Abstract
Lactobacillus delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis are both used in the dairy industry as homofermentative lactic acid bacteria in the production of fermented milk products. After selective pressure for the fast fermentation of milk in the manufacture of yogurts, L. delbrueckii subsp. bulgaricus loses its ability to regulate lac operon expression. A series of mutations led to the constitutive expression of the lac genes. A complex of insertion sequence (IS) elements (ISL4 inside ISL5), inserted at the border of the lac promoter, induced the loss of the palindromic structure of one of the operators likely involved in the binding of regulatory factors. A lac repressor gene was discovered downstream of the β-galactosidase gene of L. delbrueckii subsp. lactis and was shown to be inactivated by several mutations in L. delbrueckii subsp. bulgaricus. Regulatory mechanisms of the lac gene expression of L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis were compared by heterologous expression in Lactococcus lactis of the two lac promoters in front of a reporter gene (β-glucuronidase) in the presence or absence of the lac repressor gene. Insertion of the complex of IS elements in the lac promoter of L. delbrueckii subsp. bulgaricus increased the promoter's activity but did not prevent repressor binding; rather, it increased the affinity of the repressor for the promoter. Inactivation of the lac repressor by mutations was then necessary to induce the constitutive expression of the lac genes in L. delbrueckii subsp. bulgaricus.
The two phenotypically related subspecies of Lactobacillus delbrueckii, L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis, are gram-positive, facultative anaerobes, and homofermentative lactic acid bacteria. They are economically very important because of their use in milk fermentation to convert lactose to lactic acid for food product preservation. Since the domestication of milk-producing animals by humans, microorganisms that can acidify milk in a reasonable time and yield tasty products were subjected to high selective pressure for an efficient utilization of lactose, the unique sugar found in milk. L. delbrueckii subsp. bulgaricus was probably selected, together with Streptococcus thermophilus, as the typical dairy starter strain for yogurt production. This selection may be the result of the fast and reliable fermentation capacity of L. delbrueckii subsp. bulgaricus versus L. delbrueckii subsp. lactis, as well as its robust resistance at low pH values. In many countries, it is now even required by law to use L. delbrueckii subsp. bulgaricus for the production of yogurt. L. delbrueckii subsp. lactis is found in a variety of cheese products, where it contributes to the fermentation process in combination with a diversity of other lactic acid bacteria.
The two enzymes involved in the metabolism of lactose were isolated and characterized from L. delbrueckii subsp. bulgaricus N299, a variant of the type strain ATCC 11842. They comprise a lactose antiport permease (lacS) involved in the uptake of the sugar and a β-galactosidase (lacZ) for the hydrolysis of lactose into glucose and galactose (13, 23). The presence of a phosphoenolpyruvate lactose phosphotransferase system with a phospho-β-galactosidase regulating sugar uptake and hydrolysis, as found in many other dairy lactic acid bacteria, has never been identified in an L. delbrueckii strain. On the contrary, expression of β-galactosidase in L. delbrueckii subsp. bulgaricus N299 was shown to be constitutive (13). The lactose operon itself was found to present genetic instabilities, resulting in large deletions at the level of the lac operon locus (16). Characterization of such deletions resulted in the identification of an active nonreplicative IS element, ISL3, located 1.2 kb downstream of the end of the lacZ gene (4). Two genes coding for enzymes involved in the metabolism of tRNA, an asparagine tRNA ligase (asnS1) and an aspartate-ammonia ligase (asnA), were localized just downstream of this IS element (10, 11). Next to these RNA genes is a gene coding for the cell wall- anchored protease (prtB) (5) essential for digestion of casein during growth in milk.
In this study, we extended sequence analysis of the lactose operon in different strains of L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis. We show here how unregulated expression of the lac operon in L. delbrueckii subsp. bulgaricus evolved from an intact regulatory gene expression system discovered in L. delbrueckii subsp. lactis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
All bacterial strains and plasmids used in this study are listed in Table 1. Lactobacilli were grown in MRS broth (Difco Laboratories) or in BHI broth (Oxoid Unipath, Basingstoke, England) at 42°C. Lactococcus lactis was cultivated in M17 broth (Difco) supplemented with 0.5% glucose (GM17). Plasmids pNZ272 and pNZ273 (19) were maintained in Lactococcus lactis MG1363 (3) by the addition of 10 μg of chloramphenicol/ml. Histochemical screening for gusA-positive clones was performed with 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gluc; Sigma) at a final concentration of 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or phenotypea | Source or reference |
|---|---|---|
| Lactobacillus delbrueckii subsp. lactis | ||
| LL44 (NCC936) | Inducible lac operon | Our collection |
| LL44/6 (NCC2727) | ΔlacR, constitutive lac operon | NCC936; this work |
| N62 (NCC181) | Inducible lac operon | NCDO270 |
| N141 (NCC2506) | Inducible lac operon | Our collection |
| LB68 (NCC653) | Inducible lac operon | ATCC 21815 |
| Lactobacillus delbrueckii subsp. bulgaricus | ||
| N299 (NCC2515) | Constitutive lac operon | ATCC 11842T |
| YL5 (NCC408) | Constitutive lac operon | Our collection |
| Lactococcus lactis | ||
| MG1363 | Plasmid-free derivative of NCDO 712, lac mutant | 3 |
| Plasmids | ||
| pNZ272 | Camr, promoterless gusA gene, shuttle E. coli; gram positive | 20 |
| pNZ273 | Camr, pNZ272 derivative | 20 |
| pLL110 | pNZ272 with LL44 lac promoter | This work |
| pLL112 | pNZ272 with LL44 lac promoter, LL44 lacR gene | This work |
| pLL113 | pNZ273 with N299 lac promoter | This work |
| pLL116 | pNZ273 with N299 lac promoter, LL44 lacR gene | This work |
Camr, chloramphenicol resistant.
DNA manipulations.
Total lactobacillus DNA was prepared by a modification of the spooling method (2). Bacteria grown in MRS broth were collected by centrifugation, washed once with 1 M NaCl, and incubated for 1 h at 37°C in the presence of proteinase K (250 μg/ml) and pronase E (500 μg/ml). Cells were washed twice in TE10 (10 mM Tris-HCl, pH 8.0; 1 mM EDTA), resuspended in TE50 (50 mM Tris, pH 8.0; 10 mM EDTA) containing mutanolysin (125 μg/ml) and lysozyme (1 mg/ml), and incubated for 1 h at 37°C. One volume of 0.5% sodium dodecyl sulfate was added to lyse the cells. Finally, proteinase K was added to 200 μg/ml, and the solution was further incubated for 30 min at 65°C. The DNA was extracted with phenol, precipitated with ethanol, spooled out on a sterile toothpick, washed in 70% ethanol, and drained. The DNA was then dissolved in TE50 in the presence of 200 μg of RNase A/ml, chloroform extracted, reprecipitated in ethanol, and spooled again. The purified DNA was dissolved in TE10 to give a final concentration of 500 to 1,000 μg/ml. Plasmid DNA was isolated from Lactococcus lactis with the QiaPrep 8 Miniprep Kit (Qiagen) with a first incubation step in 10 mM Tris-HCl (pH 8.0)-1 mM EDTA containing 1 mg of lysozyme/ml for 30 min at 37°C. DNA fragments were isolated from agarose gels with the Wizard PCR Preps DNA Purification System (Promega). DNA amplifications were performed in a total volume of 50 μl containing 0.2 mM concentrations of each deoxynucleotide triphosphate (Pharmacia Biotech), 1 μM concentrations of each primer (Microsynth GmbH, Balgach, Switzerland), standard buffers, and polymerase from the Expand Long Template PCR System (Boehringer, Mannheim, Germany) or 0.5 U of SuperTaq polymerase (Endotel, Allschwil, Switzerland). DNA amplifications (by PCR) were performed for 35 cycles of 1 min at 95°C, 2 min at 55°C, and 1 min/kb at 72°C with a DNA thermocycler (Perkin-Elmer). DNA sequences were determined directly by PCR by using the VISTRA Thermo Sequenase Sequencing kit (Amersham) on a DNA LICOR sequencer (model 4000; MWG-Biotech). DNA sequences were analyzed with the help of the Wisconsin Sequence Analysis Package from the Genetics Computer Group (University of Wisconsin).
Amplification of the lac promoter and the lacR gene.
The region upstream of the lac operon of different L. delbrueckii subsp. lactis and L. delbrueckii subsp. bulgaricus strains was amplified with the forward primer 2005 (5"-ACAGTGACTTAAACTGG), located close to the stop codon of the lacA gene, and the reverse primer 1994 (5"-ACGTCGTTGCCAAAGGC), located close to the start codon of the lacS gene. The region downstream of the lac operon was amplified with the primer 2030 (5"-CGCCTGGTGATTCAGCC), located close to the stop codon of the lacZ gene, and the reverse primer 946 (5"-AGCTTTACGGGGAAGTCGGG), located close to the stop codon of the asnS1 gene, which is in the reverse orientation compared to lacZ.
Construction of the expression vectors.
The lac promoters of L. delbrueckii subsp. lactis LL44 and L. delbrueckii subsp. bulgaricus N299 were amplified with forward primers containing an EcoRI site (underlined), i.e., 2409 (5"-ATATTAGAATTCAGTGACTTAAACTGG), located close to the stop codon of lacA gene, and 2410 (5"-ATATTAGAATTCAAGAGGCTATATCGC), located close to the end of ISL5, and the reverse primer 250 (5"-GGTTAATGCCGCCAAAGT), located at position 325 of the lacS gene. The amplification products were cleaved with EcoRI and BspEI (blunt) and cloned in pNZ272 or in pNZ273 restricted with EcoRI and AvaII (blunt) in front of the promoterless gusA gene (plasmids pLL110 and 113). The lacR gene was amplified from L. delbrueckii subsp. lactis LL44 with primers containing an XhoI site (underlined): forward primer 3174 (5"-ATAAATCTCGAGTGGTGATTCAGCC), located close to the stop codon of the lacZ gene, and reverse primer 3033b (5"-ATATTACTCGAGACAGAATGCAGCC), located close to the stop codon of asnS1 gene. The amplified product and the plasmids pLL110 and pLL113 were digested with XhoI and ligated (plasmids pLL112 and pLL116). All of the plasmids were used to transform directly Lactococcus lactis MG1363 by electroporation (7).
Isolation of spontaneous lacR mutants.
L. delbrueckii subsp. lactis LL44 (NCC936) was incubated overnight at 42°C in MRS broth containing 10% lactose. The culture was stored refrigerated for 24 h and subcultured in MRS broth containing 10% lactose for 7 h at 42°C. A dilution of the culture was plated on Reinforced Clostridial Medium (Oxoid) containing glucose and 200 μg of X-Gal (5-bromo-4-chloro-3-indolyl-beta-d-galactopyranoside; Sigma)/ml. Plates were incubated for 72 h at 40°C under microaerophilic conditions (BBL).
β-Galactosidase assays.
L. delbrueckii subsp. lactis and L. delbrueckii subsp. bulgaricus were grown overnight in MRS broth and used to inoculate BHI medium containing 0.5% lactose, 0.5% glucose, or 0.5% glucose plus 0.5% lactose. Cells were grown at 42°C to an optical density at 600 nm (OD600) of ca. 1.0, harvested, and resuspended in 0.2 ml of distilled water containing 0.1% Triton X-100. Cells were incubated for 30 min at room temperature, centrifuged, and resuspended at different concentrations in 500 μl of potassium phosphate (pH 7.5) containing 50 μg of ONPG (o-nitrophenyl-β-d-galactopyranoside; Sigma)/ml. After 20 min of incubation at 42°C, 200-μl aliquots were mixed with 0.8 ml of 400 mM Na2CO3-50 mM EDTA to stop the reaction. The OD of the mixture was measured after centrifugation at 420 nm, and the β-galactosidase activity in units (U) was calculated as follows: U = (OD420 × d)/(3.5 × t × OD600), where d is the dilution factor, 3.5 is the millimolar extinction coefficient of the ortho-nitrophenol, and t is the reaction time.
β-Glucuronidase assays.
Transformed Lactococcus lactis cells were grown overnight in M17 broth containing 0.5% mannose and 10 μg of chloramphenicol/ml and used to inoculate M17 containing 0.5% mannose, 0.5% lactose, 0.5% glucose, or 0.5% glucose plus 0.5% lactose. Cells were grown at 30°C to an OD660 of ca. 1.0, harvested, and resuspended in 2 ml of distilled water containing 0.1% Triton X-100. Cells were incubated for 30 min at room temperature, centrifuged, and resuspended in 2 ml of 50 mM NaHPO4 (pH 7.0)-10 mM β-mercaptoethanol-1 mM EDTA-0.1% Triton X-100-50 μg of para-nitro-β-d-glucuronic acid (Clontech)/ml. After 1 h of incubation at 37°C, 500-μl aliquots were drawn, and the reaction was stopped with 0.5 ml of 2 mM Na2CO3. The OD of the reaction mixture was measured after centrifugation at 415 nm, and the β-glucuronidase activity (in units) was calculated as follows: U = (OD415 × d)/(18 × t), where t is the reaction time in minutes, 18 is the millimolar extinction coefficient of the para-nitrophenol, and d is the dilution factor. The protein content of the culture was determined by using a Bio-Rad protein assay with bovine serum albumin as the standard.
RESULTS
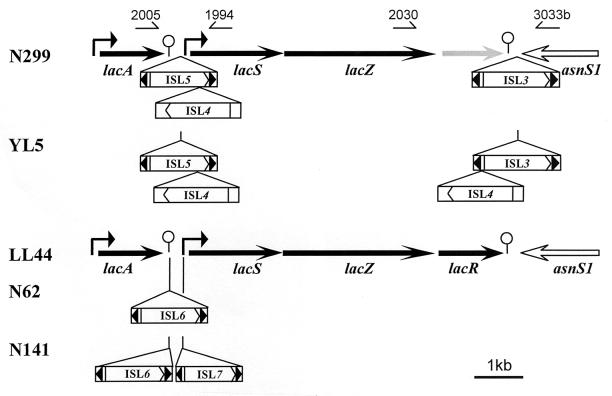
Structural analysis of the L. delbrueckii subsp. bulgaricus lac operon. Existing information about the sequence of the lactose operon (lacS and lacZ) in L. delbrueckii subsp. bulgaricus N299 was extended by chromosomal crawling by using DNA amplification (21) and sequencing of the regions located upstream and downstream of the operon. Thereby, several open reading frames (ORFs) were identified (Fig. 1). Upstream, a gene coding for a peptide of 189 amino acids, showed 50% sequence identity to the thiogalactosyl-transacetylase (lacA) gene of Escherichia coli (6) (Table 2) and is in the same orientation as the lactose genes. The presence of a putative promoter and a potential rho-independent terminator suggests that this gene is independently transcribed from the lac operon. Between this gene and the promoter of lacS appeared ORFs in both orientations showing low homologies to transposases. This finding suggests the presence of at least two putative new insertion sequences (IS elements), ISL4 and ISL5. At this stage, however, it was not possible to determine the delineations of these newly identified IS elements.
FIG. 1.
Schematic representation of the lac operon in different L. delbrueckii subsp. bulgaricus (N299 and YL5) and L. delbrueckii subsp. lactis (LL44, N62, and N141) strains. The different ORFs are represented by arrows, and the IS elements are represented by boxes. The broken arrows indicate the promoters, the arrowheads inside the IS elements show the terminal inverted repeats, and small arrows point to primers used for DNA amplification.
TABLE 2.
Characteristics and GenBank accession numbers of the different sequences involved in this studya
| Name | GenBank no. | Size (bp) | ORF (aa) | Length of IRs (bp) | Homology (gene, organism, % identity, GenBank no.)b |
|---|---|---|---|---|---|
| lacSZ | M55068 | 5,015 | 627, 1008 | Permease and β-galactosidase (lacS and lacZ) (13) | |
| asnS1 | X89438 | 1,298 | 432 | Asn tRNA ligase (10) | |
| ISL3 | X79114 | 1,492 | 433 | 38 | Transposase (4) |
| lacA | AY040211 | 570 | 189 | Acetyltransferase, yeast, 54% in 188 aa, P40892 | |
| lacR | AY040212 | 1,121 | 332 | Gal repressor, Streptococcus mutans, 36% in 332 aa, JC5310 | |
| ISL4 | AY040213 | 1,658 | 386 | ATP-binding cassette, Streptococcus crista, 44% in 265 aa, U96166 | |
| ISL5 | AY040214 | 1,590 | 240 | 65 | Transposase, Bacteroides fragilis, 28% in 240 aa, U75371 |
| ISL4/5 | AY040218 | 3,264 | 386 | 65 | Mutated ISL5 in the promoter of the L. delbrueckii subsp. bulgaricus lac operon |
| ISL6 | AY040215 | 1,458 | 420 | 24 | Transposase, Streptococcus agalactiae, 49% in 263 aa, M22449 |
| ISL7 | AY040216 | 1,239 | 349 | 28 | Transposase, Clostridium perfringens, 48% in 352 aa, X71844 |
IR, inverted repeat; aa, amino acids.
The source or reference is indicated in parentheses.
The region downstream of the lac operon was amplified with primers designed from lacZ and ISL3 and sequenced, but only stretches of putative ORFs in the different reading frames showed significant homologies to repressor molecules. This indicated the presence of remnants of the lacR gene, which were oriented in the same direction as the lactose genes. Thus, the presence of several IS elements close to the lac promoter and remnants of a potential repressor gene suggested that expression of the lac operon in L. delbrueckii was once regulated. This prompted us to investigate this genetic locus in related L. delbrueckii strains.
Structural analysis of the lac operon in other strains of L. delbrueckii.
The structure of the lac operon was analyzed in different strains of L. delbrueckii in order to identify the functional structures responsible for the control of lac gene expression. Five strains were chosen from among sixty-four, which had been studied in detail in the scope of a taxonomic study of the L. delbrueckii group (J. E. Germond, L. Lapiere, M. Delley, and B. Mollet, unpublished data). Two regions were amplified with primers designed from lacA and lacS for the region upstream and from lacZ and asnS1 for the region downstream of the lac operon (Fig. 1). In both cases, the size of the amplification products differed from strain to strain and revealed several different structures.
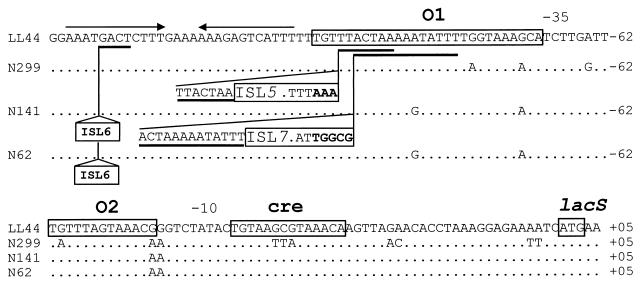
The amplification product obtained from the region upstream of the lac operon in L. delbrueckii subsp. lactis LL44 was the shortest with 200 bp. Its sequence revealed, upstream of the ribosome-binding site of the lacS gene, the presence of three palindromes (Fig. 2 and Fig. 3). Two are homologous to operators (O1 and O2) involved in the binding of specific regulatory factors such as repressors (12, 17). The third is homologous to the catabolite regulatory element (cre) involved in nonspecific carbon catabolite regulation (8, 14, 15). The operator O1 is directly preceded by the stem-loop structure of a rho-independent terminator for the newly identified lacA gene.
FIG. 2.
Alignment of the sequence of the lac promoters with their operators (O1 and O2) and the catabolite responsive element (cre) in different strains of L. delbrueckii subsp. lactis (LL44, N141, and N62) and L. delbrueckii subsp. bulgaricus (N299). The dots are nucleotides that are the same as in strain LL44, nucleotides in boldface indicate changes in O1 due to IS element insertions, direct repeats of the insertion sites are underlined, and arrows show the rho independent terminator of lacA. Sequences are numbered from the start codon of lacS.
FIG. 3.
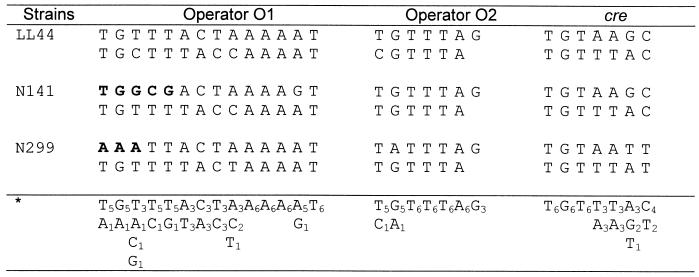
Alignment of the half-site sequences of the operators (O1 and O2) and the catabolite responsive element (cre) of different L. dubrueckii strains. Nucleotide changes due to IS element insertions are indicated in boldface. ∗, consensus sequences.
In other strains, the amplification products were longer, and their sequences revealed the presence of several new putative IS elements. In L. delbrueckii subsp. lactis strains N62 and N141, a new IS element, ISL6, was localized exactly in the rho-independent terminator of lacA. It contains 24-bp long-terminal inverted repeats and duplicated the sequence GACT at its integration site (Fig. 2 and Table 2). This feature of inverted terminal repeats is characteristic of IS elements and is involved in the process of excision and insertion of the element. Next to ISL6, another new IS element, ISL7, was localized at the border of the operator O1 in L. delbrueckii subsp. lactis N141. It contains 28-bp long-terminal inverted repeats and duplicated the sequence ACTAAAAATATTT at its integration site (Fig. 2 and Table 2). Finally, the IS element free sequence of the lac promoter allowed us to localize exactly the complex structure of the two IS elements detected upstream of the lac operon in the L. delbrueckii subsp. bulgaricus N299. This structure is integrated at the border of the operator O1 by duplication of the sequence TTACTAA and has 65-bp long-terminal inverted repeats (Fig. 2 and Table 2). Thus, it should be constituted of one IS element, ISL5, in which another IS element, ISL4, is integrated. At this stage, however, it was not possible to determine the delineations of ISL4.
The amplification product, obtained from the region downstream of the lac operon, was the same for all strains of L. delbrueckii subsp. lactis analyzed. Sequencing of the product obtained from strain LL44 revealed the presence of only one ORF coding for a peptide of 332 amino acids located 52 bp downstream and in the same orientation as the lacZ gene (Fig. 1). The peptide showed 32% sequence identity to the ebgR gene of E. coli (24); this gene codes for a repressor belonging to the lacI family of regulators and was called lacR (Fig. 4). Its sequence predicts the helix-turn-helix characteristic of repressor molecules (1, 18), and its gene is likely transcribed as part of the lac operon since no promoter sequence could be identified. This structure is unusual, since lacR genes are generally found outside the lac operon they control and under their own promoter, as in Lactococcus lactis (25). The amplification products were longer in L. delbrueckii subsp. bulgaricus strains. Sequencing of these products revealed that the lacR gene was inactivated by a series of deletion or insertion events, which are described elsewhere (Germond et al., unpublished). These mutations led to the fragmentation of the gene in several ORFs in the different reading frames. The first of these events, the deletion of a single nucleotide 18 bp downstream of the start codon, led to a premature stop before the end of the helix-turn-helix motif (Fig. 4). The previously described IS element, ISL3, was found downstream of this pseudo lacR gene (4) (Fig. 1 and Table 2). In L. delbrueckii subsp. bulgaricus YL5, in addition, the newly identified ISL4 was found inserted into an A-rich region of the promoter of the ISL3 transposase gene (Fig. 1). ISL4 has no terminal inverted repeats and had been inserted without duplication of its integration site (Table 2). Thus, the delineation of ISL4 present in the complex structure of two IS elements upstream of the lac operon in the L. delbrueckii subsp. bulgaricus N299 was exactly localized. ISL4 was inserted close to the end of the transposase coding sequence of ISL5, leading to inactivation of ILS5. Sequencing of an ISL4-free ISL5, found in another strain of L. delbrueckii subsp. bulgaricus (LB68) revealed that the ISL5 containing ISL4 acquired a series of deletions and/or insertions. These mutations prevented ISL5 from being transposed and stabilized its presence at this locus. All strains of L. delbrueckii subsp. bulgaricus available from the Nestlé Culture Collection were shown to have this complex of ISL4 and ISL5 in the lac promoter and to demonstrate constitutive expression of the lac genes (Germond et al., unpublished). All of the sequences described here have been deposited in the GenBank database (Table 2).
FIG. 4.
Nucleotide and amino acid sequences of the L. delbrueckii subsp. lactis LL44 lacR gene. Start and stop codons and the ribosome-binding site are boxed. Arrows show the putative rho-independent terminators. The large open arrow indicates the insertion site of ISL3, and the large bars indicate the helix-turn-helix motif. Single nucleotide deletions in N299 and LL44/6 are indicated by open and filled arrowheads, respectively, and the small bars point out premature stop codons in these mutated lacR genes.
Regulation of β-galactosidase expression in vivo.
The different strains of L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis were grown in MRS broth in the presence of either lactose or glucose, and the β-galactosidase activity was measured (Table 3). β-Galactosidase activity was observed in the presence of lactose and glucose with both L. delbrueckii subsp. bulgaricus N299 and YL5, indicating that the regulation of the lac gene expression was lost and that β-galactosidase production is constitutive, whereas with L. delbrueckii subsp. lactis LL44, N62, and N141 β-galactosidase activity was detected only in the presence of lactose, indicating that the production of β-galactosidase was repressed in the absence of lactose.
TABLE 3.
β-Galactosidase activities in different strains of L. delbrueckii grown in MRS broth
| Strain | Presence (+) or absence (−) of activity
|
|
|---|---|---|
| Lactose | Glucose | |
| N299 | + | + |
| YL5 | + | + |
| LL44 | + | − |
| N62 | + | − |
| N141 | + | − |
In order to determine the functionality of the identified lac repressor and its involvement in the regulation of the lac gene expression, spontaneous constitutive mutants of the L. delbrueckii subsp. lactis LL44 were screened on agar plates containing glucose and X-Gal. One colony appeared blue (>104 screened), indicating production of β-galactosidase in the presence of glucose and thereby constitutive expression of the lac genes. This mutant (LL44/6), its parent strain (LL44), and L. delbrueckii subsp. bulgaricus N299 were grown in MRS broth in the presence of glucose, lactose, or both sugars, and the β-galactosidase activity was measured (Table 4). β-Galactosidase activity was observed only in the presence of lactose for LL44, whereas for the mutant LL44/6 and for N299, β-galactosidase activity was recorded in the presence of both sugars, indicating that β-galactosidase production can no longer be repressed in the absence of lactose. Sequencing of the lacR gene of the LL44/6 mutant revealed a single nucleotide deletion 722 bp downstream of the start codon, leading to a premature stop after 212 amino acids (Fig. 4). These results confirmed that the isolated repressor gene is responsible for the regulation of lac gene expression.
TABLE 4.
Activities of β-galactosidase in different strains of L. delbrueckii grown in MRS broth
| Sugar(s) addeda | Mean activity ± SD (mU/OD600)b in:
|
||
|---|---|---|---|
| LL44 | LL44/6 | N299 | |
| 0.5% Lac | 117.1 ± 26.9 | 237.1 ± 14.3 | 142.6 ± 9.7 |
| 0.5% Lac + 0.5% Glu | 126.8 ± 10.3 | 240.6 ± 25.1 | 357.2 ± 86.2 |
| 0.5% Glu | 4.0 ± 0.9 | 342.2 ± 22.2 | 343.4 ± 80.5 |
Lac, lactose; Glu, glucose.
Mean of five experiments.
Functionality of the lac promoter.
The three palindromes found in the different lac promoters analyzed were aligned as half-site sequences (Fig. 3) and were shown to be conserved in the different strains analyzed. These structures involved in the binding of transcription regulatory factors have in general the conserved dinucleotides TG/AC at their extremities (12, 17). In L. delbrueckii several IS elements were found integrated at the 5"end of the O1 operator. For L. delbrueckii subsp. lactis N141, insertion of ISL7 at the border of the first operator (O1) has modified the sequence of the palindrome but has kept the two important terminal nucleotides, whereas in L. delbrueckii subsp. bulgaricus N299 and YL5 the insertion of ISL5 in the same region modified the sequence of the 5"end of the palindrome (Fig. 3). Loss of the conserved TG motif of the O1 operator could induce a constitutive production of the enzymes coded by the lac operon. Beside insertion of IS elements, several base changes occurred in the lac promoter sequence of the different strains analyzed. In L. delbrueckii subsp. bulgaricus N299, three of these mutations modified the important central CG motif of the catabolite regulatory element (cre) (Fig. 2 and 3) (8). This modification of the cre sequence could prevent binding of the regulatory factor (CcpA complex) in the presence of glucose. The result will be a loss of glucose catabolite repression and an unrestrained production of β-galactosidase.
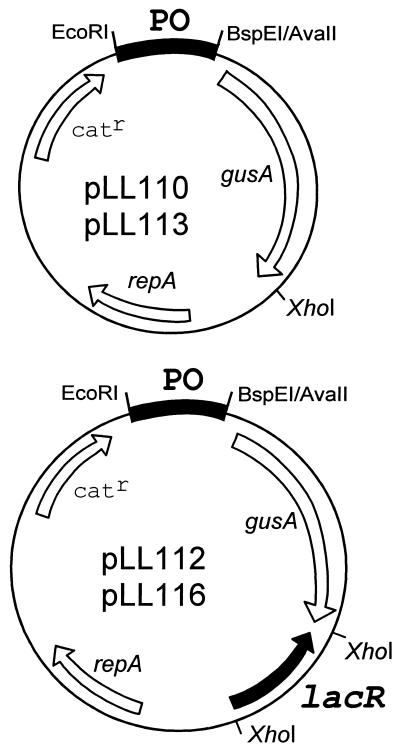
In order to determine the importance of the different nucleotide changes in their lac operon on the regulation of the lac gene expression, the lac promoters of L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis were cloned in front of a reporter gene in the presence or absence of the lac repressor. The lac promoters of L. delbrueckii subsp. bulgaricus N299 and L. delbrueckii subsp. lactis LL44 were amplified with primers in ISL5 and lacA, respectively, and lacS (Fig. 1). The amplification products were then cloned in the pNZ272 (20) in front of the promoterless β-glucuronidase (gusA) gene (Fig. 5). The resulting plasmids, pLL110 and pLL113, respectively, were transformed in a plasmid free strain of Lactococcus lactis MG1363. The transformed cells were grown in the presence of different sugars, and the β-glucuronidase activity was measured (Table 5). The results showed that both L. delbrueckii subsp. bulgaricus and L. delbrueckii subsp. lactis lac promoters were able to induce the production of β-glucuronidase in Lactococcus lactis. The same activity was observed in the presence of mannose and lactose for each promoter but was two times higher for the L. delbrueckii subsp. bulgaricus promoter. It can be argued that the mutation induced by ISL5 insertion likely led to a more active promoter. The presence of glucose led only to a strong reduction of the β-glucuronidase activity with the L. delbrueckii subsp. lactis LL44 promoter. This observation indicates that the LL44 promoter is subjected to glucose catabolite regulation in Lactococcus lactis, whereas no such reduction of β-glucuronidase was observed with the N299 promoter in which the cre sequence was shown to be mutated. (Fig. 2).
FIG. 5.
Physical map of the plasmids used for the study on the regulation of the L. delbrueckii lac promoters.
TABLE 5.
Activities of the β-glucuronidase gene (gusA)a
| Sugar(s) addedb | Mean activity ± SD (mU/mg of protein)c with:
|
||||
|---|---|---|---|---|---|
| No lacR
|
LL44 lacR
|
||||
| pLL110 (LL44) | pLL113 (N299) | pLL112 (LL44) | pLL116 (N299) | ||
| 0.5% Man | 2.01 ± 0.80 | 3.58 ± 0.05 | 0.05 ± 0.05 | 0.07 ± 0.07 | |
| 0.05% Lac | 1.56 ± 0.44 | 3.20 ± 0.41 | 0.34 ± 0.11 | 0.07 ± 0.08 | |
| 0.5% Lac | 2.01 ± 1.26 | 3.41 ± 0.94 | 2.71 ± 0.75 | 1.11 ± 0.13 | |
| 0.5% Glu | 0.51 ± 0.37 | 2.23 ± 0.20 | 0.05 ± 0.02 | 0.06 ± 0.06 | |
| 0.5% Glu + 0.5% Lac | 0.35 ± 0.14 | 2.80 ± 0.76 | 0.33 ± 0.16 | 0.20 ± 0.07 | |
The gene was fused to different L. delbrueckii lac promoters in the absence or presence of lacR genes and cloned in Lactococcus lactis MG1363 grown in M17.
Man, mannose; Lac, lactose; Glu, glucose.
Mean of three experiments.
The next step was to analyze the activity of the newly identified LL44 lac repressor on both promoters. The lacR gene was amplified from L. delbrueckii subsp. lactis LL44 with primers in lacZ and asnS1 (Fig. 1). The product of amplification was cloned in both plasmids containing the promoters of LL44 and N299 in the XhoI site located downstream of the gusA gene, yielding pLL112 and pLL116, respectively (Fig. 5). In both cases, the lacR gene was inserted in the reverse orientation compared to the gusA gene. Both were then cloned in Lactococcus lactis, and the β-glucuronidase activity was measured in the presence of various sugars (Table 5). With both promoters, the β-glucuronidase activity was produced only when bacteria were grown in the presence of lactose and was repressed with the other sugars. The LL44 lac repressor is thus able to repress both promoters. However, induction of β-glucuronidase activity by lactose was different for each promoter. For the LL44 promoter, β-glucuronidase activity measured in the presence of lactose is similar in the presence or absence of repressor, whereas for the N299 promoter, it is three times lower in the presence of the repressor. These results indicate that the lac repressor probably controls the lac promoter of L. delbrueckii subsp. bulgaricus N299 more strongly than the lac promoter of L. delbrueckii subsp. lactis LL44. The lacR gene was also cloned in the same orientation as the gusA gene expressed from the N299 promoter, likely inducing a higher production of repressor. In this case, β-glucuronidase activity could hardly be induced, even in the presence of 1% lactose (data not shown). Thus, the mutation induced by integration of the complex of two IS elements in the operator O1 does not prevent the regulation of the promoter by the repressor but induces an even tighter repression of its activity. The conserved TG motif of the palindrome seems to not be essential for the control of the promoter by the repressor in L. delbrueckii.
DISCUSSION
In L. delbrueckii subsp. bulgaricus N299, the presence of numerous IS-like structures (IS elements) in the lac promoter and remnants of a repressor gene downstream of the lacZ gene strongly indicated that the lac operon could be subjected to genetic instability and was once regulated. In order to identify the original regulatory elements, the regions upstream and downstream of the lac operon were analyzed in different strains of L. delbrueckii. A gene coding for a repressor (lacR) was found downstream of the lacZ gene in L. delbrueckii subsp. lactis. Its sequence allowed us to define the different insertion or deletion events which led to its inactivation in L. delbrueckii subsp. bulgaricus. Loss of lac repressor production would thus be sufficient to induce the constitutive expression of the lac genes in L. delbrueckii subsp. bulgaricus. However, other genetic events occurred in the promoter region, which could be responsible for the constitutive expression of the lac genes. Several IS elements were found inserted at the 5" end of the first operator (O1). In some L. delbrueckii subsp. lactis strains, ISL7, was inserted with minor modifications of the palindromic structure of the O1 operator. Whereas in all L. delbrueckii subsp. bulgaricus, a complex of two IS elements inserted into each other, ISL4/5, induced a major modification of the palindromic structure of the O1 operator. Loss of the palindromic structure of the O1 operator in L. delbrueckii subsp. bulgaricus could have been the prerequisite for a more efficient and constitutive activity of the promoter, by modifying the binding of regulatory factors such as repressors as well as enhancers.
To rule out these possibilities, both lac promoters were cloned in front of the β-glucuronidase gene (gusA), transformed in Lactococcus lactis since no transformation system exists for L. delbrueckii. Both promoters were shown to be active in Lactococcus lactis. Cloning of the lacR gene on the same plasmid resulted in repression in Lactococcus lactis of the activity of both promoters. The activity of the L. delbrueckii subsp. bulgaricus lac promoter, which acquired several mutations, was even strongly repressed. One can speculate regarding which mutations occurred first. One possibility is that the promoter mutated, providing better access to enhancer factors of RNA polymerase, but with a stronger affinity also for the repressor. In this case, a lacR mutant would have been rapidly selected. It has been shown that a single nucleotide deletion at the beginning of this gene is present in all L. delbrueckii subsp. bulgaricus strains. Other mutations are observed only in some strains and could have appeared during the speciation of L. delbrueckii subsp. bulgaricus (Germond et al., unpublished). Another possibility is that two independent mutations occurred first in the repressor gene to induce a constitutive production of the lac genes and second in the promoter to induce a higher activity.
Another interesting selective advantage for higher expression of lac genes in L. delbrueckii subsp. bulgaricus is the accumulation of mutations in the catabolite responsive element responsible for nonspecific carbon catabolite regulation induced by the CcpA phosphorylated Hpr-like complex (9). In Lactococcus lactis, the cloned L. delbrueckii subsp. bulgaricus promoter was effectively shown not to be under carbon catabolite repression in the presence of glucose, whereas in the same conditions the activity of the L. delbrueckii subsp. lactis promoter was reduced by a factor of 4 or 5. However, this cre element should not play a role, because it was shown recently that even though a protein homologous to the CcpA factor was found in L. delbrueckii subsp. lactis, no significant carbon catabolite regulation could be observed in this strain (22). This indicates that a factor that allows glucose catabolite regulation to be active in L. delbrueckii is missing. Indeed, this repression activity was not observed, since the β-galactosidase activity in the L. delbrueckii subsp. lactis lacR mutant LL44/6 was the same in the presence of glucose and lactose. The absence of repressor in this mutant would have left an active glucose catabolite regulation.
From these results we conclude that, due to its selection for fast growth in milk for yogurt production, L. delbrueckii subsp. bulgaricus optimized its metabolism for an efficient utilization of the milk sugar. L. delbrueckii subsp. lactis, however, was not primarily selected for fast growth in milk but for more varied metabolic capabilities for the production of specific metabolites such as flavors and proteolytic enzymes for the maturation of cheese products.
Acknowledgments
This work was supported by a grant of the EU Biotech II under contract nbr BIO4CT960439 (CH-OFES 96.0018).
We gratefully acknowledge Elizabeth Prior for reviewing the manuscript.
REFERENCES
- 1.Brennan, R., and B. Matthews. 1989. The helix-turn-helix DNA binding motif. J. Biol. Chem. 264:1903-1906. [PubMed] [Google Scholar]
- 2.Delley, M., B. Mollet, and H. Hottinger. 1990. DNA probe for Lactobacillus delbrueckii. Appl. Environ. Microbiol. 56:1967-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasson, M. J. 1983. Genetic transfer systems in lactic acid bacteria. Antonie Leeuwenhoek 49:275-282. [DOI] [PubMed] [Google Scholar]
- 4.Germond, J. E., L. Lapierre, M. Delley, and B. Mollet. 1995. A new mobile genetic element in Lactobacillus delbrueckii subsp. bulgaricus. Mol. Gen. Genet. 248:407-416. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert, C., D. Atlan, B. Blanc, R. Portailer, J. E. Germond, L. Lapierre, and B. Mollet. 1996. A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. J. Bacteriol. 178:3059-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hediger, M. A., D. F. Johnson, D. P. Nierlich, and I. Zabin. 1985. DNA sequence of the lactose operon: the lacA gene and the transcriptional termination region. Proc. Natl. Acad. Sci. USA 82:6414-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holo, H. Y., and I. F. Nes. 1989. High frequency transformation by electroporation of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hueck, C. J., and W. Hillen. 1995. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the gram-positive bacteria? Mol. Microbiol. 15:395-401. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic, I., O. Egeter, and R. Bruckner. 2001. Analysis of catabolite control protein A-dependent repression in Staphylococcus xylosus by a genomic reporter gene system. J. Bacteriol. 183:580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, S. I., J. E. Germond, D. Pridmore, and D. Soll. 1996. Lactobacillus bulgaricus asparagine synthetase and asparaginyl-tRNA synthetase: coregulation by transcription antitermination? J. Bacteriol. 178:2459-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, S. I., M. Nalaskowska, J. E. Germond, D. Pridmore, and D. Soll. 1996. Asn-tRNA in Lactobacillus bulgaricus is formed by asparaginylation of tRNA and not by transamidation of Asp-tRNA. Nucleic Acids Res. 24:2648-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehming, N., J. Sartorius, M. Niemoller, G. Genenger, B. Wilcken-Bergmann, and B. Muller-Hill. 1987. The interaction of the recognition helix of lac repressor with lac operator. EMBO J. 6:3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leong-Morgenthaler, P., M. C. Zwahlen, and H. Hottinger. 1991. Lactose metabolism in Lactobacillus bulgaricus: analysis of the primary structure and expression of the genes involved. J. Bacteriol. 173:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lokman, B. C., R. J. Leer, R. van Sorge, and P. H. Pouwels. 1994. Promoter analysis and transcriptional regulation of Lactobacillus pentosus genes involved in xylose catabolism. Mol. Gen. Genet. 245:117-125. [DOI] [PubMed] [Google Scholar]
- 15.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollet, B., and M. Delley. 1990. Spontaneous deletion formation within the β-galactosidase gene of Lactobacillus bulgaricus. J. Bacteriol. 172:5670-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muiznieks, I., and R. Schmitt. 1994. Role of two operators in regulating the plasmid-borne raf operon of Escherichia coli. Mol. Gen. Genet. 242:90-99. [DOI] [PubMed] [Google Scholar]
- 18.Nauta, A., D. van Sinderen, H. Karsens, E. Smit, G. Venema, and J. Kok. 1996. Inducible gene expression mediated by a repressor-operator system isolated from Lactococcus lactis bacteriophage r1t. Mol. Microbiol. 19:1331-1341. [DOI] [PubMed] [Google Scholar]
- 19.Platteeuw, C., I. Alen-Boerrigter, S. van Schalkwijk, and W. M. de Vos. 1996. Food-grade cloning and expression system for Lactococcus lactis. Appl. Environ. Microbiol. 62:1008-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platteeuw, C., G. Simons, and W. M. de Vos. 1994. Use of the Escherichia coli beta-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ. Microbiol. 60:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Schick, J., B. Weber, J. R. Klein, and B. Henrich. 1999. pepR1, a CcpA-like transcription regulator of Lactobacillus delbrueckii subsp. lactis. Microbiology 145(Pt. 11):3147-3154. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt, B. F., R. M. Adams, C. Requadt, S. Power, and S. E. Mainzer. 1989. Expression and nucleotide sequence of the Lactobacillus bulgaricus β-galactosidase gene cloned in Escherichia coli. J. Bacteriol. 171:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokes, H. W., and B. G. Hall. 1985. Sequence of the ebgR gene of Escherichia coli: evidence that the EBG and LAC operons are descended from a common ancestor. Mol. Biol. E vol. 2:478-483. [DOI] [PubMed] [Google Scholar]
- 25.van Rooijen, R. J., M. J. Gasson, and W. M. de Vos. 1992. Characterization of the Lactococcus lactis lactose operon promoter: contribution of flanking sequences and LacR repressor to promoter activity. J. Bacteriol. 174:2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]