I. Ryu. 1983. Salmonella typhimurium LT2 strains which are r− m+ for all three chromosomally located systems of DNA restriction and modification. J. Bacteriol. 156:471-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clegg, S., and D. L. Swenson. 1994. Salmonella fimbriae, p. 105-114. In P. Klemm (ed.), Fimbriae: adhesion, genetics, biogenesis, and vaccines. CRC Press, Boca Raton, Fla.
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagelar assembly in Salmonella typhimurium. J. Bacteriol. 173:2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancox, L. S., K.-S. Yeh, and S. Clegg. 1998. Construction and characterization of type 1 non-fimbriate and non-adhesive mutants of Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 19:289-296. [DOI] [PubMed] [Google Scholar]
- 9.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. ASM Press, Washington, D.C.
- 10.Hultgren, S. J., S. Normark, and S. N. Abraham. 1991. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu. Rev. Microbiol. 45:383-415. [DOI] [PubMed] [Google Scholar]
- 11.Li, X., D. Rasko, A., C. V. Lockatell, D. E. Johnson, and H. L. T. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Old, D. C., and J. P. Duguid. 1970. Selective outgrowth of fimbriate bacteria in static liquid medium. J. Bacteriol. 103:447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappleye, C. A., and J. R. Roth. 1997. A Tn10 derivative (T-POP) for isolation and insertions with conditional (tetracycline-dependent) phenotypes. J. Bacteriol. 179:5827-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swenson, D. L., and S. Clegg. 1992. Identification of ancillary fim genes affecting fimA expression in Salmonella typhimurium. J. Bacteriol. 174:7697-7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thanassi, D. G., and S. J. Hultgren. 2000. Assembly of complex organelles: pilus biogenesis in gram-negative bacteria as a model system. Methods 20:111-126. [DOI] [PubMed] [Google Scholar]
- 16.Tinker, J. K., L. S. Hancox, and S. Clegg. 2001. FimW is a negative regulator affecting type 1 fimbrial expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinker, J. K., and S. Clegg. 2000. Characterization of FimY as a coactivator of type 1 fimbrial expression in Salmonella enterica serovar Typhimurium. Infect. Immun. 68:3305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh, K. S., L. S. Hancox, and S. Clegg. 1995. Construction and characterization of a fimZ mutant of Salmonella typhimurium. J. Bacteriol. 177:6861-6865. [DOI] [PMC free article] [PubMed] [Google Scholar]