Abstract
Experiments were undertaken to examine gene transfer in Mycoplasma pulmonis. Parent strains containing transposon-based tetracycline and chloramphenicol resistance markers were combined to allow transfer of markers. Two mating protocols were developed. The first consisted of coincubating the strains in broth culture for extended periods of time. The second protocol consisted of a brief incubation of the combined strains in a 50% solution of polyethylene glycol. Using either protocol, progeny that had acquired antibiotic resistance markers from both parents were obtained. Analysis of the progeny indicated that only the transposon and not flanking genomic DNA was transferred to the recipient cell. Gene transfer was DNase resistant and probably the result of conjugation or cell fusion.
Transformation, transduction, and conjugation are the three primary mechanisms of horizontal gene exchange in bacteria. Because mollicutes lack a cell wall and are bound by a single membrane, a process involving membrane fusion is a fourth potential mechanism for gene exchange between these organisms. The first reports of chromosomal gene transfer in the class Mollicutes was in Spiroplasma citri (2, 3). Gene exchange has also been reported in Mycoplasma pulmonis (26), but it was later shown that the organism used for this study was actually Acholeplasma (11).
Exchange of chromosomal DNA between cells of the genus Mycoplasma has not been previously demonstrated. Mycoplasmas probably do not acquire DNA by natural transformation (12, 17). Although mycoplasma phages are known to exist, transduction has not been described. Several mycoplasmas can acquire the conjugative transposon Tn916 by mating with an enterococcal donor, but conjugal transfer of any genetic element, including Tn916, from a mycoplasmal donor has not been described (12, 17). However, circumstantial evidence suggests that horizontal gene exchange has occurred between species of Mycoplasma. The IS1221 insertion sequence element is found in M. hyorhinis, M. hyopneumoniae, and M. flocculare (19). These three species are all parasites of swine, and IS1221 could have spread by horizontal gene exchange in the animal host. The pMGA genes of M. gallisepticum have closely related genes in M. imitans and M. synoviae but not in phylogenetically related mycoplasmas from humans (27, 28). These three species are avian pathogens, leading again to the suggestion of horizontal gene exchange in animal hosts.
Genetic markers have recently become available to study gene exchange in the murine pathogen M. pulmonis. The tetM determinant of transposon Tn916 has been a widely used antibiotic resistance marker in many mycoplasmas, including M. pulmonis (12, 17). We constructed a chloramphenicol acetyltransferase gene (cat) that functions in M. pulmonis and described the use of transposon Tn4001 as a delivery vehicle to insert the cat and tetM genes into the mycoplasma chromosome (15a). Two parent strains, one with tetM and the other with cat, were mixed in broth and incubated together in the absence of antibiotic selection, followed by incubation on agar with double antibiotic selection. Mating progeny that were resistant to both antibiotics were obtained at a very low frequency. Gene transfer was resistant to DNase, required both parents to be viable, and could be stimulated by polyethylene glycol (PEG). The strains of M. pulmonis used in this study are thought to be free of phages. Thus, cell fusion or conjugation is the most likely mechanism of gene transfer.
MATERIALS AND METHODS
Mycoplasma strains and antibiotics.
Mycoplasmas were grown in broth medium as described previously (15a). M. pulmonis strain KD735-15 (KD) is a derivative of strain UAB 6510 (4), which has been shown to be virulent in rats (6). Strain CT causes severe respiratory disease in mice (5, 9). Genetic markers were introduced into both strains by PEG-mediated transformation with plasmids containing Tn916 (pAM120), Tn4001T (contains tetM), or Tn4001C (contains the cat gene) as previously described (13, 15a). Tetracycline (3 μg/ml) and chloramphenicol (15 μg/ml) were used for antibiotic selection for cells containing tetM and cat, respectively. A summary of the parent strains used for mating experiments is provided in Table 1.
TABLE 1.
M. pulmonis strains used in this study
| Strain | Characteristics |
|---|---|
| CT | Virulent pathogen of mice; no transposon |
| CT182 | CT containing Tn4001T inserted into Mypu_5290 (encoding VsaH) at nucleotide position 652759 |
| CT186 | CT containing Tn4001T inserted at nucleotide position 678012, disrupting Mypu_5470 |
| CT247 | CT containing Tn4001T inserted at nucleotide position 640094, disrupting Mypu_5220 (encoding LipB) |
| CT G5 | CT containing Tn4001C at undetermined location |
| KD735-15 | Derivative of strain UAB 6510; no transposon |
| KD-T2 | KD735-15 containing Tn916 at undetermined location |
| KD305 | KD735-15 containing Tn4001T at undetermined location |
| IVC-3 | KD735-15 containing Tn4001C at undetermined location |
Broth (natural) mating protocol.
M. pulmonis strains containing either Tn916, Tn4001T, or Tn4001C were grown in separate cultures containing the appropriate antibiotic to late-logarithmic or stationary-growth phase. Cells were harvested by centrifugation and suspended in the original volume of fresh medium without antibiotic selection. For mating of tetracycline-resistant KD with chloramphenicol-resistant KD, equal volumes (1 ml, usually ca. 108 CFU) of each parent culture were mixed. For mating between KD and CT, 10 ml of CT were mixed with 1 ml of KD to compensate for the generally low titers of CT (usually ca. 107 CFU/ml). The mixed culture was incubated overnight (ca. 16 h) unless indicated otherwise. Mating progeny were assayed on mycoplasma agar (15a) selecting for resistance to both tetracycline and chloramphenicol. Colonies were picked, propagated in 1 ml of mycoplasma broth, and stored at −80°C for later analysis.
PEG-mediated mating protocol.
M. pulmonis cultures (1.5 ml for CT and 150 μl for KD) were grown to late-logarithmic or stationary-growth phase as described above, combined in a single tube, washed once in phosphate-buffered saline (PBS), and suspended in a final volume of 15 μl. The parental strains were combined in one microcentrifuge tube containing 135 μl of PEG (PEG 8000 or PEG 3350, Sigma; 50% [wt/vol] solution in PBS unless stated otherwise). Some experiments also included 10 mM MgCl2 in the PEG-cell mixture. After vortexing to mix the cells and PEG, the mixture set at ambient temperature for 1 min. Fresh medium was added to dilute the mixture 10-fold, followed by a 2-h incubation at 37°C. Cells were harvested by centrifugation and suspended in 1 ml of medium. Mating progeny were assayed on mycoplasma agar and preserved at −80°C as described above for further analysis.
Heat killing of M. pulmonis parents.
M. pulmonis cultures were grown to late-logarithmic or stationary-growth phase, and a sample was removed to assay CFU prior to heat killing. A culture of one of the parental strains (CT or KD) was placed in a water bath for 15 min at 50°C. Samples were removed and assayed for CFU to confirm effective heat killing. The heat-killed culture was added to either natural or PEG-mediated mating mixtures as described above.
Nuclease treatment of mating mixtures.
DNase I (200 μg/ml; Sigma) was added at the time parent strains were mixed and coincubated in nonselective broth to determine whether gene transfer was nuclease resistant.
PCR analysis of mating progeny.
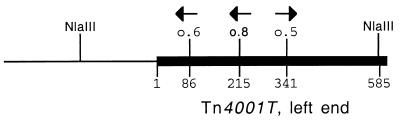
M. pulmonis genomic DNA was isolated by using the Easy-DNA Kit (Invitrogen, Carlsbad, Calif.). PCR amplification of several genes, including tetM, cat, p93 (KD specific), and lipB (CT specific), was used to examine the genotype of the mating progeny (Table 2). For the parent strains and for some mating progeny, the precise location of Tn4001T was determined by the inverse PCR strategy diagrammed in Fig. 1. The inverse PCR methods were as described previously (1, 23, 30). Briefly, genomic DNA was digested with the restriction enzyme NlaIII, the restriction fragments were circularized by incubation with T4 DNA ligase, and the ligation products were PCR amplified with the primers o.5 and o.8. The nucleotide sequence of the junction between the transposon and the mycoplasma chromosome was determined by using o.6 as the primer. Sequence reactions used automated dye terminator methods at the Iowa State University DNA Synthesis and Sequencing Facility, Ames. Sequence analysis was performed by using MacVector Sequencher software, and the basic local alignment search tool (BLAST [http://genolist.pasteur.fr/MypuList/]) for comparison to the complete CT genome.
TABLE 2.
Oligonucleotides and PCR conditions
| Pair | Expected product (annealing temp [°C]) | Primers |
|---|---|---|
| 1 | 296-bp lipB fragment (60) | 5"-CAAAAAGAATCAACTAACTTGTCTG |
| 5"-TGCTTGTTTTCAGAAATTACAGC | ||
| 2 | 390-bp p93 fragment (60) | 5"-AATTAGATTTGAAATCAATGATCC |
| 5"-ATTCTAAAGCGTAATTTCCTTCAG | ||
| 3 | 398-bp tetM fragment (55) | 5"-TTATCAACGGTTTATCAGG |
| 5"-CGTATATATGCAAGACG | ||
| 4 | 700-bp cat gene fragment (55) | 5"-GGAGGTACCATGGAGAAAAAAATCAC |
| 5"-CACTTCTCGAGGCGTAGCACCAGG | ||
| 5 | 267-bp product containing the junction of Tn4001T and the CT182 genome (50) | 5"-ACCGATTCTAAATCTACACTGAG (o.8) |
| 5"-GAGGAAGTGGCAAAACTGAAACTC | ||
| 6 | 415-bp product containing the junction of Tn4001T and the CT186 genome (65) | 5"-AGGACTGCATAACATCTTCCGCAG (o.6) |
| 5"-TTCCAGGCTTTGATGATGAAGTTG | ||
| 7 | 607-bp product containing the junction of Tn4001T and the CT247 genome (50) | o.8 |
| 5"-TGCTTGTTTTCAGAAATTACAGC | ||
| 8 | Inverse PCR product to determine nucleotide position of Tn4001T in CT transformants (65) | o.8 |
| 5"-CCCAATCCCATAGCCATACCTATC (o.5) |
FIG. 1.
Schematic diagram of the junction between the left end of Tn4001T (thick line) and M. pulmonis genomic DNA (thin line) showing the location of the o.5, o.6, and o.8 primer binding sites (the arrows indicate directionality) used to determine the nucleotide position of the transposon in the genome by inverse PCR. The numbers refer to nucleotide positions, with position 1 being the first nucleotide of Tn4001T. The NlaIII site shown in M. pulmonis genomic sequences represents the NlaIII site that is closest to the left end of Tn4001T for the particular transformant being analyzed.
For parent strains for which the precise nucleotide location of Tn4001T in the chromosome was determined, primers were designed to directly amplify the junction between the transposon and the mycoplasma chromosome. These primer pairs (summarized in Table 2) were used to determine by PCR whether flanking DNA in addition to the transposon was transferred to the mating progeny. Standard 25-μl PCRs were subjected to previously described cycling parameters (29) by using 30 amplification cycles and the annealing temperatures provided in Table 2.
RESULTS
Gene transfer in the absence of PEG.
Several pilot experiments were performed to identify conditions under which gene transfer in M. pulmonis could be detected in the absence of PEG (natural mating). The parent strains for these experiments, KD-T2 and IVC-3, were both derived from KD735-15. Experiments designed to investigate gene transfer between cells grown on agar were not successful. Gene transfer in broth was rare and was detected in only a minority of the experiments if the mixture of parent strains was incubated for 6 h or less prior to applying antibiotic selection on agar. It was not until the parent strain mixtures were incubated overnight that gene transfer was consistently obtained. Thus, the mating protocol used for most experiments included overnight incubation. Also, gene transfer was more consistent when the cell density of the parent strains was high. Therefore, cultures of the parent strains were often grown to high titers (>108 CFU/ml) and likely in stationary-growth phase prior to being mixed. Conditions were not identified in which gene transfer was detectable if either parent was heat killed.
A variety of parent strains derived from KD and CT were examined for their ability to undergo gene transfer (Table 3). Both the tetM and cat markers for many experiments resided on Tn4001 (Table 3, experiments 4 to 8). Thus, gene transfer was independent of the conjugative properties of Tn916 (8). Gene transfer occurred between KD and CT regardless of whether tetM was in KD and cat in CT (Table 3, experiment 9) or vice versa (Table 3, experiments 6 to 8). Gene transfer was independent of the particular site where a transposon was located in the parental M. pulmonis chromosome. Gene transfer occurred in the presence of high concentrations of DNase I (Table 3, experiments 3 and 6). Also, growth of M. pulmonis strains in the presence of 10 μg of the Tn916-containing plasmid pAM120/ml did not result in any tetracycline-resistant progeny. Therefore, gene transfer did not result from natural transformation.
TABLE 3.
Summary of gene transfer experiments
| Expt | Protocola | Parent
|
Parent 1 (CFU/ml) | Parent 2 (CFU/ml) | No. of progeny colonies | Presence or absenceb of:
|
||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Mg2+ | DNase I | |||||
| 1 | Nat. | IVC-3 Tn4001C | KD-T2 Tn916 | 2.2 × 108 | 1.1 × 108 | 4 | − | − |
| 2 | Nat. | IVC-3 Tn4001C | KD-T2 Tn916 | 2.2 × 108 | 6 × 109 | 2 | − | − |
| 3 | Nat. | IVC-3 Tn4001C | KD-T2 Tn916 | 4 × 108 | 8 × 108 | 9 | − | − |
| 3 | Nat. | IVC-3 Tn4001C | KD-T2 Tn916 | 4 × 108 | 8 × 108 | 8 | − | + |
| 4 | Nat. | IVC-3 Tn4001C | KD305 Tn4001T | 6.0 × 108 | 1.3 × 109 | 10 | − | − |
| 5 | Nat. | IVC-3 Tn4001C | KD305 Tn4001T | NDc | ND | 2 | − | − |
| 6 | Nat. | CT186 Tn4001T | IVC-3 Tn4001C | ND | ND | 8 | − | − |
| 6 | Nat. | CT186 Tn4001T | IVC-3 Tn4001C | ND | ND | 9 | − | + |
| 7 | Nat. | CT182 Tn4001T | IVC-3 Tn4001C | 7.0 × 106 | 1.8 × 109 | 7 | − | − |
| 8 | Nat. | CT247 Tn4001T | IVC-3 Tn4001C | 3.0 × 107 | 2.7 × 109 | 5 | − | − |
| 9 | Nat. | CT G5 Tn4001C | KD-T2 Tn916 | 6.0 × 106 | 1.0 × 108 | 11 | − | − |
| 10 | 50% PEG 3350 | IVC-3 Tn4001C | KD-T2 Tn916 | ND | ND | 1,100 | − | − |
| 10 | 50% PEG 8000 | IVC-3 Tn4001C | KD-T2 Tn916 | ND | ND | 700 | − | − |
| 11 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 6 × 107 | 6 × 107 | >3,000 | − | − |
| 11 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 6 × 107 | 6 × 107 | >3,000 | + | − |
| 11 | 5% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 6 × 107 | 6 × 107 | 0 | − | − |
| 11 | 5% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 6 × 107 | 6 × 107 | 0 | + | − |
| 12 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | ND | ND | 96 | − | − |
| 12 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | ND | ND | 80 | + | − |
| 13 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 2.5 × 107 | 4.0 × 107 | >3,000 | − | − |
| 13 | 50% PEG 3350 | CT186 Tn4001T | IVC-3 Tn4001C | 2.5 × 107 | 4.0 × 107 | >3,000 | − | + |
| 14 | 50% PEG 3350 | CT182 Tn4001T | IVC-3 Tn4001C | 4.5 × 106 | 8.5 × 107 | 2 | − | − |
| 15 | 50% PEG 3350 | CT247 Tn4001T | IVC-3 Tn4001C | 1.6 × 105 | 1.2 × 108 | 16 | − | − |
Mating was done by the natural broth method (Nat.) or was PEG mediated at the indicated percentage and type of PEG.
The presence (+) or absence (−) of Mg2+ and DNase I is shown.
ND, not determined.
In three independent experiments not shown in Table 3, mating between strains CT G5 and CT186 was examined. No progeny that were resistant to both tetracycline and chloramphenicol were obtained. In each one of these experiments, there were 106 to 107 CFU of each parent in the mating mixture. Thus, the lack of mating progeny may have been a result of a low density of parent cells.
PEG-mediated gene transfer.
PEG is thought to enhance fusion of mycoplasma membranes, possibly with a requirement for magnesium ions (Mg2+) (31). PEG was previously shown to enhance gene transfer in S. citri but to have no effect in Acholeplasma (2, 26). Using the PEG-mediated protocol described in Materials and Methods, gene transfer in M. pulmonis was readily detected at comparable frequencies with a 50% solution of either PEG 3350 or PEG 8000 (Table 3, experiment 10). Low concentrations of PEG (5%) did not promote gene transfer, and the presence of MgCl2 had no effect regardless of the PEG concentration (Table 3, experiments 11 and 12). The inclusion of DNase I in the PEG-cell mixture also did not affect gene transfer (Table 3, experiment 13).
Genetic analysis of mating progeny.
A series of PCR experiments was performed to examine the extent of gene transfer between CT and KD. Primer pairs that would amplify an internal fragment of the cat and tetM genes were used to confirm that gene transfer had in fact occurred. By PCR analysis, all of the progeny described in this study had both the tet and cat markers and had not acquired antibiotic resistance by spontaneous mutation. The lipB and p93 genes are specific for CT and KD, respectively (29). Thus, primer pairs that amplified an internal fragment of these genes were used to determine whether progeny colonies had a KD genetic background, a CT background, or a chimera of both parental genomes. The results of these PCR experiments are summarized in Table 4. No mating progeny had both p93 and lipB, indicating that neither of these genes, which are unlinked to the transposon, was transferred to progeny cells. For the natural matings without PEG, all of the analyzed progeny had the p93 gene but lacked lipB. Therefore, CT served as a donor for gene transfer to KD recipients. For the PEG matings, about half of the analyzed progeny also were KD recipients. The other progeny had lipB but not p93 and are considered to be CT recipients. For each of the KD recipients, the junction between the CT genome and Tn4001T was not transferred to KD. Therefore, gene transfer apparently involved transfer of Tn4001T but not flanking CT sequences, a finding indicative of transposition of Tn4001T into the recipient genome.
TABLE 4.
PCR analysis of KD × CT matings and the direction of gene transfera
| Mating | Total no. of progeny analyzed | No.
|
|
|---|---|---|---|
| KD to CTb | CT to KDc | ||
| IVC-3 × CT182 (Nat.) | 6 | 0 | 6 |
| IVC-3 × CT182 (PEG) | 3 | 1 | 2 |
| IVC-3 × CT186 (Nat.) | 8 | 0 | 8 |
| IVC-3 × CT186 (PEG) | 3 | 0 | 3 |
| IVC-3 × CT247 (Nat.) | 5 | 0 | 5 |
| IVC-3 × CT247 (PEG) | 15 | 10 | 5 |
The primer pairs for these PCR experiments are presented in Table 2.
CT recipients were each PCR positive for cat (primer pair 4), tetM (pair 3), and lipB (pair 1) and retained the original Tn4001T-mycoplasmal genome junction of the CT parent (pair 5, 6, or 7, as appropriate). CT recipients were PCR negative for p93 (pair 2).
KD recipients were each PCR positive for cat, tetM, and p93 and lacked the Tn4001T-mycoplasmal genome junction found in the CT parent. KD recipients were PCR negative for lipB.
The possibility of transposition of Tn4001T from CT donors to representative KD recipients was further examined by nucleotide sequence analysis of inverse PCR products containing the junction between Tn4001T and the KD genome. The nucleotide sequence was compared to that of the CT complete genome to determine the site of Tn4001T insertion. These data are summarized in Table 5. Each of four progeny obtained from experiment 7 of Table 3 had Tn4001T inserted into the genome at a different site, which was distinct from the site of the parent strain (nucleotide position 652759 in CT182). One of these progeny apparently had two copies of Tn4001T, located at two different genomic sites. Three progeny from experiment 15 of Table 3 and two from experiment 8 also had Tn4001T at sites distinct from the parental CT247 strain and distinct from one another. The sequence obtained for one of these progeny did not match the CT genome sequence, indicating that Tn4001T had transposed into a region of the KD genome that is missing in CT. The finding that no two progeny had Tn4001T at the same site in the KD genome suggests that the progeny arose from independent mating events and are not siblings.
TABLE 5.
Genome location of Tn4001T transferred to IVC-3 recipients
| CT parent | Expta | Tn4001T nucleotide position(s) |
|---|---|---|
| CT182 | 7 | 77847 and 580731b |
| CT182 | 7 | 709875 |
| CT182 | 7 | 529246 |
| CT182 | 7 | 914001 |
| CT182 | 14 | 83281 |
| CT186 | 6 | 79030 |
| CT186 | 6 | 205991 |
| CT247 | 15 | 87533 |
| CT247 | 15 | 32636 |
| CT247 | 15 | 206410 |
| CT247 | 8 | 284708 |
| CT247 | 8 | KD-specific sequencec |
As provided in Table 3.
This progeny clone had two inverse PCR products, a finding consistent with two copies of Tn4001T in the IVC-3 genome as indicated.
The sequence adjacent to Tn4001T in this progeny clone had no match in the CT genome sequence.
DISCUSSION
It is highly unlikely that the mechanism of gene transfer in M. pulmonis by the natural route (i.e., no PEG) is transformation or transduction. The resistance of gene transfer to DNase I, the requirement that both parent strains be viable, and the failure of purified plasmid to transform cultures of M. pulmonis all argue against transformation. M. pulmonis is susceptible to infection by mycoplasma virus P1 (14). However, P1 is not thought to be lysogenic, and the KD and CT strains lack P1 DNA (32). Therefore, gene transfer was not a result of transduction by this phage. Even if the KD strain harbors an unknown lysogenic phage, it would be expected that KD cells might be resistant to superinfection by this phage. Therefore, gene transfer from KD to KD (Table 3, experiments 1 to 5) might be expected to not occur if transduction was the mechanism. Also, the CT genome sequence lacks any putative prophages (7). Gene transfer from CT to KD was independent of the site of Tn4001T in the CT genome and apparently did not involve the transfer of any DNA (hypothetical phage) other than the transposon.
One possible mechanism of gene transfer is conjugation, the transfer of DNA from donor to recipient cells in direct contact. In the absence of PEG, gene transfer was unidirectional from CT to KD. Although this result might suggest conjugation as a mechanism, the CT genome sequence lacks recognizable genes associated with conjugation in other bacteria. The frequency of gene transfer was low, suggesting that a highly evolved mechanism for gene transfer may not exist. The apparent unidirectionality of gene transfer may be a result of the dynamics of cell growth during overnight incubation of the combined cultures. KD grows more rapidly than CT. The doubling times of KD and CT are ca. 90 and 120 min, respectively (16). Thus, KD may have been more efficient at expression of a recently acquired antibiotic resistance gene than was CT.
Another possible mechanism of gene transfer is fusion, which we define as the partial or complete combining of the cytoplasm of two cells as a result of membrane fusion. Broth cultures of most species of mycoplasma, including M. pulmonis, often contain chains or clusters of cells that might be amenable to occasional fusion events. Because mycoplasmas lack a cell wall, fusion between mycoplasmas may perhaps be similar to protoplast fusion of gram-positive bacteria (22). Two cells may fuse to become a single cell with two chromosomes. Transposition of a transposon from one chromosome to another, followed by cell division to segregate the chromosomes into separate daughter cells, would account for the mating progeny obtained in this study. As above, the apparent unidirectionality of gene transfer from CT to KD may result from the superior growth of KD.
It is not known whether gene transfer in the absence of PEG occurs by the same mechanism as gene transfer by the PEG method. PEG can stimulate the transformation of many mycoplasmas, including M. pulmonis (13, 15). However, the insensitivity of the PEG-mediated gene transfer to DNase I argues against transformation as the mechanism. PEG can stimulate protoplast fusion as well as fusion between mycoplasma membranes (22, 31), a finding consistent with a cell fusion model of gene transfer. However, it is also possible that PEG would stimulate conjugation, if it exists in this system.
M. pulmonis probably lacks a robust homologous recombination system. Attempts to specifically target M. pulmonis genes for mutagenesis by transformation with plasmids that contain an internal fragment of the gene to be targeted have not succeeded using strategies that were successful in other mollicutes (18, 20, 23). A lack of homologous recombination would restrict the incorporation of transferred genes into the recipient chromosome. Perhaps the only genes that could be recombined were those that did not require homologous recombination, e.g., genes located on a transposable element. Even if homologous recombination exists in M. pulmonis, it may be much less efficient than transposition of Tn4001 and Tn916 and have gone undetected in the current study. It would be interesting to perform mating experiments similar to those of the current study but in a species of Mycoplasma for which homologous recombination is known to be active, such as M. genitalium (10).
Tn4001 can be used as a vector to insert genes into the mycoplasmal chromosome. For example, Tn4001 has been used to express lacZ in M. pulmonis, M. arthritidis, and M. gallisepticum and cytadherence-associated genes in M. pneumoniae (15a, 21, 24, 25). The protocols developed in the current study could be used to transfer genes within Tn4001, such as lacZ, from one strain of M. pulmonis to another. If interspecies gene transfer proves feasible, this approach might significantly impact studies on mycoplasmal genetics.
Acknowledgments
This work was supported by Public Health Service grants GM51126 and AI41113.
C. Todd French and Amy M. Teachman contributed equally to this work.
We thank Portia Caldwell and Tajuana Johnson for technical assistance and Brenda Clapper for helpful comments.
REFERENCES
- 1.Barnes, M. H., P. M. Tarantino, Jr., P. Spacclapoli, N. C. Brown, H. Yu, and K. Dybvig. 1994. DNA polymerase III of Mycoplasma pulmonis: isolation and characterization of the enzyme and its structural gene, polC. Mol. Microbiol. 13:843-854. [DOI] [PubMed] [Google Scholar]
- 2.Barroso, G., and J. Labarere. 1988. Chromosomal gene transfer in Spiroplasma citri. Science 241:959-961. [DOI] [PubMed] [Google Scholar]
- 3.Barroso, G., J.-C. Salvado, and J. Labarere. 1990. Influence of genetic markers and of the fusing agent polyethylene glycol on chromosomal transfer in Spiroplasma citri. Curr. Microbiol. 20:53-56. [Google Scholar]
- 4.Bhugra, B., and K. Dybvig. 1992. High-frequency rearrangements in the chromosome of Mycoplasma pulmonis correlate with phenotypic switching. Mol. Microbiol. 6:1149-1154. [DOI] [PubMed] [Google Scholar]
- 5.Cartner, S. C., J. W. Simecka, J. R. Lindsey, G. H. Cassell, and J. K. Davis. 1995. Chronic respiratory mycoplasmosis in C3H/HeN and C57BL/6N mice: lesion severity and antibody response. Infect. Immun. 63:4138-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell, G. H., and J. K. Davis. 1978. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect. Immun. 21:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambaud, I., R. Heilig, S. Ferris, V. Barbe, D. Samson, F. Galisson, I. Moszer, K. Dybvig, H. Wroblewski, A. Viari, E. P. C. Rocha, and A. Blanchard. 2001. Mycoplasma pulmonis pathogenicity strategies revealed by hypervariable domains in the complete genome. Nucleic Acids Res. 29:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clewell, D. B. 1986. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu. Rev. Microbiol. 40:635-659. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, M. K., J. R. Lindsey, R. F. Parker, J. G. Tully, and G. H. Cassell. 1988. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect. Immun. 56:2156-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhandayuthapani, S., W. G. Rasmussen, and J. B. Baseman. 1999. Disruption of gene mg218 of Mycoplasma genitalium through homologous recombination leads to an adherence-deficient phenotype. Proc. Natl. Acad. Sci. USA 96:5227-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dybvig, K. 1993. The genetics and basic biology of Mycoplasma pulmonis: how much is actually Acholeplasma? Plasmid 30:176-178. [DOI] [PubMed] [Google Scholar]
- 12.Dybvig, K. 1990. Mycoplasmal genetics. Annu. Rev. Microbiol. 44:81-104. [DOI] [PubMed] [Google Scholar]
- 13.Dybvig, K., and J. Alderete. 1988. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid 20:33-41. [DOI] [PubMed] [Google Scholar]
- 14.Dybvig, K., J. Alderete, and G. H. Cassell. 1988. Adsorption of Mycoplasma pulmonis virus P1 to host cells. J. Bacteriol. 170:4373-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dybvig, K., and G. H. Cassell. 1987. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science 235:1392-1394. [DOI] [PubMed] [Google Scholar]
- 15a.Dybvig, K., C. T. French, and L. L. Voelker. 2000. Construction and use of derivatives of transposon Tn4001 that function in Mycoplasma pulmonis and Mycoplasma arthritidis. J. Bacteriol. 182:4343-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dybvig, K., J. W. Simecka, H. L. Watson, and G. H. Cassell. 1989. High-frequency variation in Mycoplasma pulmonis colony size. J. Bacteriol. 171:5165-5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dybvig, K., and L. L. Voelker. 1996. Molecular biology of mycoplasmas. Annu. Rev. Microbiol. 50:25-57. [DOI] [PubMed] [Google Scholar]
- 18.Dybvig, K., and A. Woodard. 1992. Construction of recA mutants of Acholeplasma laidlawii by insertional inactivation with a homologous DNA fragment. Plasmid 28:262-266. [DOI] [PubMed] [Google Scholar]
- 19.Ferrell, R. V., M. B. Heidari, K. S. Wise, and M. A. McIntosh. 1989. A Mycoplasma genetic element resembling prokaryotic insertion sequences. Mol. Microbiol. 3:957-967. [DOI] [PubMed] [Google Scholar]
- 20.Gaurivand, P., F. Laigret, E. Verdin, M. Garnier, and J. M. Bove. 2000. Fructose operon mutants of Spiroplasma citri. Microbiology 146:2229-2236. [DOI] [PubMed] [Google Scholar]
- 21.Hahn, T.-W., K. A. Krebes, and D. C. Krause. 1996. Expression in M. pneumoniae of the recombinant gene encoding the cytadherence-associated protein HMW1 and identification of HMW4 as a product. Mol. Microbiol. 19:1085-1093. [DOI] [PubMed] [Google Scholar]
- 22.Hopwood, D. A. 1981. Genetic studies with bacterial protoplasts. Annu. Rev. Microbiol. 35:237-272. [DOI] [PubMed] [Google Scholar]
- 23.King, K. W., A. Woodard, and K. Dybvig. 1994. Cloning and characterization of the recA genes from Mycoplasma pulmonis and M. mycoides subsp. mycoides. Plasmid 139:111-115. [DOI] [PubMed] [Google Scholar]
- 24.Knudtson, K. L., and F. C. Minion. 1993. Construction of Tn4001lac derivatives to be used as promoter probe vectors in mycoplasmas. Gene 137:217-222. [DOI] [PubMed] [Google Scholar]
- 25.Liu, L., K. Dybvig, V. S. Panangala, V. L. van Santen, and C. T. French. 2000. The GAA trinucleotide repeat region regulates the M9/pMGA gene expression in Mycoplasma gallisepticum. Infect. Immun. 68:871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahairas, G. G., C. Jian, and F. C. Minion. 1990. Genetic exchange of transposon and integrative plasmid markers in Mycoplasma pulmonis. J. Bacteriol. 172:2267-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markham, P. F., M. F. Duffy, M. D. Glew, and G. F. Browning. 1999. A gene family in Mycoplasma imitans closely related to the pMGA family of Mycoplasma gallisepticum. Microbiology 145:2095-2103. [DOI] [PubMed] [Google Scholar]
- 28.Noormohammadi, A. H., P. F. Markham, M. F. Duffy, K. G. Whithear, and G. F. Browning. 1998. Multigene families encoding the major hemagglutinins in phylogenetically distinct mycoplasmas. Infect. Immun. 66:3470-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, X., H. Yu, J. Gumulak, C. T. French, N. Zou, and K. Dybvig. 2000. Gene rearrangements in the vsa locus of Mycoplasma pulmonis. J. Bacteriol. 182:2900-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitaraman, R., and K. Dybvig. 1997. The hsd loci of Mycoplasma pulmonis: organization, rearrangements and expression of genes. Mol. Microbiol. 26:109-120. [DOI] [PubMed] [Google Scholar]
- 31.Tarshis, M., M. Salman, and S. Rottem. 1991. Fusion of mycoplasmas: the formation of cell hybrids. FEMS Microbiol. Lett. 82:67-72. [DOI] [PubMed] [Google Scholar]
- 32.Tu, A.-H. T., L. L. Voelker, X. Shen, and K. Dybvig. 2001. Complete nucleotide sequence of the mycoplasma virus P1 genome. Plasmid 45:122-126. [DOI] [PubMed] [Google Scholar]