Abstract
The production of virulence factors and carbapenem antibiotic in the phytopathogen Erwinia carotovora is under the control of quorum sensing. The quorum-sensing signaling molecule, N-(3-oxohexanoyl)-l-homoserine lactone (OHHL), accumulates in log-phase culture supernatants of E. carotovora but diminishes in concentration during the stationary phase. In this study, we show that the diminution in OHHL was not due to sequestration of the ligand by the cells, although some partitioning did occur. Rather, it was caused by degradation of the molecule. The rate of stationary-phase degradation of OHHL was as rapid as the rate of log-phase accumulation of the ligand, but it was nonenzymatic and led to a decrease in the expression of selected genes known to be under the control of quorum sensing. The degradation of OHHL was dependent on the pH of the supernatant, which increased as the growth curve progressed in cultures grown in Luria-Bertani medium from pH 7 to ∼8.5. OHHL became unstable over a narrow pH range (pH 7 to 8). Instability was increased at high temperatures even at neutral pH but could be prevented at the growth temperature (30°C) by buffering the samples at pH 6.8. These results may provide a rationale for the observation that an early response of plants which are under attack by Erwinia is to activate a proton pump which alkalizes the site of infection to a pH of >8.2.
Erwinia carotovora subsp. carotovora is a plant pathogen which causes soft rot in a number of economically important crops, such as potatoes, carrots, and beets (20, 21). The symptoms of soft rot are caused by the action of certain classes of exoenzymes (pectinases, pectate lyases, cellulases, and proteases) which are secreted by the bacteria and lead to tissue maceration (6, 8, 20). Production of these exoenzymes is under the control of quorum sensing in E. carotovora subsp. carotovora; i.e., they are not synthesized until the population cell density exceeds a certain value (22). It has been hypothesized that this strategy enables the bacteria to evade the host defense response until the pathogens have achieved sufficient numbers to overwhelm it (24). Thus, an offensive by Erwinia has two phases: a “passive” prequorum phase, during which the bacteria multiply but do not macerate the plant tissue, and an “aggressive” postquorum phase characterized by the concerted synthesis of a barrage of exoenzymes and other products, including the antibiotic, carbapen-2-em-3-carboxylic acid (referred to here as carbapenem). This compound is synthesized by the products of the car gene cluster (carA to carH [15]) and perhaps might function to prevent other, opportunistic bacterial species from colonizing the nutrient-rich site of infection (1). Carbapenem is stable for several days at 5°C in the pH range 7 to 9 but is rapidly inactivated by heating, freezing, or acid conditions (18). The synthesis of carbapenem in E. carotovora subsp. carotovora is strictly under the control of quorum sensing (3). Indeed, it was during a screen of carbapenem-negative (Car−) mutants that the gene (carI) encoding the enzyme responsible for synthesis of the quorum-sensing signaling molecules in E. carotovora subsp. carotovora was discovered (2, 13, 28). The CarI protein synthesizes one major signal-ing molecule, N-(3-oxohexanoyl)-l-homoserine lactone (OHHL), together with smaller quantities of the unsubstituted hexanoyl and butanoyl homoserine lactone (HSL) derivatives (HHL and BHL, respectively). The transcription of virulence determinant genes is downregulated in a carI background but can be reactivated by the addition of synthetic l-OHHL. The physiological role of the minor HSLs is unclear.
OHHL activates the transcription of the car gene cluster by binding to a LuxR homolog called CarR (14, 16). The CarR protein is a transcriptional activator that becomes functional when the ligand binds. In vitro, purified CarR is a homodimer, which binds one molecule of OHHL per monomer with a Kd of ca. 2 μM (31). Ligand-binding causes CarR to self-associate, forming a multimeric complex that binds to the DNA upstream of the first gene in the car cluster, carA (12, 14, 15, 32). The precise mechanism by which the DNA-CarR-OHHL complex activates transcription is not known, although it probably involves the formation of favorable contacts with RNA polymerase. Exoenzyme production is normal in a carR background and so, by inference, a different LuxR homolog (or similar HSL receptor) may be involved in controlling the production of these virulence factors (14).
Given the central role played by OHHL in controlling virulence, we would expect that its concentration is tightly regulated. On the basis of this assumption, we set out to measure how the concentration of this ligand changes through the growth curve in cultures of E. carotovora subsp. carotovora. An earlier report (12) showed that OHHL levels rise during the log phase of growth and then appear to decline during the stationary phase. This decline appeared to be slow but was not well represented in terms of datum points. The authors of this study inferred that the loss of OHHL was not due to chemical instability of the ligand. In the present study, we investigated directly what happens to OHHL levels during the stationary phase (i.e., after the population has achieved a “quorum”). At the same time, we also wanted to examine whether changes in the level of OHHL correlated with changes in a quorum-sensing-dependent phenotype (carbapenem production). Contrary to our expectation, we found that OHHL levels rapidly decline during the stationary phase, indicating that E. carotovora subsp. carotovora not only synthesizes this ligand but is also responsible for its degradation.
MATERIALS AND METHODS
Bacterial strains and media.
All E. carotovora subsp. carotovora strains used in the current study are from the lab stock and are derivatives of E. carotovora subsp. carotovora MS1, which is a Lac−, Car+, Tn10-cured derivative of ATTn10 (ATCC 39048::Tn10, Tetr Car+, restrictionless, modificationless [3]). GB7 is a carA::TnphoA"-2 mutant of MS1 (7). jbC1 is a carI::TnphoA"-2 derivative of MS1. The β-lactam-supersensitive Escherichia coli strain, ESS, has been described previously (15). The Chromobacterium violaceum HSL biosensor strain (CV026) is a kanamycin-resistant (Kanr) cviI::mini-Tn5 derivative of ATCC 31532 (13). The E. coli HSL indicator strain, JM109(pSB401), has been described previously (34). Plasmid pDAH330 (14), which confers resistance to chloramphenicol, was introduced to JM109(pSB401) by electroporation by with a Bio-Rad Gene Pulser.
Dehydrated media and agar were from Difco. The medium used throughout this study was Luria-Bertani (LB) broth (0.5% yeast extract, 1% tryptone, 1% NaCl). All other chemicals were of laboratory grade. Antibiotics were used at the indicated concentrations. l-OHHL was purchased as N-(β-ketocaproyl)-l-HSL from the Sigma Chemical Co. (St. Louis, Mo.) and was stored as a 4.7 mM stock solution at −20°C in acetonitrile. l-HHL was a generous gift from B. W. Bycroft (University of Nottingham, Nottingham, United Kingdom) and was stored as a 5 mM solution in acetonitrile at −20°C.
Growth curves and culture conditions.
Unless otherwise stated, overnight cultures of E. carotovora subsp. carotovora were grown in LB medium and used to inoculate 400 ml of prewarmed (30°C) LB medium in a 2-liter baffled glass flask. Routinely, the starting optical density at 600 nm (OD600) of the cultures was between 0.02 and 0.03. The cultures were grown with good aeration in an orbital shaker (140 rpm) at 30°C for 18 h. During this time, aliquots of culture were removed every hour for measurement of cell density (i.e., the OD600) and for the preparation of supernatant and cell samples. The supernatant and cell samples were prepared by sedimenting the bacteria in a benchtop centrifuge. The supernatant was removed without disturbing the pellet and, unless it was to be used for carbapenem determination, was immediately frozen at −20°C. Residual fluid was removed from around the cell pellet by aspiration, and the cells were immediately frozen. Supernatant for carbapenem determination was kept on ice until use (which was routinely 18 h after the start of each growth curve).
Anaerobic cultures were grown at 30°C in smooth-walled flasks containing 200 ml of LB medium overlain by an equal volume of sterile paraffin. Sterile glass beads (5 mm in diameter) were added to the cultures so that, on gentle shaking in a water bath at 100 rpm, they rolled around the bottom of the flask, preventing the cells from settling.
The E. coli strains were grown at 37°C on both solid and liquid media. C. violaceum CV026 was grown on solid medium at 25°C and in LB medium at 30°C.
Carbapenem assay.
Carbapenem assays were performed immediately after each growth curve was completed. Molten LB agar (1.6%, 400 ml) was poured into a square bioassay dish (24 by 24 cm) and allowed to solidify. The agar base was then overlaid with LB agar (0.6%, 100 ml) containing 100 μl of an overnight culture of E. coli ESS. After this had solidified, holes were punched through both agar layers by using a number 3 cork borer, and plugs of agar were removed to leave behind wells (ca. 20 per plate). Into each well was placed 250 μl of supernatant. Once all of the wells were filled, the plate was incubated for 48 h at 30°C. The relative amount of carbapenem present in each sample was estimated from the area of the clear zones around each well.
OHHL assay.
Routinely, supernatants were diluted 100-fold in LB medium containing 50 μg of chloramphenicol/ml. The samples were then dispensed in aliquots (100 μl) into black-walled 96-well microtiter plates. A range of OHHL standards (1 to 1,000 nM; also in LB-chloramphenicol) were dispensed into the plates where appropriate. Each sample was then mixed with an equal volume of E. coli indicator cells (JM109 containing pSB401 [Tetr, luxR luxCDABE] [30, 34]) and pDAH330 (to confer chloramphenicol resistance [14]) which had been grown to an OD600 of 1 at 37°C in LB medium containing 10 μg of tetracycline and 50 μg of chloramphenicol/ml. The samples were then incubated at 30°C for 2 h. Light emission was measured by using an Anthos Lucy 1 luminometer.
Western blot analysis.
Cells from a culture of MS1 were harvested by centrifugation. The cell pellets were resuspended to an OD600 of 1.0 in 50 mM Tris-HCl (pH 8.0) and then boiled for 5 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Portions (5 μl, corresponding to ca. 5 × 106 cells) of each sample were loaded onto 10 to 20% polyacrylamide gradient gels and electrophoresed in Tris-glycine buffer. The proteins were then transferred to polyvinylidene difluoride membrane by electroblotting. The membranes were then incubated with rabbit polyclonal antibodies raised against either CarA or CarB. The secondary antibody used was horseradish peroxidase-coupled donkey polyclonal anti-rabbit immunoglobulin G. The bands were revealed by enhanced chemiluminescence.
Extraction of OHHL from cell pellets.
Cell pellets taken from throughout the growth curve were extracted with 1 ml of cold (4°C) ethanol for 48 h. The extracted samples were centrifuged, and the top 0.5 ml of each solution was removed and placed in a clean tube. The ethanol from these samples was then evaporated in a SpeedVac, and the residue was redissolved in 100 μl of LB medium containing 50 μg of chloramphenicol/ml. The OHHL content of the sample was then measured as described above.
Extraction of OHHL from culture supernatants.
Our method is based on that of Shaw et al. (26) with modifications. Portions (0.8 ml) of cell-free supernatants were extracted three times with 0.5 ml of ethyl acetate. The extracts from each time point were pooled, and the solvent was evaporated in a SpeedVac. The residue was dissolved in 15 μl of acetonitrile and spotted onto a C18 reversed-phase thin-layer chromatography (TLC) plate. Spots of pure synthetic HHL and OHHL were also applied to allow us to identify these HSLs in the samples. The plate was developed in a 60% methanol-40% water mix. The positions of the HSL spots were revealed by overlaying the TLC plate with LB agar (0.6%) containing 0.5 ml of an overnight culture of CV026.
RESULTS
OHHL levels decrease in stationary-phase cultures of E. carotovora subsp. carotovora.
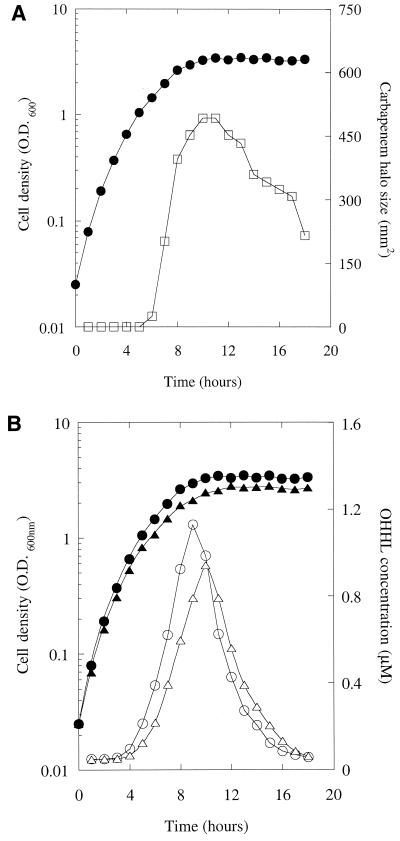
The growth curve of an MS1 culture and the carbapenem content of the culture supernatant are shown in Fig. 1A. During the first 5 h of growth, the cell density increased exponentially, with a doubling time of 43 ± 2 min. The growth rate then decreased until the cells entered the stationary phase at ca. 10 h. After this point, no further change in population cell density occurred. In the same culture, detectable carbapenem production began after 6 h and rose rapidly until the cells entered the stationary phase. Thereafter, the amount of carbapenem in the supernatant declined. The decline in carbapenem production suggested that the amount of OHHL in the supernatant may also be decreasing during this part of the growth curve. This was indeed the case (Fig. 1B). Initially, the OHHL concentration rose during the mid-log phase of the growth curve and increased linearly (R2 = 0.99) with the population cell density. However, as the cells entered the stationary phase, the OHHL concentration reached a maximal value (ca. 1.2 μM) and then decreased as rapidly as it had accumulated.
FIG. 1.
Carbapenem and OHHL production through the growth curve of a wild-type E. carotovora subsp. carotovora culture. Aliquots of an MS1 culture were withdrawn every hour through the growth curve for the measurement of cell density (•) and the carbapenem (□) (A) or OHHL concentration (○) (B). Panel B also shows the growth curve (▴) and the OHHL concentration (▵) of a culture of GB7.
One caveat in the experiments described above is that the carbapenem produced by MS1 might have caused lysis of the E. coli cells used in the lux bioassay, thereby giving rise to the apparent decrease in OHHL levels. To test this, we measured OHHL levels through the growth curve of GB7 (Fig. 1B), which is a Car− MS1 derivative in which the carA gene has been interrupted by a lacZ transcriptional fusion (33). The OHHL levels through the growth curve of GB7 were similar to those of MS1 (Fig. 1B). (The slightly later peak of OHHL production in the GB7 culture compared to the MS1 culture probably reflects the reduced growth rate of the mutant, which had a doubling time of 47 min.) We conclude that the carbapenem produced by MS1 does not affect the lux bioassay.
The results in Fig. 1 suggest that the OHHL levels in cultures of E. carotovora subsp. carotovora decline rapidly during the stationary phase of growth. However, alternative explanations exist. For example, the E. carotovora subsp. carotovora cells may produce a compound during the stationary phase that reduces light production in the lux bioassay. We therefore used a second approach to monitor HSL levels through the growth curve. HSLs were extracted from cell-free culture supernatants into ethyl acetate. The extracts were then applied to a reversed-phase TLC plate and developed in one dimension. The location of the OHHL spots was revealed by overlaying the plate with a thin layer of soft agar containing CV026 (which produces the violet pigment, violacein, in the presence of certain HSLs). This experiment also allowed us to monitor growth phase-dependent changes in the level of a minor HSL produced by CarI, HHL, which is detected by CV026. We found that both OHHL and HHL decline during the stationary phase of growth (data not shown).
Expression of CarA and CarB through the growth curve.
To see whether the decrease in carbapenem levels in stationary-phase MS1 cultures was accompanied by a corresponding decrease in the carbapenem biosynthetic enzymes, we used Western blot analysis to measure the amount of CarA and CarB in the cells through the growth curve (data not shown). Expression of both proteins was detected 5 h into the growth curve and reached an apparent maximum after 8 to 10 h. After this, the levels of CarA and CarB decreased until the last time point measured (15 h), when they were present at only ca. 25% of their maximal induction values.
Sequestration is not responsible for the decrease in OHHL levels.
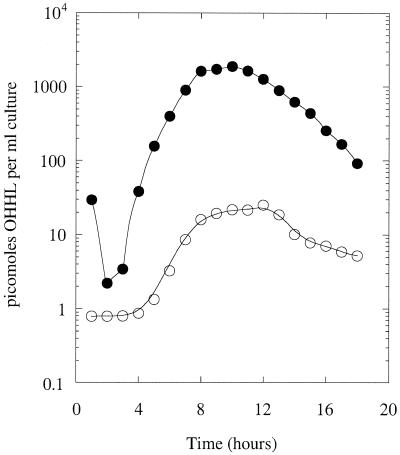
The decreased OHHL levels in stationary-phase MS1 cultures might have been due to either turnover or sequestration of the HSL. If the latter possibility were correct, the amount of OHHL sequestered in the cell pellets should increase as the OHHL declines in the supernatant. We tested this by extracting the cell pellets with cold ethanol and then measuring the amount of OHHL in each extract (Fig. 2). This was compared to the OHHL content of the corresponding culture supernatant. The OHHL was distributed between the cells and supernatant. Although the distribution appeared to vary somewhat through the growth curve, when we plotted the amount of sequestered OHHL per unit of cell density we found that there was an excellent correspondence between the ligand concentration in the bulk medium and in the cells (data not shown). Thus, the sequestered OHHL is in simple equilibrium with the bulk-phase ligand. These data show that, although the cells do sequester some of the HSL, this is not responsible for the disappearance of OHHL from the stationary phase supernatants.
FIG. 2.
Sequestration of OHHL by wild-type E. carotovora subsp. carotovora cells. The amount of OHHL in the supernatant (•) and in the cells (○) from a culture of MS1 was measured throughout the growth curve. The high initial value in the supernatant is due to carryover from the sample used to inoculate the culture.
OHHL turnover in a carI mutant.
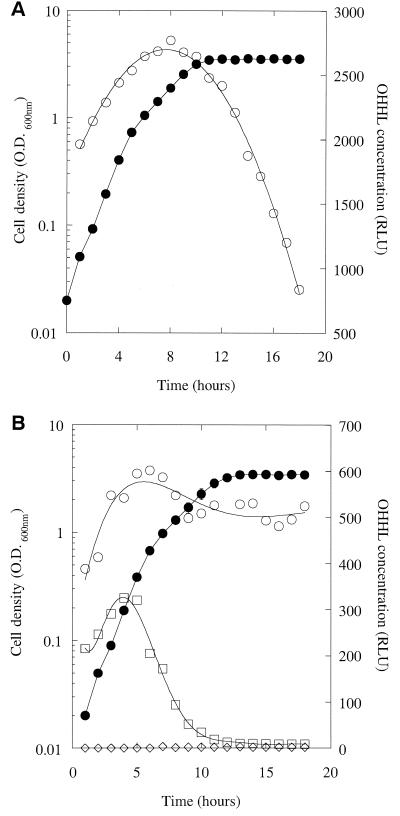
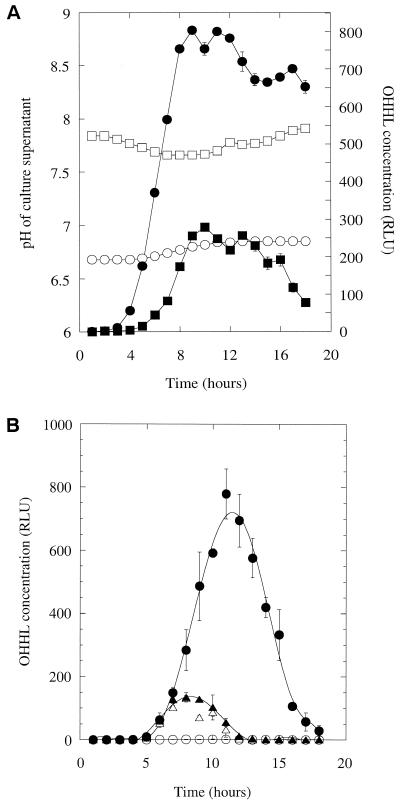
The experiments described above are complicated by the fact that in cultures of MS1, OHHL is both synthesized and degraded. To try and simplify the situation, we examined how the concentration of synthetic l-OHHL, added at the start to a culture of a carI mutant (jbC1), changed in the supernatant as the growth curve progressed (Fig. 3A). During the early part of the growth curve, the OHHL concentration reported by the lux bioassay appeared to increase in a linear fashion (R 2 = 0.99) until it reached a peak 10 h into the growth curve. Thereafter, the apparent OHHL concentration declined. The initial increase in apparent OHHL levels reported by the bioassay was unexpected. One possibility was that the cells used to inoculate the culture rapidly sequestered some of the added OHHL and then gradually released the bound ligand as the growth curve progressed. However, the corresponding samples of jbC1 cells from this growth curve contained very little sequestered OHHL (data not shown), so this was not the case. A second possibility was that the responsiveness of the bioassay changed as the growth curve progressed. To test this, we took supernatant samples through the growth curve from a culture of jbC1 without added OHHL. Synthetic l-OHHL (2 μM) was then added to each supernatant, and the samples were assayed immediately (Fig. 3B). The apparent OHHL concentration was low in the first time point sample and then increased and leveled off after a few hours. This shows two things. First, as the E. carotovora subsp. carotovora cells grow, they produce a compound in the log phase that enhances light production in the lux bioassay. Second, the reduction in apparent OHHL concentration in the latter parts of the growth curve in Fig. 3A reflects a real decline in the concentration of the HSL.
FIG. 3.
(A) Degradation of synthetic OHHL by a carI mutant. Synthetic OHHL (4.7 μM) was added to a culture of the carI mutant, jbC1. Aliquots of the culture were withdrawn every hour throughout the growth curve (•), and the OHHL content of the supernatant was measured (○). (B) OHHL stability in conditioned medium. Cell-free supernatant was prepared from throughout the growth curve (•) of a jbC1 culture. Some of this supernatant was retained for the measurement of endogenous OHHL (◊). Synthetic OHHL (2 μM) was added to the remaining samples, which were then divided into two portions. The OHHL content of one set of these portions was measured immediately (○), while the other was boiled for 10 min before being assayed (□).
OHHL is sensitive to pH and temperature.
Next, we investigated whether OHHL could be degraded or modified by a cell-associated factor (e.g., an enzyme). In our earliest attempts at this, we mixed cells with synthetic OHHL and allowed the reaction mixtures to stand for 2 h at 30°C. The cell suspensions were then boiled 10 min to stop any further HSL turnover. These reaction mixtures showed a very substantial (ca. >50%) loss of OHHL (data not shown). However, when the same experiment was performed without boiling, no degradation of OHHL was seen (data not shown). We subsequently found that the HSL is rapidly inactivated by boiling, even in water or dilute buffers, indicating that OHHL is thermosensitive. To see if longer-term exposure to lower temperatures might be responsible for the loss of OHHL from stationary-phase E. carotovora subsp. carotovora cultures, we repeated the experiment of Holden et al. (12) and added synthetic OHHL to sterile LB medium for 18 h at 30°C. This had no effect on the activity of the compound in the lux bioassay (data not shown). However, the thermostability of OHHL was altered when the HSL was added to conditioned media from the carI mutant, jbC1 (Fig. 3B). Clearly, something present in the mid- to late-log-phase supernatants decreased the thermostability of OHHL.
We also examined, over a range of pH and salt concentrations, whether cell-free sonicated extracts of stationary-phase E. carotovora subsp. carotovora cells could turn over synthetic l-OHHL. Although we obtained no evidence for the existence of an enzyme capable of degrading OHHL (in either the cells or in the culture supernatants), these experiments showed that OHHL is sensitive to pH over the physiological range.
OHHL depletion in stationary-phase cultures is due to alkalization of the medium.
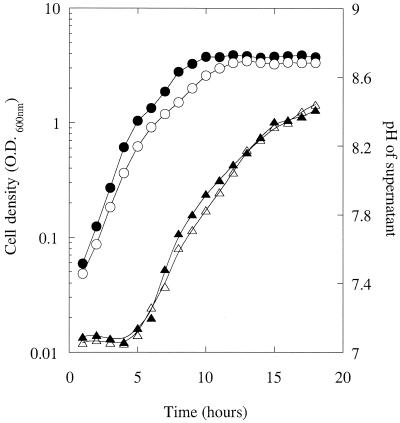
The pH of the supernatants used to obtain the data in Fig. 3A was measured. The pH of the culture was relatively constant (ca. pH 7.05) for the first 5 h of growth and then began to increase, reaching a maximal value of pH 8.45 after 18 h (Fig. 4). The pH values of jbC1 supernatants grown in the absence of OHHL were superimposable with those grown in the presence of the ligand. Very similar changes in pH were observed through the growth curve for MS1 supernatants. Similar observations were made for MS1 grown on solid medim: when MS1 was streaked onto LB agar containing a pH indicator (phenol red, 0.001% [wt/vol]), the agar turned red as the colonies grew, indicating that its pH was >8.2 (data not shown).
FIG. 4.
pH changes in the culture supernatants throughout the growth curve. The growth curves (circles) and supernatant pH values (triangles) of an MS1 culture (solid symbols) and of the jbC1 culture in Fig. 5 (open symbols) are shown.
To test whether the change in pH might be responsible for the reduction in OHHL levels during the stationary phase, we repeated the experiment in Fig. 3B, with one modification. Instead of measuring the OHHL concentration immediately after the addition of the ligand, we added the HSL and then left the sample for 18 h (the duration of the original growth curve) at 30°C before the assay. (Chloramphenicol was also added to prevent any bacterial growth; this did not affect the subsequent OHHL assay because the lux reporter strain contained a plasmid, pDAH330, encoding resistance to this antibiotic.) In the samples corresponding to the first few hours of the growth curve, the apparent OHHL concentration increased, as in Fig. 3A. However, as the pH of the samples began to rise, the OHHL concentration reported by the bioassay decreased sharply. Indeed, over the pH range 7.4 to 8.0, ca. 75% of the OHHL was degraded (Fig. 5). It should be recalled that in these, and in the earlier experiments, the samples were diluted 100-fold into fresh LB medium to bring the OHHL concentration within the linear range of the assay. Thus, the pH of all of the samples in the OHHL assay was fixed at that of LB medium (ca. pH 7.0).
FIG. 5.
Degradation of OHHL in conditioned medium. Synthetic OHHL (2 μM) and chloramphenicol (50 μg/ml) were added to jbC1 culture supernatants which had been harvested throughout the growth curve. The mixtures were incubated for 18 h at 30°C before measurement of the OHHL concentration (▴) and pH (▵) of the samples. Error bars represent the standard error of the mean from two independent cultures.
By necessity, in all of the above experiments, the supernatant samples taken at each time point through the growth curve were immediately frozen (at −20°C) until they were assayed. However, for any given set of supernatants, we found that the OHHL concentration was the same when assayed 1 day or 1 week after being harvested (data not shown). Thus, freezing appears to stop (or very greatly reduce) the rate of OHHL degradation in the samples.
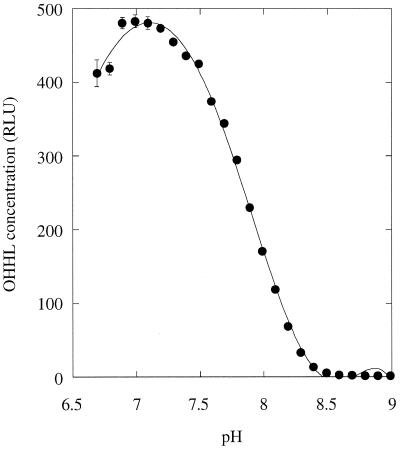
The data in Fig. 5 show that there is a good correlation between OHHL levels in MS1 culture supernatants and the pH of the medium. To prove that the change in pH was responsible for the OHHL degradation, we examined the stability of 2 μM OHHL in solutions of 100 mM potassium phosphate buffer over a range of pH values. The reaction mixtures were incubated at 30°C for 7 h and then diluted 100-fold into LB medium just prior to assay. Nearly all of the ligand was degraded in passing from pH 7.2 to 8.2, with 50% degradation occurring at pH 7.85 (Fig. 6). We conclude that OHHL is degraded in mildly alkaline conditions.
FIG. 6.
pH-dependent degradation of OHHL. Solutions of OHHL (2 μM) were prepared in 100 mM potassium phosphate buffer at the indicated pH and incubated at 30°C for 7 h. The samples were then diluted 100-fold in LB medium and assayed for OHHL content.
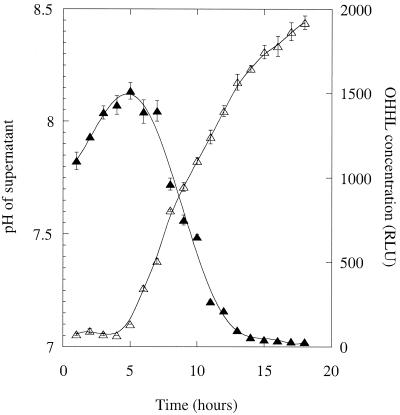
The results above suggested that degradation of OHHL might be prevented in solutions buffered at a pH of <7 and enhanced in solutions buffered at pH 7.5 or above. To test this, we performed two types of experiment. In the first, we grew cultures of MS1 in LB medium buffered at different pH values with 100 mM potassium phosphate. (This was the lowest potassium phosphate concentration that was effective at buffering throughout the 18 h of the experiment and yet did not affect the growth curves of the cultures.) Figure 7A shows how the pH and OHHL at each time point in supernatants from cultures initially buffered at pH 6.7 and at pH 7.7 varied through the growth curve. At the lower pH, the OHHL levels are higher and decline much more slowly than those obtained from the culture at the higher pH. We conclude that OHHL turnover is slowed down at pH 6.7. In our second approach, four identical sets of supernatant were taken throughout the growth curve of GB7. After the samples were defrosted, two of these sets of supernatant were buffered with potassium phosphate (200 mM final concentration) so that their pH was fixed at pH 6.8 or 8.3. All four sets of supernatant (buffered and unbuffered) were then placed at 30°C for 18 h. After this, potassium phosphate (200 mM) was added to the two unbuffered samples so that their pH was set to pH 6.8 or 8.3. The four sets of supernatant now contained the same concentration of buffer, but two had been buffered from the start of the experiment and two had the buffer added only at the end. The amount of OHHL in each set of supernatants was then measured. (Control experiments showed that, although 200 mM potassium phosphate reduced [by ca. 10 to 15%] light production in the bioassay, the response of the assay was the same at pH 6.8 and at pH 8.3.) We found that when the samples were buffered at pH 6.8 from the start, their OHHL levels remained high (Fig. 7B). However, incubation at pH 8.3 from the start led to the complete disappearance of OHHL. In samples which were buffered only at the end of the experiment, detectable but only small quantities of OHHL remained, independent of the pH (Fig. 7B). We conclude that the degradation of OHHL in culture supernatants can be prevented or enhanced by buffering the samples at pH 6.8 or at pH 8.3 (respectively).
FIG. 7.
(A) Buffering of growing cultures alters OHHL turnover. Cultures of MS1 were grown in LB medium buffered with 100 mM potassium phosphate at pH 6.7 (circles) or at pH 7.7 (squares). The OHHL levels (solid symbols) and pH values (open symbols) at each time point are shown. (B) pH-dependent degradation of OHHL in conditioned medium. Supernatants taken throughout the growth curve of GB7 were buffered with 200 mM potassium phosphate at pH 6.8 (filled symbols) or pH 8.3 (open symbols) either before (circles) or after (triangles) an 18 h of incubation at 30°C. The residual OHHL concentration in each set of supernatants is shown. Error bars represent the standard error of the mean from two independent cultures.
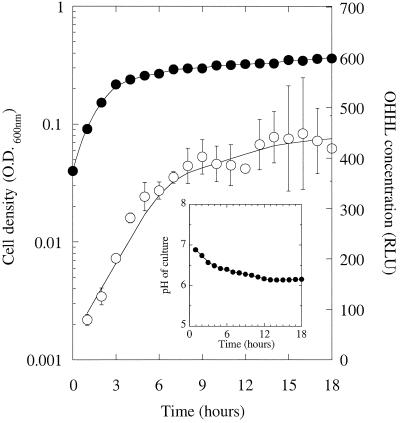
OHHL levels do not decline in anaerobic conditions.
One way of preventing alkalization in complex (peptide-based) media is to grow the cells in anaerobic conditions (see Discussion). We did this by inoculating a smooth-walled flask of LB medium with MS1 and then overlaying the culture with sterile paraffin oil. The flasks were gently agitated to prevent the cells from clumping and sedimenting. (A few sterile glass beads were added to each flask to facilitate this gentle mixing.) The cell density and OHHL content of the cultures was measured every hour (Fig. 8). Although the cultures rapidly reached stationary phase, the OHHL concentration increased and then leveled off rather than declining. The pH of the cultures at each time point was also measured (Fig. 8, inset). Unlike the aerobically grown cultures, the pH during growth in anaerobic conditions decreased from pH 7 to 6.2 over the course of the experiment. We conclude that during anaerobic growth on LB medium, OHHL is stabilized because the medium becomes slightly acidic.
FIG. 8.
OHHL does not decline in stationary-phase anaerobic cultures. A culture of MS1 was grown in LB medium in anaerobic conditions as described in Materials and Methods. Symbols: •, cell density; ○, OHHL level. (Inset) pH of the culture supernatant through the growth curve.
DISCUSSION
In this study, we present four major findings. First, we demonstrate that the concentration of OHHL in E. carotovora subsp. carotovora culture supernatants rapidly decreases in the stationary phase of the growth curve. Second, this decrease in OHHL affects levels of the carbapenem biosynthetic enzymes, which are encoded by genes under the control of the quorum-sensing system in E. carotovora subsp. carotovora (12, 14). Third, we show that the stationary-phase decrease in OHHL is due to nonenzymatic turnover of the ligand. Fourth, this nonenzymatic degradation of OHHL is shown to involve alkalization of the growth medium. In the following discussion, we address the implications of these findings in greater detail.
It was reported in an earlier study (12) that OHHL levels slowly decline in stationary-phase cultures of E. carotovora subsp. carotovora and that this decline is not due to chemical instability of the molecule. In contrast, we found that OHHL is rapidly degraded in cultures of E. carotovora subsp. carotovora and is unstable in the physiological pH range. Do these results have any physiological relevance? An intriguing finding which may shed some light on this question is that one of the first recognizable responses of plants to E. carotovora subsp. carotovora infection is to increase the pH of the apoplastic fluid around the site of infection (17). This is achieved by activating a very rapid H+ influx into the plant cells during the early stages of infection (prior to any signs of tissue maceration) which causes the intercellular pH to increase from a pH of <6.4 to a pH of >8.2 (4, 5). It is possible that this response might have evolved in order to enhance HSL degradation, thereby slowing down the production of virulence factors. In principle, such nonenzymatic HSL degradation might be offset by the bacteria through increased synthesis of the ligand. Indeed, some organisms are known to employ positive feedback as a means of rapidly elevating the HSL concentration to very high levels. However, there is no evidence that this is the case for E. carotovora subsp. carotovora (G. P. C. Salmond and M. Welch, unpublished data).
At first glance, our results may seem paradoxical. After all, why should an organism alkalize its own growth medium and, at the same time, use a base-sensitive molecule as an intercellular signal? The answer to this probably lies (at least partially) in the differences between aerobic and anaerobic metabolism. Although aerobic metabolism in complex media is known to result in alkalization due to the preferential use of weak acids as carbon sources (27), anaerobic metabolism does the opposite, and results in net production of weak acids. Presumably, the prevention of alkalization in anaerobic conditions accounts for the persistence of OHHL during the stationary phase in Fig. 8, and helps to explain why soft rot occurs much more readily when oxygen is limiting (21). Another factor that affects the pH of a culture is the carbon source. While aerobic growth on a peptide-based medium such as LB medium and on weak acids such as acetate results in alkalization, growth on carbohydrates such as glucose, glycerol, and sucrose does the opposite and acidifies the culture (J. T. Byers and M. Welch, unpublished observations). The persistence of OHHL in a culture is therefore likely to be strongly dependent on the availability of oxygen and on the nature of the substrate being metabolized.
Our findings also suggest that thermophiles and alkalophiles, although they may use other compounds, are unlikely to use HSLs as signaling molecules. Even rather mild alkaline conditions may disfavor the use of HSLs as signaling agents. This may explain why organisms such as E. coli, which live in the intestine (pH 7.6, 37°C [31]), use a non-HSL molecule (a probable furanone derivative of 4,5-dihydroxy-2-3-pentanedione [25]) for quorum sensing. However, many marine bacteria do use HSLs, in spite of the fact that the pH of seawater is high (pH 7.8 to 8.2 [11]). One reason for this apparent discrepancy may be that the temperature of seawater is some 20 to 30°C lower than the temperature of the gut, which should reduce the rate of HSL turnover ca. four- to eightfold. Furthermore, the data in Fig. 6 suggest that the half-life of OHHL at pH 7.85 is quite long (7 h at 30°C), although at pH values substantially higher than this the stability of OHHL rapidly declines. Indeed, at pH 8.5 the half-life of OHHL is just 30 min (M. Welch, unpublished data). Hence, the use of HSLs as signaling molecules may depend on the combined effects of pH and temperature. Superficially, our finding that OHHL is thermolabile appears to contradict an earlier report by Eberhard et al. (10), who noted that the molecule is stable to heating at 140°C for 10 min. However, this result was obtained by heating a thin, dry film of OHHL in air, where no hydrolysis could occur, while the current work was done in aqueous solutions.
In previous work, we and others (19, 23, 32) have suggested that HSLs, due to their amphipathic nature, may partition from the bulk phase into the membrane compartment(s) of cells. Our results verify that cells sequester OHHL and, further, show that the sequestered ligand is in rapid (on the time scale of gene expression) equilibrium with the HSL in the bulk phase. However, we conclude that sequestration is not involved in the loss of OHHL from stationary-phase E. carotovora subsp. carotovora cultures, nor does it serve as an effective buffer against this loss. Nevertheless, if we assume that a typical E. carotovora subsp. carotovora cell has a volume of 5 × 10−16 liters and that an OD of 1 corresponds to 109 cells per ml, we calculate that the concentration of sequestered OHHL in the cells is at least sixfold higher than that of the bulk supernatant (Welch, unpublished). Presumably, most of this sequestered OHHL is in the inner membrane, so the local concentration of the ligand at the cytoplasm-membrane interface may be greater still (23). We speculate that, with its potential to increase the dynamic range (and/or sensitivity) of the system, the physiological effect of partitioning might be far greater than previously envisaged. Indeed, partitioning might account for the observation that carbapenem production in MS1 was high even though the maximal OHHL concentration in the supernatant (ca. 1 μM) was substantially less than the Kd of the CarR-OHHL complex (2 μM).
We found that basic conditions, in addition to breaking down OHHL, also cause HHL degradation. If we assume that a similar mechanism operates in each case, this suggests that the 3-oxo moiety is not involved in the reaction. The most likely mechanisms are either hydrolysis of the amide bond linking the lactone ring to the acyl chain or hydrolysis of the ester bond within the lactone ring. The latter bond is more labile, and delactonization of OHHL has been shown to occur in vitro at pH 12 (10), resulting in the formation of a product with little or no biological signaling activity (9). However, attempts to reform the lactone ring by briefly acidifying MS1 supernatants with HCl (to pH 1.5), followed by neutralization to pH 6.8 with potassium phosphate, were unsuccessful (Welch, unpublished).
The data in Fig. 3A show that E. carotovora subsp. carotovora cultures, prior to the onset of significant alkalization of the medium, produce one or more compounds which affect the response of the lux bioassay toward OHHL. This compound(s) is present even in the supernatants of a carI mutant, suggesting that it is not an HSL. However, the compound(s) alone is not a signaling molecule(s) per se since, in the absence of OHHL, the supernatants from a carI mutant did not activate light production in the bioassay. We conclude that this unidentified factor acts to increase the sensitivity of the bioassay toward OHHL. Apropos, the presence of this compound in culture supernatants is likely to complicate precise quantification of OHHL levels by using the lux bioassay, although not more than a factor of ∼2-fold (cf. Fig. 3A).
Concluding remarks.
Our results suggest that the resistance of plants against soft rot might be enhanced if the plant could be engineered to constitutively express gene products that alkalize the apoplastic space, such as the proton pump discussed above, or perhaps a bicarbonate or ammonia transporter (assuming that these modifications have no adverse effects on the growth yield and quality of the final product). This type of approach may complement the recently developed strategy of expressing in planta a Bacillus enzyme which has the ability to break down HSLs (9).
Acknowledgments
J.T.B. was supported by a studentship from the BBSRC. Work in the lab of G.P.C.S. is funded by the BBSRC and is covered by MAFF license no. PHL177/3963(10/2001). M.W. is a Royal Society University Research Fellow and thanks the Royal Society for its support.
We thank B. W. Bycroft (University of Nottingham, Nottingham, United Kingdom) for generously providing the HSLs used in this study. We also thank N. Whitehead for stimulating discussions.
REFERENCES
- 1.Axelrood, P. E., M. Rella, and M. N. Schroth. 1988. Role of antibiosis in competition of Erwinia strains in potato infection courts. J. Bacteriol. 54:1222-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainton, N. J., B. W. Bycroft, S.-R. Chhabra, P. Stead, I. Gledhill, P. J. Hill, C. E. D. Rees, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1992. A general role for the lux autoinducer in bacterial cell signaling: control of antibiotic synthesis in Erwinia. Gene 116:87-92. [DOI] [PubMed] [Google Scholar]
- 3.Bainton, N. J., P. Stead, S.-R. Chhabra, B. W. Bycroft, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1992. N-(3-Oxohexanoyl)-l-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem. J. 288:997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, C. J., M. M. Atkinson, M. A. Roy, and A. Collmer. 1986. Inhibition of the hypersensitive response in tobacco by pectate lyases. Physiol. Mol. Plant Pathol. 29:217-225. [Google Scholar]
- 5.Baker, C. J., N. Mock, M. M. Atkinson, and S. W. Hutcheson. 1990. Inhibition of the hypersensitive response in tobacco by pectate lyase digests of cell wall and of polygalacturonic acid. Physiol. Mol. Plant Pathol. 37:133-167. [Google Scholar]
- 6.Barras, F., F. van Gijsegem, and A. K. Chatterjee. 1994. Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annu. Rev. Phytopathol. 32:201-234. [Google Scholar]
- 7.Bosgelmez, G. 1999. Regulation of carbapenem antibiotic synthesis genes in Erwinia carotovora subspecies carotovora. Ph.D. thesis. University of Cambridge, Cambridge, United Kingdom.
- 8.Collmer. A., and N. T. Keen. 1986. The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24:383-409. [Google Scholar]
- 9.Dong, Y.-H., L.-H. Wang, J.-L. Xu, H.-B. Zhang, X.-F. Zhang, and L.-H. Zhang. 2001. Quenching quorum-sensing dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and N. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 11.Hawley, G. G. (ed.). 1981. The condensed chemical dictionary, 10th ed. Van Nostrand Reinhold Company, New York, N.Y.
- 12.Holden, M. T. G., S. J. McGowan, B. W. Bycroft, G. S. A. B. Stewart, P. Williams, and G. P. C. Salmond. 1998. Cryptic carbapenem antibiotic production genes are widespread in Erwinia carotovora: facile trans activation by the CarR transcriptional regulator. Microbiology 144:1495-1508. [DOI] [PubMed] [Google Scholar]
- 13.McClean, K. H., M. K. Winson, L. Fish., A. Taylor, S.-R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 14.McGowan, S. J., M. Sebaihia, S. Jones, B. Yu, N. Bainton, P. F. Chan, B. W. Bycroft, G. S. A. B. Stewart, P. Williams, and G. P. C. Salmond. 1995. Carbapenem antibiotic production in Erwinia carotovora is regulated by CarR, a homologue of the LuxR transcriptional regulator. Microbiology 141:541-550. [DOI] [PubMed] [Google Scholar]
- 15.McGowan, S. M., M. Sebaihia, S. O'Leary, K. R. Hardie, P. Williams, G. S. A. B. Stewart, B. W. Bycroft, and G. P. C. Salmond. 1997. Analysis of the carbapenem gene cluster of Erwinia carotovora: definition of the antibiotic biosynthetic genes and evidence for a novel β-lactam resistance mechanism. Mol. Microbiol. 26:545-556. [DOI] [PubMed] [Google Scholar]
- 16.McGowan, S. J., M. Sebaihia, L. E. Porter, G. S. A. B. Stewart, P. Williams, B. W. Bycroft, and G. P. C. Salmond. 1996. Analysis of bacterial carbapenem antibiotic production genes reveals a novel β-lactam biosynthesis pathway. Mol. Microbiol. 22:415-426. [DOI] [PubMed] [Google Scholar]
- 17.Nachin, L., and F. Barras. 2000. External pH: an environmental signal that helps to rationalize pel gene duplication in Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 13:882-886. [DOI] [PubMed] [Google Scholar]
- 18.Parker, W. L., M. L. Rathnum, J. S. Wells, Jr., W. H. Trejo, P. A. Principe, and R. B. Sykes. 1982. SQ 27,860, a simple carbapenem produced by species of Serratia and Erwinia. J. Antibiot. 35:653-660. [DOI] [PubMed] [Google Scholar]
- 19.Pearson, J. P., C. van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pérombelon, M. C. M. 1992. The genus Erwinia, p. 2899-2921. In H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. III. Springer-Verlag, London, England. [Google Scholar]
- 21.Pérombelon, M. C. M., and A. Kelman. 1980. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 18:361-387. [Google Scholar]
- 22.Pirhonen, M., D. Flego, R. Heikinheimo, and E. T. Palva. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 12:2467-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin, Y., Z.-Q. Luo, A. J. Smyth, P. Gao, S. Beck-von-Bodman, and S. K. Farrand. 2000. Quorum sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 19:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salmond, G. P. C., B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1995. The bacterial “enigma”: cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 25.Schauder, S., K. Shokat, M. G. Surette, and B. Bassler. 2001. The LuxS family of autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 26.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slonczewski, J. L., T. N. Gonzalez, F. M. Bartholemew, and N. J. Holt. 1987. Mu d-directed lacZ fusions regulated by low pH in Escherichia coli. J. Bacteriol. 169:3001-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swift, S., M. K. Winson, P. F. Chan, N. J. Bainton, M. Birdsall, P. J. Reeves, C. E. D. Rees, S.-R. Chhabra, P. J. Hill, J. P. Throup, B. W. Bycroft, G. P. C. Salmond, P. Williams, and G. S. A. B. Stewart. 1993. A novel strategy for the isolation of luxI homologues: evidence for the widespread distribution of a luxR/luxI superfamily in enteric bacteria. Mol. Microbiol. 10:511-520. [DOI] [PubMed] [Google Scholar]
- 29.Thomson, N. R., M. A. Crow, S. J. McGowan, A. Cox, and G. P. C. Salmond. 2000. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36:539-556. [DOI] [PubMed] [Google Scholar]
- 30.Throup, J. P., M. Camara, G. S. Briggs, M. K. Winson, S.-R. Chhabra, B. W. Bycroft, P. Williams, and G. S. A. B. Stewart. 1995. Characterisation of the yenI/yenR locus from Yersinia enetrocolitica mediating the synthesis of two N-acylhomoserine lactone signal molecules. Mol. Microbiol. 17:345-356. [DOI] [PubMed] [Google Scholar]
- 31.Tortora, G. J., and S. R. Grabowski (ed.). 1993. Principles of anatomy and physiology, 7th ed. Harper-Collins, New York, N.Y.
- 32.Welch, M., D. E. Todd, N. A. Whitehead, S. J. McGowan, B. W. Bycroft, and G. P. C. Salmond. 2000. N-Acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 19:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilmes-Riesenberg, M. R., and B. L. Wanner. 1992. TnphoA and TnphoA" elements for making a switching fusions for study of transcription, translation, and cell surface localization. J. Bacteriol. 174:4558-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S.-R. Chhabra, B. W. Bycroft, P. Williams, and G. S. A. B. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]