Abstract
Efficient clearance of synaptically released glutamate from the extracellular space is an absolute requirement for maintaining information processing in the central nervous system. In the cerebellum, clearance of glutamate relies on uptake by Bergmann glial cells and Purkinje cells (PCs). Uptake by PCs can be monitored by recording the synaptic transport current (STC) mediated by the PC-specific transporter excitatory amino acid transporter 4 (EAAT4). The slow time course of the PC STC has been used to argue that glutamate clearance is protracted. We find, however, that the time course of the STC is not affected by altering the amount of glutamate released at individual synapses or by partial transporter blockade, manipulations that would be expected to change the duration of the extracellular glutamate transient. Ion substitution experiments and kinetic modeling of the PC transporter current suggest that physiological levels of intracellular Na+ and glutamate slow the cycling rate of transporters and thereby lengthen the time course of STCs. The model predicts that PC transporters bind glutamate quickly but that the actual cycling rate of EAAT4 in physiological conditions is slow; therefore, the STC reflects the intrinsic kinetics of the glutamate transporter, not the rate of glutamate clearance.
Keywords: cerebellum, climbing fiber, parallel fiber, Purkinje
Synaptic and extrasynaptic receptor activation depends on both the peak concentration of glutamate after release and the rate of glutamate clearance. Excitatory amino acid transporters (EAATs) transport glutamate across plasma membranes and are responsible for regulating the extracellular levels of glutamate. In the cerebellum, Purkinje cells (PCs) receive two types of excitatory input: a strong input from a single climbing fiber (CF) consisting of several hundred individual synapses and tens of thousands weak synapses made by parallel fibers (PFs). The CF-PC synapse has a high release probability (Pr) and can release multiple vesicles at individual synapses in response to an action potential (1–3). This multivesicular release at CF-PC synapses leads to very high glutamate concentrations (≈10 mM) at postsynaptic receptors that can diffuse to and activate perisynaptic receptors and transporters (2, 4). Synaptic currents evoked in PCs by CF stimulation are mediated by α-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and to a much smaller extent by kainate receptors and EAATs (5–8). The portion mediated by EAATs, the synaptic transport current (STC), results from the cotransport of three Na+, one H+, and one glutamate molecule into the cell and the countertransport of one K+ molecule (9). In addition to this movement of ions, glutamate transporters also allow anions to flow across the membrane in a channel-like mode (10, 11). The STC can be enhanced by chaotropic anions, such as nitrate, which are more permeant than chloride. This anion-enhanced STC has been used to estimate the amount of glutamate taken up by PCs after glutamate is synaptically released (7). Similar estimates were reached by using the stoichiometric current, which indicates that either current can be used to quantify PC uptake (12).
The PC STC is slower than transporter currents evoked in PC outside-out patches by short applications of l-glutamate (decay time constants of ≈60 ms for STCs compared with 8 ms for patch responses) (7, 13). This difference led to the suggestion that the slow decay of the STC is the result of asynchronous release, dendritic filtering, diffusion to some distantly located transporters, a slow phase of glutamate clearance at this synapse, or some combination of these factors (7, 13–17). Previously, we determined that dendritic filtering and asynchronous release do not substantially alter the time course of the faster CF-PC AMPAR-mediated excitatory postsynaptic currents (EPSCs) and therefore are unlikely to affect the slower STCs (2). In addition, immunohistochemistry of EAAT4, the glutamate transporter responsible for the PC STC, shows that its expression is highest surrounding the postsynaptic density and suggests that activation of distant transporters may not contribute significantly to the decay of the STC (8, 18). Therefore, we tested whether the long decay of the anion-enhanced PC STC depends on the shape of the glutamate transient. Based on our experimental results and kinetic modeling, we suggest that the PC STC time course is not prolonged because of diffusion or slow clearance but rather because the intrinsic kinetics of EAAT4 in physiological conditions are slow.
Results
Altering Pr Does Not Alter the Time Course of CF-Evoked STC. CF stimulation elicits synaptic currents mediated mainly by AMPARs (2, 7, 17, 19). However, with a NO–3-based internal solution and in the presence of the AMPAR and kainate receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo-[f]quinoxaline (NBQX), a small inward synaptic current persists at potentials up to +40 mV and is blocked by dl-threo-β-benzyloxyaspartic acid (TBOA), a glutamate transporter antagonist (8, 19). STCs evoked by PF or CF stimulation in rat cerebellar PCs were studied in the absence of EPSCs. The STC activates and deactivates more slowly (weighted decay time constant, 59.7 ± 16 ms; n = 30; see ref. 7) (Fig. 1) than transporter currents evoked in outside-out patches from PCs evoked by short applications of l-glutamate (decay time constant, 8 ms; see refs. 7 and 13). This finding supports the conclusion that the slow kinetics of the STC may be the result of a slow phase of glutamate clearance at this synapse (7). At CF synapses, lowering Pr decreases both the amplitude and time course of the extracellular glutamate transient (2). If the STC reflects the time course of the glutamate transient, then lowering Pr should result in a STC with a faster decay. We tested this idea by inducing synaptic depression using a paired-stimulation protocol. This protocol results in paired-pulse depression (PPD) of AMPAR EPSCs. The second EPSC decays significantly faster than the first EPSC because PPD results in part from decreased multivesicular release; thus, clearance is faster (2). Paired-pulse stimulation of CF-PC STCs (100-ms interval) also results in PPD (Fig. 1 A1). The amplitude of the second STC (STC2) was 34.4 ± 12.1% of the first STC (STC1; n = 4). However, the time course of both CF-PC STCs evoked in this manner is similar (68.1 ± 27.2 ms; n = 19; P > 0.05, ANOVA) (Fig. 1 A2), suggesting that the STC time course does not reflect the clearance rate of glutamate.
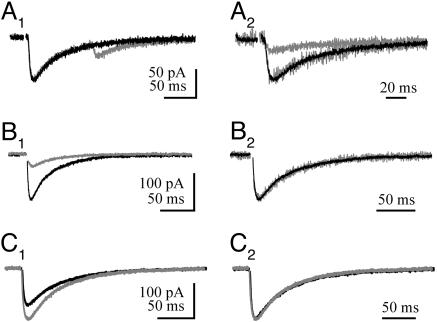
Fig. 1.
Decreasing Pr does not change STC time course. (A1) Paired-pulse stimulation of CF-PC STCs at 100-ms interstimulus intervals. (A2) Response (in A1) aligned with the stimulus artifact and normalized to the peak response. The gray trace is the second STC of a pair. (B1) CF-PC STC in control conditions and in the presence of 10 μMCd2+ (gray trace). (B2) Responses (in B1) normalized to the peak response. (C1) CF-PC STC in control condition (2 mM Ca2+) and in elevated extracellular Ca2+ (gray trace; 5 mM). (C2) Response (in C1) normalized to the peak response.
Pr was also decreased by adding a low concentration of Cd2+ to inhibit presynaptic Ca2+ channels. Bath application of Cd2+ reduces the amount of multivesicular release at individual CF synapses and thus attenuates the glutamate transient at perisynaptic EAAT4 transporters. Cd2+ (10 μM) inhibited the CF-PC STC to 27.9 ± 8.9% of control (n = 5) (Fig. 1B1) but did not affect the time course of the transporter currents (53.0 ± 22.1 ms; n = 5; P > 0.05, ANOVA) (Fig. 1B2). Lastly, extracellular Ca2+ concentration was decreased from 2 to 0.5 mM. As we switched into this extracellular solution, the amplitude of the CF-PC STC decreased and eventually disappeared into the recording noise. However, the decay time course of the STC during the solution exchange did not change (data not shown).
Increasing external Ca2+ from 2 to 5 mM enhances multivesicular release (2) and increases the peak amplitude of CF-PC STCs from cerebellar regions that are rich in EAAT4 by 45.1 ± 7.6% (n = 7) (Fig. 1C1) (19). However, the STC decay kinetics remained unchanged (69.1 ± 21.7 ms; P > 0.05, ANOVA) (Fig. 1C2). Because these manipulations of Pr alter the amplitude and the time course of the synaptic glutamate transient several fold (2), the immutable time course of the STC suggests that the intrinsic kinetics of the transporters are rate limiting, not the glutamate transient.
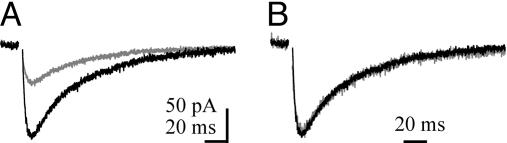
Altering the Rate of Clearance Does Not Affect the STC Time Course. We next delayed the clearance of synaptically released glutamate by partially blocking uptake. At subsaturating concentrations, transporter antagonists slow the decay time course of PC EPSCs because glutamate uptake is partially compromised (2, 14). Low concentrations of TBOA reduced the peak amplitude of the CF-PC STC to 51.1 ± 10% of control as expected for a slowly unbinding competitive antagonist (n = 8) (Fig. 2A). However, there was no detectable change in the time course of the PC STC (71.1 ± 7.2 ms; P > 0.05, ANOVA) (Fig. 2B).
Fig. 2.
Changing clearance rate does not alter the time course of CF-evoked STC. (A) CF-PC STC in control conditions and during the application of 10 μM TBOA (gray traces). (B) Response (in A) normalized to the peak response.
We also recorded CF-PC STCs at a higher temperature (34°C) because glutamate transport cycling is highly temperature dependent (Q10 ≈ 3; see ref. 20) and results in more rapid clearance (21). CF-PC STCs were larger in amplitude, decayed more rapidly, and exhibited paired-pulse depression at 34°C. Similar to our results at lower temperature, no differences in the decay time constants of STC1 and STC2 were found (22.3 ± 12 ms and 16.9 ± 9.7 ms for STC1 and STC2, respectively; n = 3; P = 0.89, t test). Although slowing clearance with TBOA has no effect on the time course of the STC, increasing the transporter cycling rate by elevating temperature speeds the decay independently of Pr. The time course of the STC, then, may depend on the transporter cycling rate.
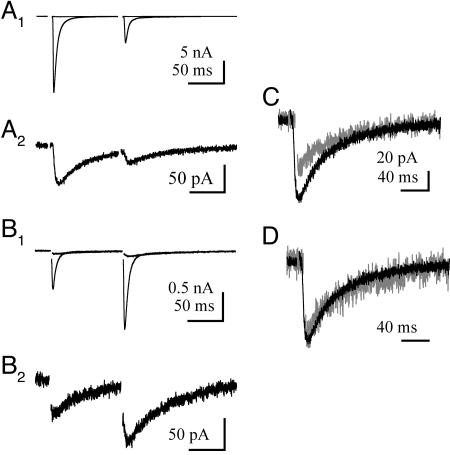
PF and CF STCs Have Similar Time Courses. Is the invariant time course of the STC a property of CF synapses or is it the same at other PC synapses? In addition to CF synapses (Fig. 3 A1 and A2), PCs also receive excitatory input from en passant synapses from PFs. PF synapses have a low Pr and facilitate during successive stimulation (Fig. 3 B1 and B2) (22). PFs synapses are less well ensheathed by Bergmann glial membranes and are more susceptible to the pooling of released glutamate from adjacent synapses (23, 24). Together, these features contribute to a synaptic glutamate transient at PF synapses that is different from that at CF synapses. Despite these differences, the decay kinetics of STCs from these two PC synapses are the same (PF-PC STC, 70.7 ± 6.7 ms; n = 6; P > 0.05, ANOVA) (Fig. 3 C and D).
Fig. 3.
PF- and CF-PC STCs have similar time course. (A1) Superimposed CF-PC EPSC and STC (in the presence of 25 μM NBQX) paired at 100-ms intervals. (A2) CF-PC STC response (in A1) expanded in size. (B1) Superimposed PF-PC EPSC and STC (in the presence of 25 μM NBQX) paired at 100-ms intervals. (C and D) Responses (first STC in A2 and B2) superimposed (C) and normalized to the peak response (D).
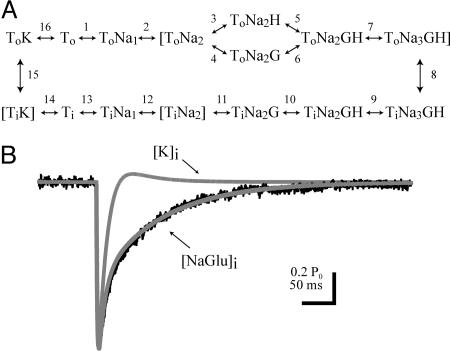
A Comprehensive Kinetic Model for the PC STC. A kinetic model of the PC transporter was developed to determine whether the STC time course could be reconciled with the faster transporter currents for PC patches (7, 13). It may be that different transporter subtypes are represented in somatic patches and synaptic recordings or that membrane excision modifies the properties of transporters in patches. However, based on previous patch experiments and the modeling below, we suggest that the kinetic difference is predominantly due to different cytoplasmic ionic compositions in the two recording conditions. Our PC transporter model is based on a kinetic model of the EAAT2 and EAAT3 subtype (25, 26) but with rate constants altered to reproduce all of the outside-out patch currents described by Otis and Jahr (13) (Fig. 6 and Table 1, which are published as supporting information on the PNAS web site). By using this kinetic scheme (Fig. 4A), we show that simulated STCs driven by a synaptic glutamate transient that mimics the release of a single vesicle decay much more rapidly than recorded STCs (Fig. 4B, trace labeled [K]i) (2). In outside-out patches, currents mediated by either EAAT2 or PC transporters are slowed when Na+ and glutamate are substituted in the internal solution (13, 25). Therefore, we used the model to determine the range on internal Na+ and glutamate that could fit the evoked STC. Reasonable fits of the data were obtained with a range on concentrations from 50 μM glutamate and 20 mM Na+ to 2 mM glutamate and 7 mM Na+ (Fig. 4B, trace labeled [NaGlu]i). Neither ion by itself was sufficient to fit the STC. The model indicates that, in the presence of internal Na+ and glutamate, the anion current decays slowly because the transporters revisit the ToNa3GH state many times. This state gates the major anion conductance.
Fig. 4.
PC STC kinetic model. (A) Illustration of the states in the model. (B) Superimposed predicted anion currents with K+ (gray trace labeled K; 140 mM K+) or Na+ and glutamate (gray trace labeled [NaGlu]i; 10 mM Na+ and 0.5 mM glutamate) are included in the internal solution and representative CF-PC STC (black trace).
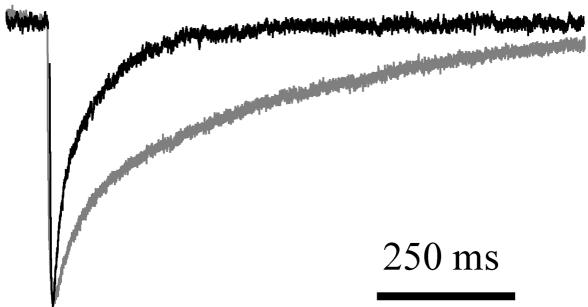
Intracellular Ions Govern the Time Course of PC STC. The model predicts that the time course of the synaptic transporter current depends on the species and concentrations of internal ions. We tested this hypothesis by comparing recordings of CF-PC STCs with internal solutions based on either 130 mM KNO3 or 130 mM NaNO3 with 10 mM glutamate. STCs recorded with the Na+ and glutamate internal were much slower to decay back to baseline (Fig. 5). The weighted decay time constant for STCs recorded with internal Na+ and glutamate was 391.2 ± 75.3 ms (n = 5) and was significantly different from the decay time constant of STCs recorded with K+-based internal solutions (100.2 ± 8.4 ms; n = 7; P = 0.001, t test) (Fig. 5 A1 and A2). Including these concentrations of internal ions in the model slow the simulated STC but not to the degree that they slow the evoked STC.
Fig. 5.
CF-PC STC time course is affected by internal ionic composition. Superimposed CF-PC STC recorded with K+ as the main internal cation (black trace) and with Na+ and glutamate (gray trace; 140 mM Na+ and 10 mM glutamate) normalized to the peak of the STC evoked with internal K+.
Discussion
The slow decay phase of PC STCs has been attributed to the slow clearance of synaptically released glutamate and the activation of distantly located transporters. However, our results suggest that, in the presence of physiological intracellular concentrations of Na+ and glutamate, the intrinsic kinetics of PC transporters are sufficient to explain the STC time course. The STC time course in PCs results from transporters visiting and revisiting Na+ and glutamate bound states as well as completing the full transport cycle and, therefore, cannot be used to estimate the lifetime of glutamate at these synapses.
PC Transporters in Physiological Conditions. Although we found that dialyzing PCs with high concentrations of Na+ and glutamate greatly prolonged the STC, when K+-based internal solutions were used, the STC did not approach the fast time course of transporter currents elicited in outside-out patches (7, 13). Because the transporter responsible for the CF-PC STC, EAAT4, is located at perisynaptic regions of dendritic spines (18), the relevant intracellular ionic composition is that within the spine. With whole-cell recordings, it is likely that incomplete dilution or active transport processes keep the intracellular milieu, especially in spines, from achieving total exchange of solutions with the recording pipette. In addition, given the high density of transporters in PC spines (18), the area of the spine membrane (27), and the stoichiometry of transport, the glutamate transporters themselves will be a significant source of intraspine Na+ and glutamate. Consequently, it may be impossible to greatly lower the normal Na+ and glutamate concentrations in PC spines by whole-cell recording.
In physiological conditions, PC transporters clear glutamate primarily by quickly binding transmitter (28). This binding is followed by either the full transporter cycle or by unbinding of glutamate to the extracellular space. The ratio of unbinding into the cytoplasm to unbinding into the extracellular space is greatly influenced by the intracellular ionic composition. The intracellular composition will also influence the cycling time, or turnover rate, of transporters. Transporters exposed to internal Na+ and glutamate will complete a full cycle more slowly as they revisit the same Na+- and glutamate-bound states a number of times before the final unbinding event. The disparate estimates of turnover rate of transporters of the same identity (10–70 ms) (29) may be explained by differences in the intracellular composition across the various preparations used. Oocytes contain ≈10 mM Na+ and 10 mM glutamate (30, 31), whereas the solution at the cytoplasmic face of outside-out patches is very close to that contained in the patch pipette, usually devoid of Na+ and glutamate. Thus, the turnover rate estimated from patches likely reflects the low Na+ and glutamate in the patch pipette and will invariably be faster than that from oocytes or intact astrocytes.
Monitoring Transmitter Concentration with Receptors and Transporters. Postsynaptic receptors (2, 32, 33) as well as extrasynaptic transporters (34) have been used to estimate the peak concentration and clearance time course of glutamate after release from synapses. Low-affinity AMPARs unbind glutamate so rapidly that the time course of the conductance approaches that of the very rapid portion of the glutamate transient. However, the low affinity also makes these receptors less effective at sensing low-concentration components of glutamate transients. High-affinity receptors, like NMDA receptors, are naturally better at reporting low concentrations (35, 36). However, because of the very slow unbinding rate of glutamate, these conductances stay active for much longer than the period that free glutamate is present (37). STCs offer a similarly distorted view of the time course of extracellular glutamate transients. In mature hippocampal astrocytes, filtering of the current mediated by transporters significantly prolongs somatically recorded STCs. To determine the underlying glutamate transient, the filter function has to be deconvolved from the recorded waveform (34). Because of this filtering and the effects of internal Na+ and glutamate, estimates of the kinetics of glutamate clearance based on the STC time course alone are highly inaccurate. We find that the high-affinity glutamate transporter EAAT4 is no exception to this rule; in physiological conditions, its time course depends on its intrinsic kinetics rather than that of the glutamate transient.
Materials and Methods
Tissue Preparation. Parasagittal cerebellar slices were prepared from rats at postnatal days 12–16 (see refs. 2 and 19). Animals were anesthetized with halothane and decapitated in accordance with the Oregon Health and Science University Institution Animal Care and Use Committee. The cerebellar vermis was dissected out and glued to the stage of a vibroslicer (Leica) in ice-cold solution containing 119 mM NaCl, 2.5 mM KCl, 2 CaCl2, 1 mM MgCl2, 1 mM NaH2PO4, 26.2 mM NaHCO2, and 11 mM glucose, bubbled with 95% O2/5% CO2. Slices 250–300 μm thick were cut and incubated at 35°C for 30 min before use in electrophysiological recordings. During recordings, the slices were superfused with the solution described above.
Electrophysiology. Whole-cell recordings with pipettes of 1–1.5 MΩ resistance were made by using infrared differential interference contrast video microscopy with a 40× water-immersion objective on an upright microscope (Zeiss Axioskop). The series resistance, as measured by the instantaneous current response to a 1- to 5-mV step with only the pipette capacitance cancelled, was usually <5 MΩ and was compensated >80%. Pipette solutions contained 130 mM XNO3, 10 mM XCl2, 10 mM Hepes, and 10 mM EGTA, where X was either Cs+ (Figs. 1, 2, 3) or K+ (Figs. 4 and 5), or 120 mM NaNO3, 10 mM NaCl, 10 mM Naglutamate, 10 mM Hepes, and 10 mM EGTA. CF-PC EPSCs were recorded at –10 mV, whereas PF-PC EPSCs were recorded at –70 mV. STCs were recorded at –70 mV in the presence of 25 μM NBQX, 100 μM picrotoxin, and 5 μM SR95531. CFs were stimulated (5–90 V, 10–200 μs) with a theta-glass pipette filled with bath solution and placed in the granule cell layer. To eliminate significant PF activation, the stimulating pipette was repositioned, and the stimulus intensity was adjusted until the current required to elicit an all-or-none response was minimized. PFs were stimulated by placing a patch pipette filled with bath solution in the molecular layer and adjusting the stimulus intensity. PF input was distinguished from CF input by the EPSC amplitude and the direction of paired-pulse modulation. For comparison between experimental conditions, the decay phase of STCs is reported as a weighted decay time constant and is the result of a double exponential fit to the decay phase of the STC back to baseline (amplitude1·decayτ1 + amplitude2·decayτ2). Synaptic currents were filtered at 2–5 kHz and digitized at 20–50 kHz by using acquisition software written in igor pro (J. S. Diamond; WaveMetrics, Lake Oswego, OR). Reported values are the mean ± SEM. Data analysis was done by using igor pro (WaveMetrics), axograph (Axon Instruments, Union City, CA), and instat (GraphPad, San Diego). Statistical significance was determined with t tests or ANOVA with Bonferroni post hoc test for the multiple experiments that were compared with control STCs (Fig. 1). Recordings were performed at room temperature (23–25°C) unless otherwise noted.
Kinetic Modeling. scop software (Simulation Resources, Berrien Springs, MI) was used to develop a kinetic model to simulate the PC STC (13, 20, 25). The kinetic model proposed by Bergles et al. (25) was used as a basis to reproduce the kinetic data obtained by Otis and Jahr (13). The current model reproduces the behavior of the PC transporter when applying various experimental protocols (see Fig. 6 and Table 1). In reaction steps the voltage dependence assigned to the forward rate constant has the form kf0exp[–λzVF/RT], and the backward rate constant has the form kb0exp[(1–λ)zVF/RT], where kf0 and kb0 are the forward and backward rate constants at 0 mV; z is the charge moved, λ designates the location of the energy barrier within the electrical field, V is the membrane potential, T = 298 K, and F and R are Faraday's constant and the gas constant, respectively. Brackets denote anion conducting states with rates β and α, as in Bergles et al. (25). For synaptic simulations, glutamate transients similar to Wadiche and Jahr (2) were used to drive the transporter kinetic scheme, although STCs were also well fitted with transients reflecting lower and longer-lasting glutamate concentrations, presumably mimicking conditions of spillover (2, 38).
Supplementary Material
Acknowledgments
We thank the members of the C.E.J. laboratory and Linda Overstreet Wadiche for comments and suggestions during this project. This work was supported by National Institute of Neurological Disorders and Stroke Grant NS40056 (to C.E.J.).
Conflict of interest statement: No conflicts declared.
Abbreviations: EAAT, excitatory amino acid transporter; PC, Purkinje cell; CF, climbing fiber; PF, parallel fiber; Pr, release probability; AMPAR, α-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; STC, synaptic transport current; EPSC, excitatory postsynaptic current; NBQX, 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline; TBOA, dl-threo-β-benzyloxyaspartic acid.
References
- 1.Silver, R. A., Momiyama, A. & Cull-Candy, S. G. (1998) J. Physiol. (London) 510, 881–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadiche, J. I. & Jahr, C. E. (2001) Neuron 32, 301–313. [DOI] [PubMed] [Google Scholar]
- 3.Foster, K. A., Kreitzer, A. C. & Regehr, W. G. (2002) Neuron 36, 1115–1126. [DOI] [PubMed] [Google Scholar]
- 4.Dzubay, J. A. & Otis, T. S. (2002) Neuron 36, 1159–1167. [DOI] [PubMed] [Google Scholar]
- 5.Konnerth, A., Llano, I. & Armstrong, C. M. (1990) Proc. Natl. Acad. Sci. USA 87, 2662–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkel, D. J., Hestrin, S., Sah, P. & Nicoll, R. A. (1990) Proc. R. Soc. London Ser. B 241, 116–121. [DOI] [PubMed] [Google Scholar]
- 7.Otis, T. S., Kavanaugh, M. P. & Jahr, C. E. (1997) Science 277, 1515–1518. [DOI] [PubMed] [Google Scholar]
- 8.Huang, Y. H., Dykes-Hoberg, M., Tanaka, K., Rothstein, J. D. & Bergles, D. E. (2004) J. Neurosci. 24, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerangue, N. & Kavanaugh, M. P. (1996) Nature 383, 634–637. [DOI] [PubMed] [Google Scholar]
- 10.Fairman, W. A., Vandenberg, R. J., Arriza, J. L., Kavanaugh, M. P. & Amara, S. G. (1995) Nature 375, 599–603. [DOI] [PubMed] [Google Scholar]
- 11.Wadiche, J. I., Amara, S. G. & Kavanaugh, M. P. (1995) Neuron 15, 721–728. [DOI] [PubMed] [Google Scholar]
- 12.Brasnjo, G. & Otis, T. S. (2004) Proc. Natl. Acad. Sci. USA 101, 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otis, T. S. & Jahr, C. E. (1998) J. Neurosci. 18, 7099–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbour, B., Keller, B. U., Llano, I. & Marty, A. (1994) Neuron 12, 1331–1343. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi, M., Kovalchuk, Y. & Attwell, D. (1995) J. Neurosci. 15, 5693–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergles, D. E., Dzubay, J. A. & Jahr, C. E. (1997) Proc. Natl. Acad. Sci. USA 94, 14821–14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auger, C. & Attwell, D. (2000) Neuron 28, 547–558. [DOI] [PubMed] [Google Scholar]
- 18.Dehnes, Y., Chaudhry, F. A., Ullensvang, K., Lehre, K. P., Storm-Mathisen, J. & Danbolt, N. C. (1998) J. Neurosci. 18, 3606–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadiche, J. I. & Jahr, C. E. (2005) Nat. Neurosci. 8, 1329–1334. [DOI] [PubMed] [Google Scholar]
- 20.Wadiche, J. I. & Kavanaugh, M. P. (1998) J. Neurosci. 18, 7650–7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min, M. Y., Rusakov, D. A. & Kullmann, D. M. (1998) Neuron 21, 561–570. [DOI] [PubMed] [Google Scholar]
- 22.Zucker, R. S. & Regehr, W. G. (2002) Annu. Rev. Physiol. 64, 355–405. [DOI] [PubMed] [Google Scholar]
- 23.Xu-Friedman, M. A., Harris, K. M. & Regehr, W. G. (2001) J. Neurosci. 21, 6666–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atluri, P. P. & Regehr, W. G. (1998) J. Neurosci. 18, 8214–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergles, D. E., Tzingounis, A. V. & Jahr, C. E. (2002) J. Neurosci. 22, 10153–10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson, H. P., Tzingounis, A. V., Koch, H. P. & Kavanaugh, M. P. (2004) Proc. Natl. Acad. Sci. USA 101, 3951–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris, K. M. & Stevens, J. K. (1988) J. Neurosci. 8, 4455–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong, G. & Jahr, C. E. (1994) Neuron 13, 1195–1203. [DOI] [PubMed] [Google Scholar]
- 29.Huang, Y. H. & Bergles, D. E. (2004) Curr. Opin. Neurobiol. 14, 346–352. [DOI] [PubMed] [Google Scholar]
- 30.Dascal, N. (1987) CRC Crit. Rev. Biochem. 22, 317–387. [DOI] [PubMed] [Google Scholar]
- 31.Zerangue, N. & Kavanaugh, M. P. (1996) J. Physiol. (London) 493, 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clements, J. D., Lester, R. A., Tong, G., Jahr, C. E. & Westbrook, G. L. (1992) Science 258, 1498–1501. [DOI] [PubMed] [Google Scholar]
- 33.Diamond, J. S. & Jahr, C. E. (1997) J. Neurosci. 17, 4672–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond, J. S. (2005) J. Neurosci. 25, 2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sah, P., Hestrin, S. & Nicoll, R. A. (1989) Science 246, 815–818. [DOI] [PubMed] [Google Scholar]
- 36.Rossi, D. J. & Slater, N. T. (1993) Neuropharmacology 32, 1239–1248. [DOI] [PubMed] [Google Scholar]
- 37.Lester, R. A., Clements, J. D., Westbrook, G. L. & Jahr, C. E. (1990) Nature 346, 565–567. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen, T. A., DiGregorio, D. A. & Silver, R. A. (2004) Neuron 42, 757–771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.