Abstract
In Pseudomonas fluorescens CHA0, an antagonist of root-pathogenic fungi, the GacS/GacA two-component system tightly controls the expression of antifungal secondary metabolites and exoenzymes at a posttranscriptional level, involving the RNA-binding protein and global regulator of secondary metabolism RsmA. This protein was purified from P. fluorescens, and RNA bound to it was converted to cDNA, which served as a probe to isolate the corresponding chromosomal locus, rsmZ. This gene encoded a regulatory RNA of 127 nucleotides and a truncated form lacking 35 nucleotides at the 3" end. Expression of rsmZ depended on GacA, increased with increasing population density, and was stimulated by the addition of a solvent-extractable extracellular signal produced by strain CHA0 at the end of exponential growth. This signal appeared to be unrelated to N-acyl-homoserine lactones. A conserved upstream element in the rsmZ promoter, but not the stress sigma factor RpoS, was involved in rsmZ expression. Overexpression of rsmZ effectively suppressed the negative effect of gacS and gacA mutations on target genes, i.e., hcnA (for hydrogen cyanide synthase) and aprA (for the major exoprotease). Mutational inactivation of rsmZ resulted in reduced expression of these target genes in the presence of added signal. Overexpression of rsmA had a similar, albeit stronger negative effect. These results support a model in which GacA upregulates the expression of regulatory RNAs, such as RsmZ of strain CHA0, in response to a bacterial signal. By a titration effect, RsmZ may then alleviate the repressing activity of RsmA on the expression of target mRNAs.
The GacS/GacA two-component system controls the expression of secondary metabolism and protein secretion in a wide variety of bacterial species (19, 39). In the biocontrol strain Pseudomonas fluorescens CHA0, which protects plant roots from pathogenic fungi, a functional GacS/GacA system is strictly required for the production of extracellular antimicrobial agents such as 2,4-diacetylphloroglucinol, pyoluteorin, and hydrogen cyanide (HCN) and the production of enzymes such as tryptophan side chain oxidase, phospholipase C, and exoprotease (24, 25, 32, 37, 48, 52).
Structural genes whose expression is controlled by GacA in strain CHA0 include hcnABC for HCN synthase (6, 8, 25), aprA for the major exoprotease (8, 48), and phlACBDE for the 2,4-diacetylphloroglucinol biosynthesis enzymes (52). Translational hcnA"-"lacZ and aprA"-"lacZ fusions have proved to be useful reporter constructs to monitor signal transduction mediated by the GacS/GacA two-component system. By contrast, a transcriptional hcnA-lacZ fusion and the hcnA promoter are not subject to control by GacA, indicating that GacA regulates expression of target genes by a posttranscriptional mechanism in P. fluorescens (5, 8).
In Erwinia carotovora subsp. carotovora, the production of exoenzymes and virulence are posttranscriptionally controlled by the RsmA/RsmB system (11, 30). The RNA-binding protein RsmA and its homolog CsrA in Escherichia coli and Salmonella enterica can act as repressors of target genes at the mRNA level and as factors favoring mRNA decay (28, 29, 45). RsmB of E. carotovora and CsrB of E. coli are regulatory RNAs that complex the RsmA and CsrA proteins and thereby are assumed to titrate these repressors, allowing the translation of target mRNAs to proceed (18, 45). Searches in the GenBank databases (including sequences from unfinished microbial sequencing projects) show that homologs of RsmA/CsrA can be found in a large number of gram-negative bacteria as well as some gram-positive bacteria, e.g., Bacillus subtilis (12, 45).
In P. fluorescens CHA0, RsmA is an important negative control element involved in the posttranscriptional control of secondary metabolism regulated by the GacS/GacA system (7, 8). Therefore, it could be postulated that an RNA analogous to RsmB/CsrB would be required to counteract the repressing effects of RsmA on target genes and that the expression of this regulatory RNA would be directly or indirectly controlled by GacA. This hypothesis has received support from the recent finding that the GacS/GacA system positively controls the expression of RsmB in E. carotovora (12, 22). However, whereas the RsmA/CsrA protein sequences are well conserved in different bacteria, such sequence conservation is not observed for the antagonistic regulatory RNAs. No homologs of RsmB/CsrB could be detected in genomic databases of bacteria not belonging to the Enterobacteriaceae.
Aarons et al. (1) have reported the isolation of a gacS and gacA multicopy suppressor in P. fluorescens strain F113. This suppressor, PrrB, is a 133-nucleotide regulatory RNA whose sequence is unrelated to that of RsmB and CsrB. However, whether PrrB interacts with RsmA and how PrrB is regulated in strain F113 have not been reported.
The aims of the present study are to characterize a regulatory RNA that binds to RsmA in P. fluorescens CHA0 and to study the regulation of this RNA, which we designated RsmZ, by the GacS/GacA system, by the stress sigma factor RpoS, and by a novel bacterial signal.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains, vectors, and oligonucleotides used in this study are listed in Table 1. Strains were routinely grown in nutrient yeast broth (NYB; 2.5% [wt/vol] nutrient broth, 0.5% [wt/vol] yeast extract) with shaking, or on nutrient agar (NA; 4% [wt/vol] blood agar base, 0.5% [wt/vol] yeast extract) amended with the following antibiotics, when required: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; streptomycin, 20 μg/ml; tetracycline, 25 μg/ml (125 μg/ml for P. fluorescens); and gentamicin, 10 μg/ml. Chloramphenicol was used at a concentration of 10 μg/ml to select P. fluorescens and to counterselect E. coli in mating experiments. When relevant, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added to plates at a final concentration of 0.02%. Routine incubation temperatures were 37°C for E. coli and 30°C for P. fluorescens. P. fluorescens was grown at 35°C to improve its capacity to accept heterologous DNA (e.g., in transformation or triparental matings with E. coli).
TABLE 1.
Bacterial strains, plasmids and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Description | Reference or source |
|---|---|---|
| E. coli | ||
| HB101, DH5α, JM105 | Laboratory strains | 50 |
| TR1-5BW3414 | ΔlacU169 csrA::Kmr | 46 |
| P. fluorescens | ||
| CHA0 | Wild type | 58 |
| CHA19 | ΔgacS | F. Carruthers |
| CHA89 | gacA::Kmr | 25 |
| CHA207 | Chromosomal hcnA"-"lacZ fusion | 8 |
| CHA89.207 | Chromosomal hcnA"-"lacZ fusion, gacA::Kmr | 8 |
| CHA805 | Chromosomal aprA"-"lacZ fusion | 8 |
| CHA806 | Chromosomal aprA"-"lacZ fusion, ΔgacS | This study |
| CHA807 | Chromosomal aprA"-"lacZ fusion, ΔgacS rsmA::ΩKmr | 8 |
| CHA808 | ΔgacS rsmA::ΩKmr | This study |
| CHA809 | rsmA::ΩKmr | This study |
| CHA810 | ΔrsmZ | This study |
| CHA811 | Chromosomal hcnA"-"lacZ fusion, ΔrsmZ | This study |
| CHA812 | Chromosomal aprA"-"lacZ fusion, ΔrsmZ | This study |
| CHA813 | Chromosomal aprA"-"lacZ fusion, ΔrpoS | This study |
| CHA814 | Chromosomal hcnA"-"lacZ fusion, ΔrpoS | This study |
| CHA815 | ΔrpoS | This study |
| Plasmids | ||
| pBLS | pBluescript II KS+ cloning vector, ColE1 replicon, Apr | Stratagene |
| pHP45ΩKm | ColE1 replicon, Apr Kmr | 14, 43 |
| pJF118EH | lacIq-Ptac expression vector, ColE1 replicon, Apr | 16 |
| pME497 | Mobilizing plasmid; IncP-1, Tra; RepA(Ts); Apr | 60 |
| pME3087, pME3088 | Suicide vectors; ColE1 replicon, IncP-1-Mob, Tcr | 58 |
| pME3849 | pME6001 with a 311-bp BamHI-SmaI rsmA fragment of P. aeruginosa PAO1 | 42 |
| pME6001 | Cloning vector, pBBR1MCS Gmr derivative | 8, 33 |
| pME6014 | pVS1-p15A shuttle vector for translational lacZ fusions, Tcr | 52 |
| pME6016 | pVS1-p15A shuttle vector for transcriptional lacZ fusions, Tcr | 52 |
| pME6031 | pVS1-p15A shuttle vector, Tcr | 20 |
| pME6032 | NruI-EcoRI lacIq-Ptac fragment of pJF118EH subcloned in [BamHI]-EcoRI-digested pME6031; lacIq-Ptac expression vector | This study |
| pME6071 | pBLS with a 1.2-kb PstI fragment of strain CHA0 carrying rsmA | This study |
| pME6072 | pME6001 with a 0.54-kb PstI/PvuII fragment of pME6071, with lacZ"-rsmA fusion | This study |
| pME6073 | pME6072 with deletion of the PstI site; rsmA under Plac promoter control | 8 |
| pME6074 | pME3087 with 1.2-kb PstI fragment of pME6071 | This study |
| pME6075 | BamHI plasmid rescued from CHA0::pME6074, with 6.8-kb genomic fragment upstream of 1.2-kb PstI fragment | This study |
| pME6078 | rsmA, histidine tagged (RsmA6H) by PCR using primers T3 and RSMA7, subcloned in XhoI-[EcoRI]-cut pME6032 | This study |
| pME6079 | 8.0-kb BamHI/HindIII fragment of pME6075 in pBLS | This study |
| pME6080 | 6.0-kb SacII deletion of pME6079 | This study |
| pME6081 | pME3088 carrying rsmA with ΩKm at its eighth codon | This study |
| pME6084 | pBLS with a 2.3-kb XhoI-PstI genomic fragment of strain CHA0, hybridizing with a probe from pRSMCDNA7 | This study |
| pME6085 | pBLS with the 673-bp XhoI-EcoRI fragment of pME6084 | This study |
| pME6086 | 673-bp XhoI-EcoRI rsmZ fragment of pME6084 in pME3088 | This study |
| pME6087 | Plasmid rescued from CHA0::pME6086 cut with HindIII, with 1.8-kb genomic fragment upstream of rsmZ | This study |
| pME6090 | pUC6S with an rsmZ-lacZ" fusion at the +1 site | |
| pME6091 | BamHI-ClaI fragment of pME6090 with PrsmZ-lacZ" subcloned into BamHI-ClaI-digested pME6016 | This study |
| pME6092 | pME6090 deleted for box (nucleotides −196 to −157) of rsmZ promoter | This study |
| pME6093 | BamHI-ClaI fragment of pME6092 with PrsmZΔbox-lacZ subcloned into BamHI-ClaI-digested pME6016 | This study |
| pME6094 | pUC6S with an rsmZ-lacZ" fusion at the SspI site of the rsmZ promoter | This study |
| pME6095 | BamHI-ClaI fragment of pME6094 with PrsmZSspI-lacZ" subcloned in | This study |
| BamHI-ClaI-digested pME6016 | ||
| pME6096 | rsmZ under the Ptac promoter at position +37 in pME6032 | This study |
| pME6097 | pME6085 deleted for nucleotides −243 to +125 of rsmZ | This study |
| pME6099 | pME3087 containing HindIII-XhoI insert of pME6089 + XhoI-PstI insert of pME6097 | This study |
| pME6352 | pME3088 containing a 945-bp in-frame deletion of rpoS | |
| pME6359 | Deletion of pME6096 creating a Ptac-rsmZ fusion at the +1 site | This study |
| pME6522 | Source of promoterless lacZ gene | 8 |
| pRSMCDNA7 | pBLS, with a 90-bp cDNA made from RNA copurifying with RsmA6H | This study |
| pUC6S | Cloning vector, ColE1 replicon, Apr | 57 |
| Oligonucleotidesa (5"→3") | ||
| DIGRSMZ1 | TGATGGCTGTGTCTATCCGTCGAC (5" DIG-labeled), 5"-specific probe for RsmZ | |
| DIGRSMZ2 | AGGGGCGGTATGACCCGCCCACATT (5" DIG-labeled), 3"-specific probe for RsmZ | |
| PRSMCHA02 | CGACACTGCAGTGATATTAGAGAGTTCCC, reverse rsmZ, PstI site at −2 | |
| PRSMCHA03 | TCAGAATTCTGTCGACGGATAGACACAG, forward rsmZ, EcoRI site at −6 | |
| PRSMCHA04 | AACTCGAGCGGGACTTTTCGACAGACG, reverse rsmZ, XhoI site at +147 | |
| PRSMCHA05 | ACGAATTCGGTTCTCGGCTACTTCTGCG, forward rsmZ, EcoRI site at −162 | |
| PRSMCHA06 | CATGAATTCTGAGAGTTAAGCCAACTTC, reverse rsmZ, EcoRI site at −196 | |
| PRSMCHA07 | CCACAAGCTTCGTGCAATAAAAAGCC, reverse rsmZ, HindIII site at −247 | |
| PRSMCHA08 | GCCCCTAAGCTTCGTCTGTCGAAAAGTCCCG, forward rsmZ, HindIII site at +122 | |
| RPOSCHA03 | CAAACTCGAGCACTTCTTTACTGAGAGCCATTG, reverse rpoS, XhoI site at the eighth codon | |
| TACSALI | CGCTGTCGACACCACACATTATACGAGCCGA, reverse Ptac, natural SalI site at +7 | |
| RSMA7 | TTAGTGATGGTGATGGTGATGGCTTGGTTCTTCGTCC, reverse rsmA, adding 5 supplementary histidine codons to RsmA to produce RsmA6H | |
| TETA1 | AACCCAAAGGAAAGGCGCTGTC, anneals between the EcoRV and SalI sites of tetA in pME6032, oriented 5" to 3" toward the multiple cloning site | |
| T3, T7 | pBLS primers | Stratagene |
Specified restriction sites are underlined.
DNA manipulations.
Small-scale plasmid extractions were done by the cetyltrimethylammonium bromide method (13), and large-scale preparations were done by using the Qiagen Plasmid midi kit (Qiagen Inc.). Chromosomal DNA from Pseudomonas spp. was prepared as previously described (17). RNA was extracted with the High Pure RNA isolation kit (Roche Diagnostics) according to the manufacturer's recommendations. DNA manipulations were carried out as described (50). DNA fragments were purified from agarose gels with the Geneclean II Kit (Bio101, La Jolla, Calif.). Restriction sites shown in brackets indicate treatment of DNA ends with T4 DNA polymerase in the presence of deoxynucleoside triphosphates (dNTPs). DNA sequencing was performed with the Big Dye Terminator Cycle sequencing kit and an ABI-Prism 373 automatic sequencer (Applied Biosystems).
PCRs were typically carried out with 2.5 U of thermostable DNA polymerase (Extra-Pol II; Eurobio) in a reaction mixture containing 100 ng of target DNA, a 250 μM concentration of each of the four dNTPs (Roche), 10 pmol of two primers, 5 mM MgCl2, and 1× Extra-Pol buffer in a final volume of 20 μl. For the amplification reaction, 25 cycles (1 min at 94°C, 1 min at 50 to 55°C [depending on the G+C content of the primers], and 1 min at 72°C) were followed by a final elongation step of 5 min at 72°C.
Southern and Northern blots.
DNA was blotted from agarose gels to Hybond-N nylon membranes (Amersham). RNA for Northern blots was electrophoretically separated on denaturing urea-polyacrylamide gels (8.3 M urea, 8% acrylamide, 0.4% bisacrylamide) in 1× TBE buffer (50 mM Tris-borate [pH 8.3], 1 mM EDTA). The lane corresponding to the molecular weight markers (low range RNA ladder; Fermentas) was cut out, stained with TBE containing 5 μg of ethidium bromide per ml for 5 min, and photographed under UV light beside a scale reference; the rest of the gel was electroblotted at 150 mA (15 to 25 V) onto a Hybond-N membrane in TBE buffer for 10 min. All membranes were washed with 20× SSC (50) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) before nucleic acids were cross-linked by exposing the membranes to UV for 5 min. Southern and Northern hybridizations were done with digoxigenin (DIG)-labeled DNA probes or DIG-labeled oligonucleotides (Table 1; obtained from Microsynth, Switzerland) according to recommended protocols (Roche's DIG System User's Guide for Filter Hybridization). Probes were produced by replacing dNTPs in a standard PCR with DIG DNA labeling mix (Roche) before purifying the product by phenol-chloroform extraction and ethanol precipitation.
Cloning and overexpression of rsmA from P. fluorescens CHA0.
The 311-bp BamHI-SmaI insert of pME3849 carrying the Pseudomonas aeruginosa rsmA gene was used as a probe to clone a hybridizing 1.2-kb PstI genomic fragment from P. fluorescens CHA0 into pBLS, resulting in pME6071. The 0.54-kb PstI-PvuII rsmA fragment of pME6071 was inserted into PstI- and PvuII-digested pME6001. This produced, in pME6072, a translational fusion between the N-terminal part of the LacZα" peptide from pME6001 and RsmA. To allow translation of rsmA from its natural initiation codon, pME6072 was digested with PstI and treated with T4 DNA polymerase prior to ligation. The resulting plasmid, pME6073, had a 4-bp deletion interrupting lacZα" translation with a stop codon 32 bp upstream of the start codon of rsmA.
Chromosome walking upstream of rsmA.
The PstI fragment of pME6071 was subcloned into the PstI-digested suicide plasmid pME3087 to produce pME6074. After integration of pME6074 into the chromosome of strain CHA0 by triparental mating using E. coli HB101/pME497 as the mobilizing strain (60), the integrated plasmid was rescued by digesting the chromosomal DNA with BamHI and ligation. The resulting plasmid, pME6075, had an additional 6.8-kb segment upstream of the initial 1.2-kb PstI rsmA fragment; the chromosomal insert was subcloned as an 8.0-kb BamHI-HindIII fragment into pBLS, resulting in pME6079. A 6.0-kb SacII deletion of this plasmid resulted in rsmA being bordered by 0.9 kb of upstream and 1.0 kb of downstream chromosomal DNA in pME6080. This 2.1-kb SacII-PstI fragment was sequenced (GenBank accession no. AF136151, Fig. 1).
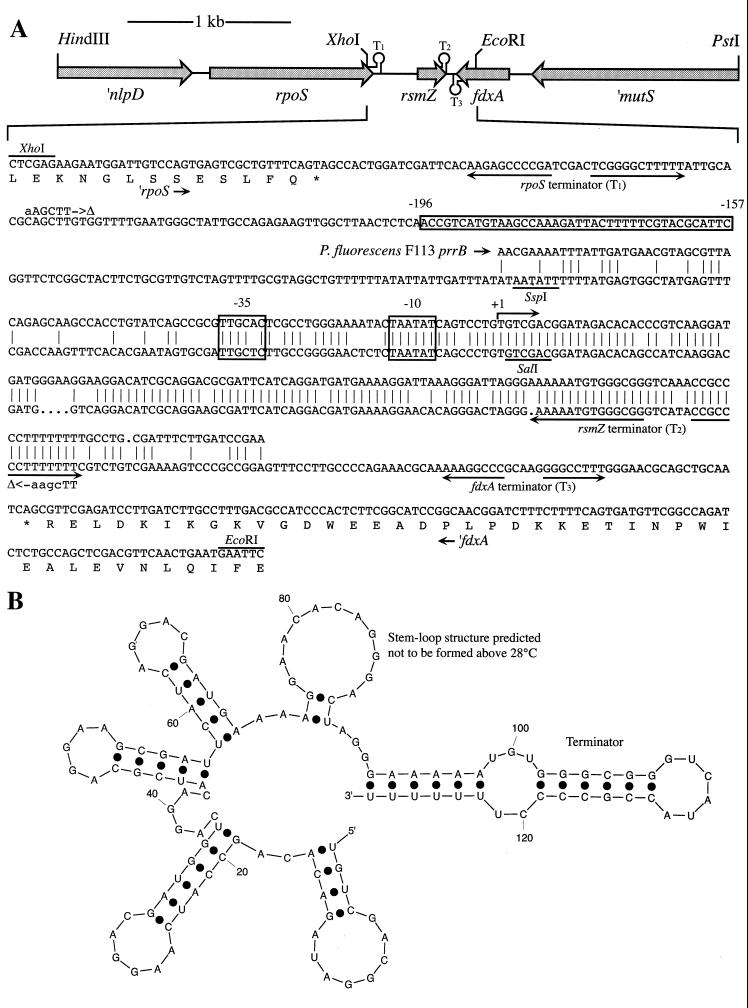
FIG. 1.
lysC-rsmA region in strain CHA0. ΩKmr, insertion in rsmA mutants CHA807, CHA808, and CHA809; T1 and T2, potential rho-independent terminators.
Chromosomal inactivation of rsmA.
The 2.0-kb ΩKmr kanamycin resistance cassette of pHP45ΩKm was inserted into the rsmA gene at the eighth codon, i.e., at the FspI site, resulting in the pME3088 derivative pME6081. rsmA::ΩKmr mutants were obtained by integration of the suicide plasmid pME6081 into the chromosome of strains CHA0, CHA19 (ΔgacS), and CHA806 (aprA"-"lacZ ΔgacS) after triparental mating as above, with selection for Kmr, tetracycline-resistant (Tcr), chloramphenicol-resistant (Cmr) recombinants. After a second crossing-over, Kmr Tcs recombinants were obtained, producing strains CHA809, CHA808, and CHA807, respectively (Table 1).
Purification of histidine-tagged RsmA and cDNA synthesis from its associated RNA.
RsmA was histidine-tagged by PCR using primers T3 and RSMA7 with pME6072 as the template. This was done by adding five histidine codons to the 3" end of rsmA (the wild-type protein already has a histidine residue at its C terminus). The purified PCR product was treated with T4 DNA polymerase to produce blunt ends, digested with PstI, and subcloned in the expression vector pME6032 (Table 1) to produce pME6078. Purification of the RsmA6H protein was performed as previously described (28): CHA0/pME6078 was grown in 200 ml of NYB at 30°C with shaking. When the optical density at 600 nm (OD600) of the culture reached 0.5, 1 mM isopropyl-β-d-thiogalactoside (IPTG) was added. The culture was further grown to an OD600 of ≈1.6. After centrifugation, RsmA6H was purified by Qiagen Ni-nitrilotriacetic acid (NTA)-agarose chromatography as recommended by the manufacturer (Qiagen QIAexpressionist handbook). The 50 mM potassium phosphate buffer, pH 8.0, containing 300 mM NaCl had the following imidazole concentrations: lysis, 10 mM; wash, 20 mM; and elution, 250 mM. The eluate from the Ni-NTA columns typically contained about 200 μg of protein, as determined by the method of Bradford (9), and about 120 μg of nucleic acids, as determined by absorption at 260 nm.
RNA was purified from the eluate by two extractions with phenol-chloroform (1:1) and once with chloroform. Salts were removed from the RNA with the High Pure RNA isolation kit (Roche) according to the manufacturer's manual. cDNA synthesis was performed as previously described (28) after polyadenylation of RNA with poly(A) polymerase (Gibco-BRL) and by using the Universal Riboclone cDNA synthesis system (Promega). The resulting blunt-ended cDNA fragments were subcloned into SmaI-digested, calf intestinal phosphatase-treated pBLS. In this way, pRSMCDNA7 and six other clones were obtained.
Cloning and overexpression of rsmZ from P. fluorescens CHA0.
A DIG probe was made from plasmid pRSMCDNA7 by PCR using oligonucleotides T3 and T7. Southern hybridization of chromosomal DNA of CHA0 digested with XhoI and PstI revealed a single 2.3-kb band, which was isolated and inserted into XhoI- and PstI-digested pBLS, producing pME6084. The fragment of interest (rsmZ) was located in pME6084 on a 673-bp XhoI-EcoRI fragment (see Fig. 3A), which was subcloned into pBLS, resulting in pME6085. A PCR product obtained from pME6085 with primers PRSMCHA03 and PRSMCHA04 was digested with EcoRI and XhoI and subcloned into EcoRI- and XhoI-cut pME6032, producing pME6096. In this plasmid, transcription from Ptac starts 37 nucleotides upstream from the EcoRI cutting site (3).
FIG. 3.
(A) The 4.1-kb region of P. fluorescens CHA0 with rpoS, rsmZ, and fdxA. The HindIII sites introduced to produce the rsmZ deletion mutant are indicated by Δ. The published prrB sequence of P. fluorescens F113 and its −35, −10, and +1 sites (1) are aligned above the rsmZ sequence. Rho-independent terminators T1, T2, and T3, predicted by the method of Brendel and Trifonov (10), are indicated by arrows beneath the sequence. The nucleotides deleted in the pME6093 reporter plasmid are shown in a box. (B) Predicted secondary structure of RsmZ using the Mfold program (63).
To make the RsmZ transcript exactly from the +1 transcription start of Ptac, a 41-bp deletion was created in pME6096 by PCR using oligonucleotides TETA1 and TACSALI. Primer TACSALI joins the −1 nucleotide of Ptac to the first seven nucleotides of rsmZ, six of which naturally form a SalI site (see Fig. 3A). Primer TETA1 is complementary to a region in the tetA tetracycline resistance marker in pME6096, annealing upstream of a SalI site. The resulting 3.4-kb product was digested with SalI and used to replace the corresponding 3.4-kb SalI fragment in pME6096, producing pME6359. Since this construct was deleterious to the growth of E. coli strains even when the tac promoter was not induced, the last subcloning step was done by transforming P. fluorescens CHA806, in which this construct did not have a detrimental effect. The Ptac-rsmZ fusion on pME6359 was checked by sequencing.
Construction of rsmZ-lacZ fusions.
Reporter plasmids were constructed carrying transcriptional rsmZ-lacZ fusions in which the +1 nucleotide of lacZ corresponds to the +1 nucleotide of the rsmZ promoter. First, the rsmZ promoter was amplified by PCR from pME6085 with primers T3 and PRSMCHA02. This fragment was digested with XhoI (located upstream of the rsmZ promoter) and PstI (artificially introduced by PRSMCHA02 in the +1 region) before being ligated to the PstI-ClaI lacZ" cassette of pME6522 and to ClaI- and SalI-digested vector pUC6S. The lacZ" fusion to the rsmZ promoter was verified by sequencing the resulting plasmid, pME6090. To complete the β-galactosidase open reading frame, the BamHI-ClaI rsmZ-lacZ" cassette from pME6090 was subcloned into BamHI- and ClaI-digested vector pME6016, resulting in plasmid pME6091. To delete nucleotides −196 to −157 of the rsmZ promoter, an inverse PCR was performed on pME6090 with primers PRSMCHA05 and PRSMCHA06 containing artificial EcoRI sites; the product was digested with EcoRI and ligated. The insert of the resulting plasmid, pME6092, was subcloned as a BamHI-ClaI fragment as above, producing pME6093.
To construct a transcriptional lacZ fusion at position −94 at the SspI site (see Fig. 3A), the XhoI-SspI 253-bp fragment of pME6084, the [PstI]-ClaI lacZ" cassette of pME6522, and pUC6S digested with ClaI and SalI were joined in pME6094. The BamHI-ClaI insert of pME6094 was then subcloned into pME6016 digested by the same enzymes to produce pME6095.
Chromosome walking upstream of rsmZ.
The XhoI-EcoRI (rsmZ) fragment of pME6084 was subcloned into XhoI- and EcoRI-digested pME3088 to produce pME6086. Plasmid rescue of pME6086 integrated in the CHA0 chromosome with HindIII resulted in the formation of plasmid pME6087, which had an additional 1.9 kb upstream of the initial 673-bp XhoI-EcoRI fragment. The HindIII-XhoI fragment carrying rpoS" of pME6087 was sequenced together with the contiguous 2.3-kb XhoI-PstI insert of pME6084 (GenBank accession number AF245440; see Fig. 3A)
Chromosomal deletion of rsmZ.
After inverse PCR on pME6085 with primers PRSMCHA06 and PRSMCHA07, the product was digested with HindIII (sites generated by the primers) and ligated. In the resulting plasmid, pME6097, 374 bp were replaced by a HindIII site, producing a deletion spanning the rsmZ promoter as well as the whole rsmZ gene but leaving intact the flanking rpoS and fdxA transcription terminators (see Fig. 3A). To produce the suicide plasmid for gene replacement, pME6099, a triple ligation was performed between pME3087 digested with PstI and HindIII, the HindIII-XhoI insert of pME6087, and the XhoI-PstI insert of pME6097. The rsmZ-negative mutants CHA810, CHA811, and CHA812 were obtained by gene replacement in strains CHA0, CHA207, and CHA805, respectively, as above for inactivation of rsmA; the introduced HindIII site in rsmZ allowed detection of the mutation by Southern blot with an XhoI-EcoRI rsmZ probe.
In-frame deletion of rpoS.
PCR with oligonucleotides RPOSCHA03 and T7 was done to amplify a 0.96-kb rpoS" fragment from pME6087. RPOSCHA03 creates an artificial XhoI site at the eighth codon of rpoS. The PCR product was digested with HindIII and XhoI and combined with the XhoI-EcoRI "rpoS-rsmZ fragment of pME6084 in suicide plasmid pME3088, resulting in an rpoS in-frame deletion of 315 codons (ΔrpoS) out of 336 in pME6352. Gene replacement of rpoS by ΔrpoS was done by double crossing-over of pME6352 in strains CHA805, CHA207, and CHA0, producing strains CHA813, CHA814, and CHA815, respectively, and verified by Southern hybridization.
Extraction of signal from cell-free culture supernatants.
Strain CHA0 was grown in 500 ml of glycerol-Casamino Acids medium (52) in a 2-liter Erlenmeyer flask for 24 h with shaking to an OD600 of 2.0 to 2.5. Cells were removed by centrifugation. The supernatant was passed through a 0.45-μm-pore-size filter, adjusted to pH 5.0, and extracted once with 200 ml and twice with 150 ml of dichloromethane. The extracts were pooled, dried with anhydrous Na2SO4, filtered through Whatman paper, evaporated to dryness, and stocked at −20°C. Extracts dissolved in 500 μl of methanol were added at 2 μl per ml of culture (see Fig. 7).
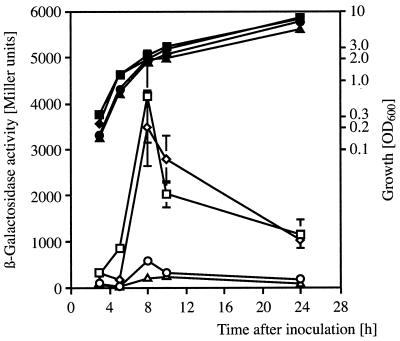
FIG. 7.
Regulation of rsmZ expression and stimulation by a signal from spent medium. β-Galactosidase activities of a transcriptional rsmZ-lacZ fusion carried by pME6091 were determined in strain CHA0 (◊, ⧫), in strain CHA0 amended with extract from spent medium (□, ▪), and in the gacA mutant CHA89 (○, •). Open symbols, β-galactosidase activity; solid symbols, OD600. Each value is the average from three different cultures ± standard deviation.
Qualitative assays of protease, tryptophan side chain oxidase, and HCN.
Proteolytic activity was detected on King's medium B agar (2% [wt/vol] Oxoid proteose peptone, 1% [vol/vol] glycerol, 6 mM MgSO4, 6 mM K2HPO4, 1.5% [wt/vol] Oxoid agar No. 1) supplemented with 0.5% Blue Gelaspher, obtained from Ivo Safarik (49). HCN production was detected as previously described (59). Tryptophan side chain oxidase was assayed as described (38).
β-Galactosidase assays.
β-Galactosidase activities were quantified by the Miller method (35), using cells permeabilized with 5% toluene.
RESULTS
Characterization of the chromosome region containing rsmA in P. fluorescens CHA0.
Previously we briefly reported the isolation of the rsmA gene from P. fluorescens CHA0 (8). When expressed from the multicopy plasmid pME6073 (8), this gene phenotypically complemented an E. coli csrA::Kmr mutant (46) for repression of glycogen synthesis (data not shown), demonstrating functional conservation of RsmA and CsrA. Using a genome walking approach (described in Materials and Methods), we found that the rsmA gene was flanked upstream by lysC (aspartokinase) and downstream by serV (seryl-tRNA, anticodon GCT) and argVα and argVβ (two arginine-tRNAs, anticodons ACG) (Fig. 1). In P. aeruginosa, the genetic neighborhood is similar (42, 54), whereas in E. coli, csrA is located upstream of the same tRNA genes, but downstream of alaS (46).
Role of RsmA in the expression of GacS/GacA-regulated genes.
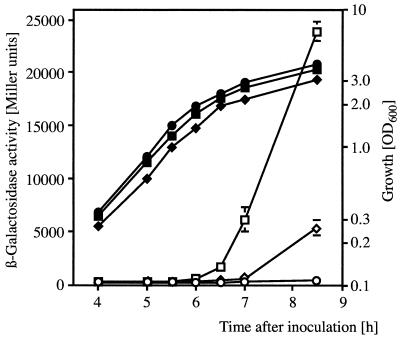
A kinetic experiment (Fig. 2) illustrates that the cell density-dependent expression of a chromosomal hcnA"-"lacZ fusion in strain CHA207 was repressed about sixfold by overexpressed rsmA. Expression of the exoprotease gene aprA in strain CHA805 (also measured by a translational lacZ fusion) paralleled that of the hcnA gene (Fig. 2), establishing a repressive effect of RsmA throughout the growth cycle. By comparison, inactivation of either gacS or gacA reduces the expression of hcnA and aprA more strongly, about 50- to 100-fold (8). When the chromosomal rsmA gene was inactivated by insertion of an ΩKm cassette (Fig. 1) in a ΔgacS background (CHA807), the effect of the gacS mutation on a chromosomal aprA"-"lacZ reporter fusion was suppressed to about 30% during the late exponential phase (corresponding to OD600 of 2.0 to 2.5) (8). Furthermore, a ΔgacS rsmA double mutant (CHA808) produced significant amounts of exoprotease, HCN, and tryptophan side chain oxidase by comparison with strain CHA19 (ΔgacS), which was negative for these phenotypes (data not shown). These results extend and corroborate the role of RsmA as a global negative control element acting downstream of GacA (8).
FIG. 2.
Regulation of aprA"-"lacZ and hcnA"-"lacZ fusions in strains overexpressing the translational repressor RsmA from pME6073 or harboring the vector pME6001 alone in NYB. □, ▪, CHA207 (hcnA"-"lacZ)/pME6001; ◊, ⧫, CHA805 (aprA"-"lacZ)/pME6001; ○, •, CHA207/pME6073; ▵, ▴, CHA805/pME6073. Open symbols, β-galactosidase activity; solid symbols, OD600. Each value is the average from three different cultures ± standard deviation.
Characterization of an RsmA-associated RNA.
To characterize RNAs that interact with RsmA in P. fluorescens CHA0, this protein was histidine tagged (RsmA6H) and purified by affinity chromatography. Following affinity chromatography, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of the eluate revealed that most (about 90%) of the protein was represented by a band of about 7.0 kDa, corresponding to the expected size of RsmA6H (data not shown). RsmA6H was biologically active in vivo; strain CHA805 (aprA"-"lacZ) containing pME6078 (lacIq Ptac-rsmA6H) formed blue colonies on X-Gal plates, whereas on the same plates amended with 1 mM IPTG, the colonies were white to light blue. Nucleic acids separated from protein by phenol-chloroform extraction could be detected by ethidium bromide staining after agarose gel electrophoresis and were resistant to a 15-min treatment with RNase-free DNase at 37°C, but were completely digested by an equivalent treatment with DNase-free RNase (data not shown), suggesting that most of the nucleic acids associated with RsmA6H were composed of RNA.
cDNA was synthesized from this RNA and cloned into pBLS, as described in Materials and Methods. A clone having an insert of 90 bp (including a 10-bp stretch of adenines at the 3" end) showed 77% identity to the regulatory RNA PrrB recently described in P. fluorescens F113 (1). Southern hybridization of the chromosome of strain CHA0 with a DIG probe made from this insert revealed a single XhoI-PstI band of about 2.3 kb. This band was subsequently subcloned in pME6084. In this construct, the fragment of interest was located on a 679-bp XhoI-EcoRI fragment (Fig. 3A). Nucleotides 1 to 176 (starting from the XhoI side) show 100% identity with the 3" end of the P. fluorescens Pf-5 rpoS gene (GenBank accession no. U34203) (51), and nucleotides 554 to 679 encode a polypeptide showing 85% identity and 93% similarity with the 41 C-terminal residues of the Azotobacter vinelandii fdxA gene product (GenBank accession no. J03521 and M63007) (26, 36). The rpoS-fdxA intergenic region shows 72% identity over 248 nucleotides with the prrB locus of P. fluorescens F113 (Fig. 3A). We conclude that the RNA bound to RsmA protein in P. fluorescens CHA0 is encoded by a gene which is related to prrB and which we designate rsmZ, to highlight the substantial sequence divergence between the two loci and the affinity of RsmZ RNA for RsmA protein.
No significant sequence similarity could be found between rsmZ and rsmB of various Erwinia strains or csrB of E. coli and S. enterica. Secondary-structure predictions, however, do reveal common features of RsmZ of strain CHA0 (Fig. 3B), PrrB of strain F113 (1), RsmB of E. carotovora (30), CsrB of E. coli (28), and CsrB of S. enterica (2). All these regulatory RNAs have several stem-loop structures, with AGG(G)A motifs in the loops. An alignment of the mapped prrB promoter with the corresponding rsmZ sequence shows sufficient similarity to predict the rsmZ transcription start (+1) site (Fig. 3A). A potential rho-independent transcription terminator (T1; Fig. 3A) is located downstream of the rpoS gene of P. fluorescens CHA0, analogous to the situation in P. aeruginosa PAO (56). A second terminator (T2) appears to be an integral part of the rsmZ transcript (see below); another terminator (T3) follows the fdxA gene (Fig. 3A).
Evidence for two RsmZ transcripts.
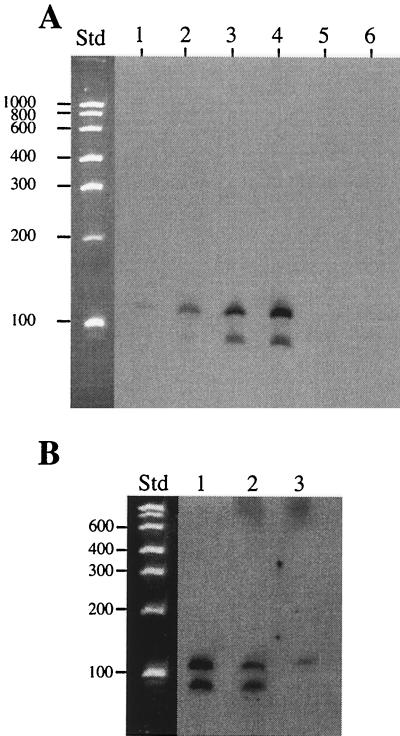
Northern blot analysis of RNA extracted from strain CHA0 at various cell densities was done with a DIG-labeled probe hybridizing to the full-length rsmZ. Two bands of about 90 and about 130 nucleotides were revealed (Fig. 4A). The intensities of both RsmZ bands increased with increasing cell densities (Fig. 4A); the highest amount was found at an OD600 of 3.4, corresponding to the transition from exponential to stationary phase (Fig. 2). No signal was detected in RNA preparations from either an rsmZ deletion mutant (used as a control) or the gacA mutant CHA89 (Fig. 4A).
FIG. 4.
(A) Northern blot of RsmZ. Total RNA was extracted from strains CHA0 (lanes 1 to 4), CHA810 (rsmZ) (lane 5), and CHA89 (gacA) (lane 6) grown in NYB and hybridized with a DIG-labeled probe covering the full-length rsmZ transcript. In each lane, 3 μg of RNA was loaded. OD600 values at the time of harvesting were, in lanes 1 to 6, 0.5, 1.0, 1.5, 3.4, 2.4, and 2.6, respectively. (B) Northern blot of total RNA extracted from strain CHA0 at an OD600 of 3.4 was hybridized with the same probe as in panel A (lane 1), with the DIG-labeled oligonucleotide DIGRSMZ1, specific for the 5" end of the transcript (lane 2), or with the DIGRSMZ2 probe, specific for the 3" end (lane 3). X-ray film exposure for chemiluminescent detection was 2 min for lane 1 and 40 min for lanes 2 and 3. Std, RNA standards; sizes are shown in bases.
To answer the question of whether the two bands both originated from the same gene (rsmZ), Northern hybridizations were performed with three different probes: one covering the whole RsmZ transcript, and two oligonucleotides hybridizing to the 5" or the 3" end of the transcript. The probe specific for the 5" end gave the same signals as did the full-length probe, while the probe specific for the 3" end hybridized only to the larger band (Fig. 4B). This result and sequence analysis suggest that RsmZ is present in the cells as a 127-nucleotide RNA, together with a 92-nucleotide molecule lacking the last 35 nucleotides of the transcript, i.e., the part corresponding to the T2 rho-independent terminator (Fig. 3B). RsmZ may be first transcribed as a single RNA molecule together with its terminator, and then this terminator may be truncated at the 3" end by a processing mechanism. Alternatively, transcription of rsmZ may stop at two sites lying 35 nucleotides apart.
Overexpression of rsmZ suppresses gacA and gacS mutations.
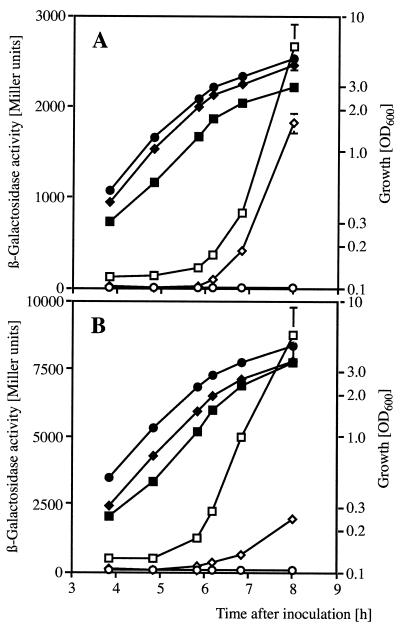
The rsmZ gene was placed under the control of the inducible tac promoter in pME6359, so that the +1 site of the promoter coincided with the first rsmZ nucleotide. This construct, which was deleterious to E. coli strains such as DH5α and JM105, was obtained in P. fluorescens. In strain CHA806 (ΔgacS aprA"-"lacZ), pME6359 had no significant effect on growth, although some slight aggregation of cells on glass walls was observed. In this strain, even without IPTG induction, β-galactosidase activities were similar to those in the corresponding wild-type strain CHA805 (gacS+ aprA"-"lacZ), indicating that excess RsmZ can restore aprA"-"lacZ expression in a gacS mutant to wild-type levels (Fig. 5A). When pME6359 was introduced into strain CHA89.207 (gacA hcnA"-"lacZ), β-galactosidase activities were much higher and occurred earlier than in the gacA+ strain CHA207 carrying the same chromosomal reporter (Fig. 5B). Thus, a surplus of RsmZ can elevate hcnA"-"lacZ expression in a gacA mutant to levels above those present in the wild type.
FIG. 5.
Suppression of gacS and gacA mutations by overexpressed RsmZ. (A) Expression of a chromosomal aprA"-"lacZ translational fusion and growth were determined in a wild-type context (◊, ⧫, CHA805/pME6032), in a gacS mutant (○, •, CHA806/pME6032), and in a gacS mutant overexpressing rsmZ (□, ▪, CHA806/pME6359). (B) Expression of a chromosomal hcnA"-"lacZ translational fusion and growth in a wild-type context (◊, ⧫, CHA207/pME6032), in a gacA mutant (○, •, CHA89.207/pME6032), and in a gacA mutant overexpressing rsmZ (□, ▪, CHA89.207/pME6359). Open symbols, β-galactosidase; solid symbols, OD600. Each value is the average from three different cultures ± standard deviation.
Expression of rsmZ depends on GacS/GacA but not on RpoS.
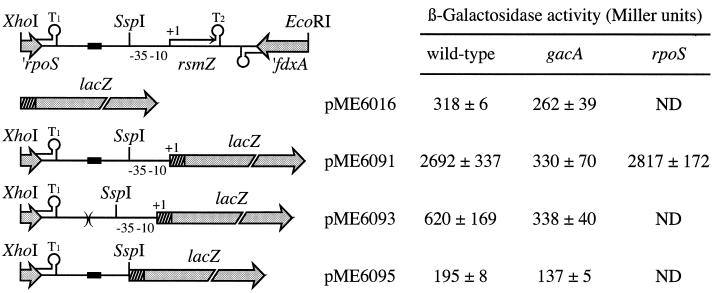
Results obtained by Northern hybridization (Fig. 4) suggest that rsmZ transcription depends on GacA and the growth phase. To quantify the regulation by GacA, the lacZ gene was placed directly under the control of the rsmZ promoter in plasmid pME6091 (Fig. 6). The resulting rsmZ-lacZ fusion was controlled by GacA, with an induction factor of about 35, after subtraction of the background activity of the vector control (pME6016; Fig. 6). The vector used in this experiment has about 5 to 7 copies (20). A transcriptional lacZ fusion constructed at −90 upstream of the transcription start was devoid of activity (pME6095; Fig. 6), indicating that there is no readthrough transcription from the upstream rpoS gene. The rpoS gene itself was not required for rsmZ-lacZ expression, in that an rpoS in-frame deletion had no effect on this reporter system (Fig. 6). The β-galactosidase activities resulting from chromosomal aprA"-"lacZ and hcnA"-"lacZ fusions were 20 to 30% higher in a ΔrpoS mutant (CHA813 and CHA814, respectively) than in the wild-type background grown to early stationary phase (data not shown), suggesting that the effects of RpoS on aprA and hcnA expression are not mediated by modulation of RsmZ expression.
FIG. 6.
Regulation of rsmZ expression. β-Galactosidase activities of transcriptional rsmZ-lacZ fusions containing the full promoter (pME6091), the promoter with the conserved upstream sequence (black box) deleted (pME6093), or no promoter (pME6095) in strains CHA0 (wild type), CHA89 (gacA), and CHA815 (rpoS). As a background control, the reporter vector pME6016 carrying the lacZ gene with its ribosome-binding site (dashed box) was used. ND, not determined. Each value is the average ± standard deviation from three different overnight cultures grown to an OD600 of ≈3.0
Examination of the rsmZ promoter region reveals an upstream sequence (positions −192 to −161; Fig. 3A) which is remarkably well conserved (≥53% identity) in P. aeruginosa, Pseudomonas syringae pv. phaseolicola, P. syringae pv. syringae, and two strains of Pseudomonas putida (see Discussion). To investigate the role of this conserved sequence in the expression of rsmZ of P. fluorescens CHA0, pME6093 was constructed, carrying an rsmZ-lacZ fusion in which nucleotides −196 to −157 upstream of the promoter were deleted (Fig. 3A). This deletion caused a marked reduction in the expression of the reporter fusion and completely abolished the control by GacA (Fig. 6). This suggests that either GacA itself or a regulatory element controlled by GacA interacts with this upstream sequence, to activate the rsmZ promoter.
Evidence for quorum-sensing control of rsmZ expression.
The rsmZ gene was expressed most strongly at the end of exponential growth, at cell densities above 2 × 109 cells/ml (OD600 ≈ 2.0). This was seen in Northern blots (Fig. 4A) as well as with an rsmZ-lacZ fusion (Fig. 7). This regulatory pattern could be the consequence of some type of quorum sensing (cell density-dependent) control involving a diffusible signal. Therefore, we searched for signal activity in cultures of the wild-type CHA0 grown to early stationary phase in NYB or rich glycerol-Casamino Acids medium. A dichloromethane extract from CHA0 culture supernatants was found to advance and to stimulate markedly the expression of the rsmZ gene (Fig. 7). Uninoculated medium did not contain the signal, and little inducing activity was found in exponentially growing cultures.
Signal activity was not affected by treatment of culture supernatants at pH 12 and 30°C for 30 min. Under these conditions, N-acyl-homoserine lactones (AHLs), which are typical cell density-related signal molecules in a range of gram-negative bacteria (55), are inactivated. Moreover, the hcnA"-"lacZ reporter strain CHA207 was not induced by addition of various AHL compounds at concentrations of 1 to 70 μM (data not shown). Furthermore, two reporter systems that detect a wide spectrum of AHLs, Chromobacterium violaceum CV026 (34) and Agrobacterium tumefaciens with a traG-lacZ fusion (53), failed to show any reaction with extracts from CHA0 culture supernatants. By contrast, when the P. aeruginosa rhlI gene encoding N-butanoyl-homoserine lactone synthase is expressed in strain CHA0, the corresponding AHL product is readily detectable (40), ruling out the possibility that some CHA0 exoproducts might mask intrinsic AHL activity. Together, these results suggest that the inducing signal of strain CHA0 is not of the AHL type. Furthermore, the apparent absence of luxS genes from pseudomonads argues against the possibility that the signal molecule of strain CHA0 could be related to autoinducer 2 (AI-2) of Vibrio harveyi and other bacteria (55).
Effect of an rsmZ null mutation on aprA and hcnA expression.
Since rsmZ expression is controlled by GacA (Fig. 4A) and since RsmZ RNA can bind RsmA, an rsmZ null mutation should lower the expression of target genes such as aprA and hcnA. Expression of aprA"-"lacZ and hcnA"-"lacZ is maximal during late exponential phase, corresponding to an OD600 of 2.0 to 2.5 (Fig. 2). However, during this phase, GacA-dependent expression of rsmZ is weak (Fig. 4A) unless the inducing signal is added (Fig. 7). With these considerations in mind, we deleted the chromosomal rsmZ gene in strain CHA810, resulting in the complete absence of RsmZ RNA (Fig. 4A). In strains carrying either a chromosomal aprA"-"lacZ fusion (CHA812) or an hcnA"-"lacZ fusion (CHA811), deletion of rsmZ had a very slight effect on the expression of these reporters at an OD600 of ≥2.0 (Fig. 8). However, when the RsmZ inducing signal was added, a clear difference of about 20% was observed between the ΔrsmZ mutant and the rsmZ+ parent at an OD of ≥2.0 (Fig. 8). These results suggest that, under inducing conditions, RsmZ can alleviate RsmA-mediated posttranscriptional repression of the two target genes.
FIG. 8.
Effect of an rsmZ deletion on expression of the aprA and hcnA genes. (A) β-Galactosidase activities of a chromosomal aprA"-"lacZ translational fusion and growth were measured in the rsmZ+ strain CHA805 without (◊, ⧫) and with extract (○, •) and in the rsmZ mutant CHA812 without (□, ▪) and with extract (▵, ▴). (B) β-Galactosidase activities of a chromosomal hcnA"-"lacZ translational fusion and growth were determined in the rsmZ+ strain CHA207 without (◊, ⧫) and with extract (○, •) and in the rsmZ mutant CHA811 without (□, ▪) and with extract (▵, ▴). Open symbols, β-galactosidase; solid symbols, OD600. Each value is the average from three different cultures ± standard deviation.
DISCUSSION
In many gram-negative bacteria, the GacS/GacA two-component system has important regulatory functions in the synthesis of extracellular products and in the adaptation to diverse environmental conditions. In animal and plant pathogens, the GacS/GacA system controls the expression of virulence factors (2, 12, 20a, 22, 39). In plant-beneficial, root-colonizing bacteria such as P. fluorescens CHA0 and Pf-5, the GacS/GacA system is required for the production of antifungal secondary metabolites and exoenzymes (1, 25, 48, 52, 58, 62). The structural genes for HCN biosynthesis (hcnABC) and exoprotease (aprA) have proved useful for studying the regulatory cascade initiated by the GacA response regulator in P. fluorescens CHA0.
We have previously obtained genetic evidence that GacA indirectly regulates the expression of these structural genes at a posttranscriptional level, involving the RNA-binding protein RsmA as a downstream regulatory element (5-8). In the present study, we have characterized a regulatory RNA termed RsmZ, which was found to bind to RsmA and, when overproduced, caused strong derepression of aprA and hcnA expression. By analogy with the RsmA(CsrA)/RsmB(CsrB) system in enteric bacteria, this derepression is thought to result from sequestration of RsmA (12, 18, 30, 45). While this work was in progress, a gacS/gacA multicopy suppressor, PrrB, was reported in P. fluorescens F113 (1). PrrB RNA has 91% sequence identity with RsmZ of P. fluorescens CHA0 (Fig. 3A).
There are several arguments for RsmZ being part of the GacA regulon. (i) Expression of rsmZ strongly depended on gacA (Fig. 4A and 6). (ii) Overexpression of rsmZ suppressed gacS and gacA mutations by >100% (Fig. 5). (iii) Expression of rsmZ was stimulated markedly by a solvent-extractable signal produced by the bacteria at high cell densities (Fig. 7). This non-AHL signal is produced under GacS/GacA control and requires a functional GacS/GacA system to exert its positive effect on secondary metabolism in strain CHA0 (our unpublished results). (iv) In the presence of added signal, an rsmZ-negative mutant showed reduced expression of the hcnA and aprA genes (Fig. 8).
These findings lend support to a model (8, 20a) whose validity has recently also been assessed in E. carotovora (12, 22). The GacS/GacA system upregulates the synthesis of the regulatory RNA RsmZ during late exponential phase; in strain CHA0 this occurs under the influence of a diffusible bacterial signal. RsmZ then binds to and titrates RsmA, preventing this protein from acting as a translational repressor and/or mRNA decay factor (45). The sigma factor RpoS does not seem to upregulate rsmZ expression (Fig. 6). Similarly, RpoS does not activate transcription of CsrB in E. coli (18). Our simplified linear model explains the facts observed in this study, but may have to be refined later for the following reasons. First, an rsmA mutation suppressed a gacS defect only to 30 to 60%, depending on the reporter construct used (8), and added signal retained a weak inducing effect in an rsmA mutant (data not shown). Therefore, it is likely that additional negative control elements exist in the GacA regulon. Second, an rsmZ mutation caused a relatively small loss of hcnA and aprA expression. Similarly, mutational inactivation of prrB in strain F113 reduces the synthesis of 2,4-diacetylphloroglucinol but has no detectable effect on HCN and exoprotease production (1), and in S. enterica csrB is not required for the expression of invasion genes but has a dramatic effect when overproduced (2).
It seems possible that RsmZ might be only one among several regulatory RNAs. Indeed, recent studies on E. coli have uncovered at least 10 new regulatory RNAs whose abundance increases towards the end of exponential growth (4, 61). Similar RNAs may exist in pseudomonads as well, and such RNAs may be elements of the GacA regulon. Our preliminary unpublished results suggest that strain CHA0 contains at least one additional RNA which copurifies with RsmA and is positively regulated by GacA. Third, the mRNA target specificity has been addressed only in the case of the hcnA 5" untranslated leader sequence, where a stretch of about 10 nucleotides surrounding the ribosome-binding site determines regulation by GacA and RsmA (8). Although a similar sequence motif can be found in the aprA leader (8), not all GacA-controlled genes appear to have this motif. Additional specificity determinants are likely to exist in the GacA regulon.
The gene organization rpoS-rsmZ-fdxA-mutS found in P. fluorescens CHA0 (Fig. 3A) is maintained in P. aeruginosa PAO (GenBank accession no. AE004782 and D26134) (15), P. syringae pv. syringae B728a (GenBank AF208000), and P. putida strains WCS358 (GenBank Y19122) (23) and Corvallis (GenBank AF178851), but not in enteric bacteria such as E. coli or Salmonella spp., in which the rpoS-mutS intergenic region is highly variable in composition and size and does not include csrB (21, 27). In Azotobacter vinelandii, fdxA is also located downstream of mutS (GenBank M63007) (26), but the genes lying further downstream are not yet known. An alignment of the rpoS-rsmZ intergenic region in fluorescent pseudomonads shows very little sequence conservation, with one exception, the rsmZ upstream box (−196 to −157; Fig. 3A). This box contains some conserved motifs (notably CNTGTAAG) which also occur upstream of the TRR locus in P. syringae pv. phaseolicola (47). TRR overexpression overrides thermoregulation of phaseolotoxin synthesis in this bacterium (47) by an unknown mechanism. It is conceivable that the TRR product is a regulatory RNA. The conserved box upstream of rsmZ in P. fluorescens CHA0 is essential for rsmZ expression and regulation by GacA (Fig. 6).
Regulatory RNAs interacting with RsmA in various bacteria appear to lack conservation of size and primary sequence, which makes detection of the corresponding genes difficult in genomic sequence databases. For example, in Enterobacteriaceae, RsmB and CsrB primary transcripts are relatively large (260 to 480 nucleotides), whereas PrrB and RsmZ of fluorescent pseudomonads are much smaller (about 130 nucleotides). Perhaps a combination of characteristic secondary structures—multiple stems—with AG(G)GAs motif in loops (1, 29, 31) accounts for the interaction of these regulatory RNAs with RsmA.
In some gram-negative bacteria, e.g., E. carotovora and P. aeruginosa, GacA positively controls the production of AHLs. By influencing the concentrations of these quorum-sensing signals, GacA can modulate the expression of exoproduct genes (11, 41, 44). However, in the same organisms, GacA control of exoproduct genes also follows an AHL-independent signal transduction pathway (11, 41). Such an AHL-independent GacA cascade appears to be responsible for the regulation of exoproducts in P. fluorescens CHA0. This strain's non-AHL signal stimulates the expression of rsmZ (Fig. 7 and 8) as well as that of the structural genes aprA, hcnA, and phlA, but does not influence housekeeping gene expression (S. Heeb, C. Gigot-Bonnefoy, M. Péchy, C. Reimmann, and D. Haas, unpublished results). The chemical nature of this signal and its mode of action are currently under investigation.
Acknowledgments
We thank Tony Romeo for the E. coli mutant strains, Ursula Schnider-Keel for the construction of some vectors used in this study, Fiona Carruthers for the construction of a gacS mutant of strain CHA0, Maria Péchy for the preparation of culture supernatant extracts, and Ivo Safarik for his kind gift of Blue Gelaspher.
Financial support was provided by the Swiss National Foundation for Scientific Research (project 31-56608.99) and the EU project QLK3-2000-31759 (ECO-SAFE).
REFERENCES
- 1.Aarons, S., A. Abbas, C. Adams, A. Fenton, and F. O'Gara. 2000. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 182:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altier, C., M. Suyemoto, A. I. Ruiz, K. D. Burnham, and R. Maurer. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 35:635-646. [DOI] [PubMed] [Google Scholar]
- 3.Amann, E., J. Brosius, and M. Ptashne. 1983. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene 25:167-178. [DOI] [PubMed] [Google Scholar]
- 4.Argaman, L., R. Hershberg, J. Vogel, G. Bejerano, E. G. H. Wagner, H. Margalit, and S. Altuvia. 2001. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 11:941-950. [DOI] [PubMed] [Google Scholar]
- 5.Blumer, C., and D. Haas. 2000. Iron regulation of the hcnABC genes encoding hydrogen cyanide synthase depends on the anaerobic regulator ANR rather than on the global activator GacA in Pseudomonas fluorescens CHA0. Microbiology 146:2417-2424. [DOI] [PubMed] [Google Scholar]
- 6.Blumer, C., and D. Haas. 2000. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol. 173:170-177. [DOI] [PubMed] [Google Scholar]
- 7.Blumer, C., and D. Haas. 2000. Multicopy suppression of a gacA mutation by the infC operon in Pseudomonas fluorescens CHA0: competition with the global translational regulator RsmA. FEMS Microbiol. Lett. 187:53-58. [DOI] [PubMed] [Google Scholar]
- 8.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Brendel, V., and E. N. Trifonov. 1984. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 12:4411-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatterjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, Y., A. Chatterjee, and A. K. Chatterjee. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and Harpin(Ecc). Mol. Plant-Microbe Interact. 14:516-526. [DOI] [PubMed] [Google Scholar]
- 13.Del Sal, G., G. Manfioletti, and C. Schneider. 1988. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 16:9878.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 15.Fujita, M., K. Tanaka, H. Takahashi, and A. Amemura. 1994. Transcription of the principal sigma-factor genes, rpoD and rpoS, in Pseudomonas aeruginosa is controlled according to the growth phase. Mol. Microbiol. 13:1071-1077. [DOI] [PubMed] [Google Scholar]
- 16.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 17.Gamper, M., B. Ganter, M. R. Polito, and D. Haas. 1992. RNA processing modulates the expression of the arcDABC operon in Pseudomonas aeruginosa. J. Mol. Biol. 226:943-957. [DOI] [PubMed] [Google Scholar]
- 18.Gudapaty, S., K. Suzuki, X. Wang, P. Babitzke, and T. Romeo. 2001. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherichia coli. J. Bacteriol. 183:6017-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas, D., C. Blumer, and C. Keel. 2000. Biocontrol ability of fluorescent pseudomonads genetically dissected: importance of positive feedback regulation. Curr. Opin. Biotechnol. 11:290-297. [DOI] [PubMed] [Google Scholar]
- 20.Heeb, S., Y. Itoh, T. Nishijyo, U. Schnider, C. Keel, J. Wade, U. Walsh, F. O'Gara, and D. Haas. 2000. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in Gram-negative, plant-associated bacteria. Mol. Plant-Microbe Interact. 13:232-237. [DOI] [PubMed] [Google Scholar]
- R20a.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other Gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 21.Herbelin, C. J., S. C. Chirillo, K. A. Melnick, and T. S. Whittam. 2000. Gene conservation and loss in the mutS-rpoS genomic region of pathogenic Escherichia coli. J. Bacteriol. 182:5381-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyytiäinen, H., M. Montesano, and E. T. Palva. 2001. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme gene expression through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 14:931-938. [DOI] [PubMed] [Google Scholar]
- 23.Kojic, M., G. Degrassi, and V. Venturi. 1999. Cloning and characterisation of the rpoS gene from plant growth-promoting Pseudomonas putida WCS358: RpoS is not involved in siderophore and homoserine lactone production. Biochim. Biophys. Acta 1489:413-420. [DOI] [PubMed] [Google Scholar]
- 24.Laville, J., C. Blumer, C. Von Schroetter, V. Gaia, G. Défago, C. Keel, and D. Haas. 1998. Characterization of the hcnABC gene cluster encoding hydrogen cyanide synthase and anaerobic regulation by ANR in the strictly aerobic biocontrol agent Pseudomonas fluorescens CHA0. J. Bacteriol. 180:3187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laville, J., C. Voisard, C. Keel, M. Maurhofer, G. Défago, and D. Haas. 1992. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc. Natl. Acad. Sci. USA 89:1562-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le, O., B. Shen, S. E. Iismaa, and B. K. Burgess. 1993. Azotobacter vinelandii mutS: nucleotide sequence and mutant analysis. J. Bacteriol. 175:7707-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeClerc, J. E., B. Li, W. L. Payne, and T. A. Cebula. 1999. Promiscuous origin of a chimeric sequence in the Escherichia coli O157:H7 genome. J. Bacteriol. 181:7614-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, M. Y., G. Gui, B. Wei, J. F. Preston III, L. Oakford, U. Yuksel, D. P. Giedroc, and T. Romeo. 1997. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272:17502-17510. [DOI] [PubMed] [Google Scholar]
- 29.Liu, M. Y., and T. Romeo. 1997. The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J. Bacteriol. 179:4639-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219-234. [DOI] [PubMed] [Google Scholar]
- 31.Ma, W. L., Y. Cui, Y. Liu, C. K. Dumenyo, A. Mukherjee, and A. K. Chatterjee. 2001. Molecular characterization of global regulatory RNA species that control pathogenicity factors in Erwinia amylovora and Erwinia herbicola pv. gypsophilae. J. Bacteriol. 183:1870-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurhofer, M., C. Hase, P. Meuwly, J. P. Metraux, and G. Défago. 1994. Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0—influence of the gacA gene and of pyoverdine production. Phytopathology 84:139-146. [Google Scholar]
- 33.Maurhofer, M., C. Reimmann, P. Schmidli-Sacherer, S. Heeb, D. Haas, and G. Défago. 1998. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology 88:678-684. [DOI] [PubMed] [Google Scholar]
- 34.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Morgan, T. V., D. J. Lundell, and B. K. Burgess. 1988. Azotobacter vinelandii ferredoxin I: cloning, sequencing, and mutant analysis. J. Biol. Chem. 263:1370-1375. [PubMed] [Google Scholar]
- 37.Oberhänsli, T., and G. Défago. 1991. Spontaneous loss of tryptophan side chain oxidase of Pseudomonas fluorescens strain CHA0—a marker for genetic instability, p. 392-398. In C. Keel, B. Koller, and G. Défago (ed.), Plant growth-promoting rhizobacteria—progress and prospects. International Organisation for Biological and Integrated Control/West Palaerarctic Regional Section (IOBC/WPRS) Bulletin XIV/8.
- 38.Oberhänsli, T., G. Défago, and D. Haas. 1991. Indole-3-acetic acid (IAA) synthesis in the biocontrol strain CHA0 of Pseudomonas fluorescens: role of tryptophan side chain oxidase. J. Gen. Microbiol. 137:2273-2279. [DOI] [PubMed] [Google Scholar]
- 39.Pernestig, A. K., O. Melefors, and D. Georgellis. 2001. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 276:225-231. [DOI] [PubMed] [Google Scholar]
- 40.Pessi, G., and D. Haas. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J. Bacteriol. 182:6940-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pessi, G., and D. Haas. 2001. Dual control of hydrogen cyanide biosynthesis by the global activator GacA in Pseudomonas aeruginosa PAO1. FEMS Microbiol. Lett. 200:73-78. [DOI] [PubMed] [Google Scholar]
- 42.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 44.Reimmann, C., M. Beyeler, A. Latifi, H. Winteler, M. Foglino, A. Lazdunski, and D. Haas. 1997. The global activator gacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309-319. [DOI] [PubMed] [Google Scholar]
- 45.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the noncoding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 46.Romeo, T., M. Gong, M. Y. Liu, and A. M. Brun-Zinkernagel. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol. 175:4744-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowley, K. B., D. E. Clements, M. Mandel, T. Humphreys, and S. S. Patil. 1993. Multiple copies of a DNA sequence from Pseudomonas syringae pathovar phaseolicola abolish thermoregulation of phaseolotoxin production. Mol. Microbiol. 8:625-635. [DOI] [PubMed] [Google Scholar]
- 48.Sacherer, P., G. Défago, and D. Haas. 1994. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 116:155-160. [DOI] [PubMed] [Google Scholar]
- 49.Safarik, I., and M. Safarikova. 1994. A modified procedure for the detection of microbial producers of extracellular proteolytic enzymes. Biotechnol. Tech. 8:627-628. [Google Scholar]
- 50.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 51.Sarniguet, A., J. Kraus, M. D. Henkels, A. M. Muehlchen, and J. E. Loper. 1995. The sigma factor ςsgr;S affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc. Natl. Acad. Sci. USA 92:12255-12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnider-Keel, U., A. Seematter, M. Maurhofer, C. Blumer, B. Duffy, C. Gigot-Bonnefoy, C. Reimmann, R. Notz, G. Défago, D. Haas, and C. Keel. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182:1215-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 55.Swift, S., J. A. Downie, N. A. Whitehead, A. M. L. Barnard, G. P. C. Salmond, and P. Williams. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45:199-270. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka, K., and H. Takahashi. 1994. Cloning, analysis and expression of an rpoS homologue gene from Pseudomonas aeruginosa PAO1. Gene 150:81-85. [DOI] [PubMed] [Google Scholar]
- 57.Vieira, J., and J. Messing. 1991. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene 100:189-194. [DOI] [PubMed] [Google Scholar]
- 58.Voisard, C., C. Bull, C. Keel, J. Laville, M. Maurhofer, U. Schnider, G. Défago, and D. Haas. 1994. Biocontrol of root diseases by Pseudomonas fluorescens CHA0: current concepts and experimental approaches, p. 67-89. In F. O'Gara, D. N. Dowling, and B. Boesten (ed.), Molecular ecology of rhizosphere microorganisms. VCH Publishers, Weinheim, Germany.
- 59.Voisard, C., C. Keel, D. Haas, and G. Défago. 1989. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 8:351-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voisard, C., M. Rella, and D. Haas. 1988. Conjugative transfer of plasmid RP1 to soil isolates of Pseudomonas fluorescens is facilitated by certain large RP1 deletions. FEMS Microbiol. Lett. 55:9-14. [Google Scholar]
- 61.Wassarman, K. M., F. Repoila, C. Rosenow, G. Storz, and S. Gottesman. 2001. Identification of novel small RNAs using comparative genomics and microarrays. Genes Dev. 15:1637-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whistler, C. A., N. A. Corbell, A. Sarniguet, W. Ream, and J. E. Loper. 1998. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor ςsgr;S and the stress response in Pseudomonas fluorescens Pf-5. J. Bacteriol. 180:6635-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuker, M. 1989. On finding all suboptimal foldings of an RNA molecule. Science 244:48-52. [DOI] [PubMed] [Google Scholar]