“I am the mob, the crowd, the mass. Do you know that all the great work of the world is done through me?”
Carl Sandburg (93)
Several hundred millennia ago, prehistoric humans learned that there is often strength in numbers. The evolution of speech likely aided in the ability of protohumans to coordinate the behavior of the group. It has recently become clear that bacteria made this discovery at least a billion years earlier, and in lieu of speech, they evolved a rich lexicon of diffusible chemical signals. In the past decade we have learned that bacteria use these signals to communicate both within and between species, and they also use a wide variety of chemical signaling molecules, signal detection apparatuses, and signal transduction mechanisms. The processes controlled by cell-cell communication among bacteria are also diverse and presumably enhance survival and optimize living in specialized niches.
The explosive growth of knowledge in this area inspired the American Society for Microbiology (ASM) to sponsor a conference entitled Cell-Cell Communication in Bacteria, held at Snowbird, Utah, in July 2001. The goal of the conference was to provide an opportunity for scientists from a variety of disciplines who all work on bacterial communication to become acquainted and to share unpublished data and insights. The purpose of this article is to review the phenomenon of cell-cell communication in bacteria and to highlight some of the most important new information shared at this meeting. The eight articles that follow this review present new research findings in this area. Bacterial cell-cell communication has also been reviewed in two recent books (20, 23).
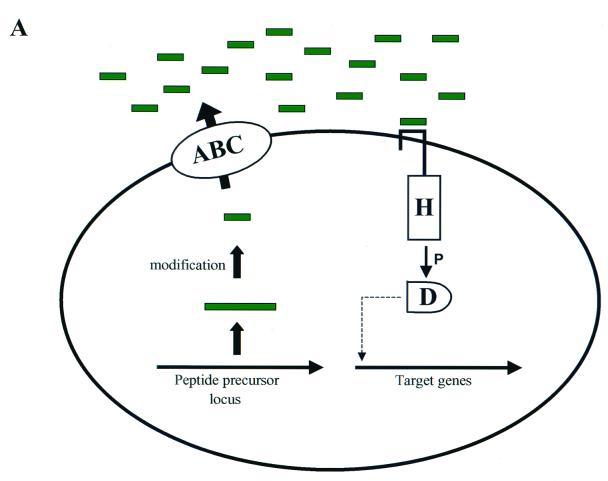
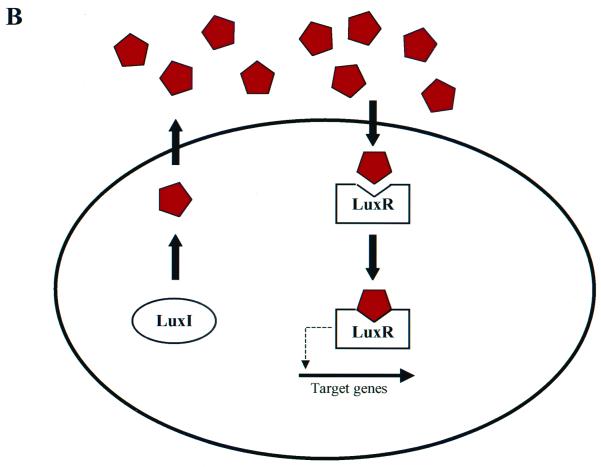
Two major classes of communication systems have been described. Gram-positive bacteria typically communicate by using processed oligopeptide signals, and signaling generally involves a two-component phosphorelay mechanism. In contrast, signaling by proteobacteria usually involves acyl-homoserine lactones (acyl-HSLs). Models showing the general features of gram-positive and gram-negative quorum-sensing circuits are presented in Fig. 1A and B, respectively. Some bacteria can use multiple signals as well as multiple types of signaling molecules to communicate. In many species of bacteria, complicated regulatory hierarchies control signal production, signal detection, and signal response.
FIG. 1.
Bacterial quorum-sensing signal transduction circuits. Generalized models illustrating the typical components used in gram-positive and gram-negative bacterial quorum-sensing systems are shown. (A) Oligopeptide-mediated quorum sensing in gram-positive bacteria. A specific precursor peptide (large green bars) is synthesized, modified, and processed. An ABC exporter complex secretes the mature oligopeptide signal (short green bars). The oligopeptide accumulates as the bacterial cell density increases. Two-component signal transduction systems are responsible for detection of the signals and relaying the information into the cell. Signal transduction occurs by a phosphorylation cascade (P). The model shows a sensor kinase and response regulator containing conserved histidine (H) and aspartate (D) residues that are the sites of phosphorylation. The signal transduction cascade results in an alteration in gene expression of specific target outputs. (B) Canonical LuxI/LuxR quorum-sensing system of gram-negative bacteria. Two conserved regulatory proteins typically control quorum sensing in gram-negative bacteria. The LuxI-like proteins are the autoinducer synthases and are responsible for catalyzing the formation of a specific acyl-HSL autoinducer molecule (red pentagons). The autoinducers freely diffuse through the bacterial cell envelope and accumulate at high cell density. The LuxR-like proteins bind their cognate autoinducers when a sufficiently high concentration of the signal has been achieved. The LuxR-autoinducer complexes also bind at target gene promoters and activate transcription.
The general theme that has emerged is that bacteria use these cell-cell communication systems to assess their cell population numbers. The argument for this assertion is that the concentration of external signal correlates with cell growth, and therefore, the signal allows bacteria to sense when they have achieved a “quorum.” The quorum contains a sufficient number of bacteria to carry out processes that necessitate the cooperation of a large number of cells in order to be effective. Presumably, acting as a quorum allows the individual bacteria in the group to benefit from the activity of the entire assembly. However, several conferees suggested that this conventional view of quorum sensing might be too simple and that this definition does not describe all forms of cell-cell signaling. It was conjectured that bacteria might require a quorum to effectively send and receive chemical signals but the information content of these signals might be for purposes other than census taking. This could explain why several organisms have multiple signaling systems. If the function of these systems is to determine cell number, then multiple systems are apparently redundant. If, on the other hand, these signals communicate other information, and if a high cell density is needed to successfully do so, then each system could well serve a unique purpose. Furthermore, cell-cell signaling is used in several forms of bacterial development, where census taking is less important than the coordination of individual cells as they create a multicellular structure. Conferee Don Clewell argued that a distinction should be made between signals used for the purpose of census taking and signals used for other purposes, such as bacterial development or the detection of conjugation recipients. He proposed that a word such as “quoromone” be adopted to designate the former class of signals.
GRAM-POSITIVE BACTERIA: SIGNALING VIA OLIGOPEPTIDES
Sporulation and competence in Bacillus subtilis.
Most bacteria have the capacity to survive for extended intervals in stationary phase. Under these conditions, some groups of bacteria form metabolically inert spores that are able to endure inimical environments for indefinite periods. The most thoroughly studied example of this phenomenon is formation of endospores by B. subtilis. During the transition from logarithmic growth to stationary phase, B. subtilis becomes hypermotile and chemotactic, and it releases a variety of hydrolytic enzymes. Antibiotics are also released, presumably to limit interspecies competition. Also, during the transition to stationary phase, B. subtilis becomes competent for the uptake and homologous integration of DNA. These processes occur preferentially at high bacterial cell population densities and are stimulated by the exchange of chemical signals (35, 106).
Endospore formation is initiated by a phosphorelay signal transduction system composed of five histidine protein kinases (KinA, KinB, KinC, KinD, and KinE) (48), an intermediate response regulator (Spo0F), a phosphotransferase (Spo0B), and a global response regulator protein (Spo0A). This phosphorelay circuit is regulated at numerous points. Most important for this discussion is that Spo0F-phosphate (Spo0F∼P) can be dephosphorylated by three specific phosphatases (RapA, RapB, and RapE), and these phosphatases are controlled by diffusible peptide signals called PhrA and PhrE (85, 87). PhrA and PhrE are specific inhibitors of RapA and RapE, respectively. These peptides are synthesized as 44-amino-acid precursors that are exported from the bacterial cytoplasm by the general secretory pathway. During export, the precursor peptides are processed, and the C-terminal pentapeptides are released as the signals. Processing was previously believed to occur in several steps. However, Marta Perego provided evidence at the conference that the precursor peptides contain unusually long signal sequences that are removed by type I signal peptidases in a single step (S. Ishikawa, L. Core, S. Stephenson, and M. Perego, Cell-Cell Communication in Bacteria meeting, abstr. S-6, 2001). This processing event is most likely common to all Phr precursor peptides, as it was shown to apply to PhrA, PhrE, and PhrC (97).
The processed oligopeptide signals are imported into the cytoplasm via an ATP-binding cassette (ABC)-type oligopeptide permease. In the cytoplasm, the Phr pentapeptides bind to the corresponding Rap phosphatases, inhibiting their activity, as has been shown in vitro for the RapA-PhrA pair (87). Perego also demonstrated that a direct interaction between RapA and its target protein, Spo0F∼P, occurs, and she showed that the complex dissociates upon dephosphorylation or the addition of the inhibitory PhrA pentapeptide. The completed genome sequence of B. subtilis shows that it contains at least nine additional genes that resemble rapA and rapB (59, 86), suggesting that other two-component regulatory systems may be controlled by dedicated protein phosphatases.
Competence in Streptococcus pneumoniae.
Most peptide signals in gram-positive signaling systems do not require import of the peptide and instead are detected by membrane-spanning two-component sensor kinases. A good example is found in the streptococci, which become genetically competent during the late exponential phase of growth and where high population densities stimulate the process. The products of the comCDE and comAB operons mediate signaling in S. pneumoniae. The comC gene encodes a 40-amino-acid precursor peptide that is exported and processed by the products of the comAB operon. The mature peptide is 17 amino acids in length and is designated CSP (41). CSP is detected by the histidine protein kinase ComB, which transfers phosphate to the response regulator ComE (44). At the conference, Leiv Havarstein reported that ComE∼P activates the expression of a dedicated sigma factor, ComX (T. Blomquist and L. S. Havarstein, Cell-Cell Communication in Bacteria meeting, abstr. 6, 2001). He also described the sequencing of the comC genes of many streptococcal strains which revealed a great diversity of signal types (pherotypes). A particular pherotype responds to low concentrations of the signal that it releases but requires a 10- to 100-fold-higher concentration of a heterologous signal for a response (42). This has an obvious adaptive significance, since it ensures that induction occurs only in the presence of closely related organisms. On the other hand, because induction can occur at high concentrations of heterologous signals, this could have important implications for horizontal transfer between commensal streptococci and the pathogenic species S. pneumoniae.
Virulence in Staphylococcus aureus.
Another example of a peptide signal used for cell-cell communication is found in the pathogen S. aureus, which causes a variety of skin and lung infections (74). This bacterium utilizes a quorum-sensing system to control the production of exotoxins that are produced in late-log-phase broth cultures. In this system, the AgrD protein is synthesized as a 46-amino-acid precursor, which appears to be exported and processed by the AgrB protein. The mature autoinducing peptide (AIP) is eight amino acids long and contains a thiolactone bond between the amino terminus and an internal cysteine residue (47, 66). AIP is detected by the AgrC two-component sensor kinase, which transduces the signal by phosphorylation of the AgrA response regulator. AgrA∼P stimulates the transcription of the agrBDCA operon, which establishes a positive autoregulatory feedback loop. AgrA∼P also activates the transcription of a divergent gene that encodes a regulatory RNA called RNAIII. RNAIII, in turn, affects the expression of other pathogenesis genes (70). S. aureus strains can be classified into four or more groups based on variations in the AIP and on compensatory variations in its receptor. The oligopeptide of one group of S. aureus strains induces pathogenesis in that group and also specifically inhibits the Agr virulence systems of the other S. aureus groups. Apparently, an invading S. aureus strain that successfully establishes its positive autoregulatory loop fends off other invading S. aureus groups by specifically inhibiting their expression of exotoxins. Conferee Richard Novick described synthetic signal analogues that are made with lactone or lactam rings rather than thiolactone rings and/or that lack the linear peptide “tail” portion of the signal (R. P. Novick, T. W. Muir, and G. Lyon, Cell-Cell Communication in Bacteria meeting, abstr. S-3, 2001). His group showed that these analogues are inactive as inducers but effective as inhibitors, suggesting that these or similar compounds could be useful in treating staphylococcal infections (64).
Conjugation in Enterococcus faecalis.
E. faecalis has long been known to use peptide signals to regulate the conjugation of certain plasmids. Conjugation recipients release an unmodified seven- to eight-amino-acid signal that is part of the signal peptide of a much longer lipoprotein. This peptide is imported via an ABC-type permease and binds to a master regulatory protein to induce the expression of the tra regulon (19, 71, 72). Conjugal donors also release this signal; however, they also release a plasmid-encoded inhibitory peptide, and this second peptide reduces the expression of the tra regulon in the absence of conjugal recipients. Conference attendee Bettina Buttaro has discovered that the stimulatory signal plays two crucial roles in plasmid biology: in addition to promoting conjugation of pCF10, it is also essential for vegetative replication of the same plasmid (B. Buttaro and M. Neloni, Cell-Cell Communication in Bacteria meeting, abstr. 14, 2001). The inhibitory peptide binds specifically to the PrgW protein, which is required for vegetative plasmid replication. Curiously, while this signal can be added exogenously to induce the tra genes, it must be produced endogenously to support plasmid replication. In a separate report, Jiro Nakayama described a second peptide signal in E. faecalis; this peptide regulates expression of a gelatinase that plays a role in pathogenicity (J. Nakayama, Y. Cao, R. Kariyama, H. Kumon, W. M. de Vos, and H. Nagasawa, Cell-Cell Communication in Bacteria meeting, abstr. 18, 2001). The signal is a cyclic lactone and has a tail, reminiscent of the thiolactone peptides of S. aureus. Interestingly, in this case, the lactone ring involves a serine residue instead of the cysteine residue found in S. aureus peptide signals.
GRAM-NEGATIVE BACTERIA: SIGNALING VIA ACYL-HSL
The Vibrio fischeri LuxI/LuxR paradigm.
One of the earliest-described bacterial cell-cell communication systems was identified in the bioluminescent marine symbiotic bacterium V. fischeri (40, 73). Two regulatory components control light production (25-27). Specifically, a protein called LuxI is responsible for production of an acyl-HSL signal molecule termed an autoinducer, and a protein called LuxR is the autoinducer sensor as well as an autoinducer-dependent transcriptional activator of the luciferase operon. Similar to what we described above for gram-positive bacteria, as an autoinducer-producing population of V. fischeri cells grows, the concentration of external autoinducer increases as a function of cell population density. When the autoinducer concentration reaches the micromolar range, the autoinducer can interact with the LuxR protein, and the LuxR-autoinducer complex binds the luciferase promoter and activates transcription. Therefore, this quorum-sensing regulatory circuit allows light production to be tightly correlated with cell population density. A puzzling aspect of this system is how the individual V. fischeri cells avoid responding to endogenously produced autoinducer molecules present in the cytoplasm. It has been proposed that LuxI could directly release newly synthesized autoinducer across the cell envelope or that LuxR might detect only extracellular autoinducer. Indeed, in support of the latter hypothesis, LuxR has been demonstrated to be peripherally associated with the cytoplasmic face of the inner membrane, a subcellular localization that could enable LuxR to preferentially detect extracellular signal (55).
For over 10 years the V. fischeri LuxI/LuxR quorum-sensing circuit was considered an isolated example of bacterial communication that had presumably evolved for a specific purpose involved in the colonization of a eukaryotic host. However, we now understand that many proteobacteria communicate by using homologous signaling circuits, as quorum-sensing systems resembling the canonical V. fischeri circuit have been shown to control gene expression in over 50 species of gram-negative bacteria (for reviews, see references 2, 16, 30, and 68). In virtually every case examined, a protein resembling LuxI synthesizes an acyl-HSL autoinducer, while a protein resembling LuxR is required for response. Although only a few of these systems have been extensively studied, it is believed that all LuxR-type proteins bind their cognate autoinducers. Many biological processes are regulated by these cell-cell communication systems, including virulence, biofilm formation, antibiotic production, and conjugation.
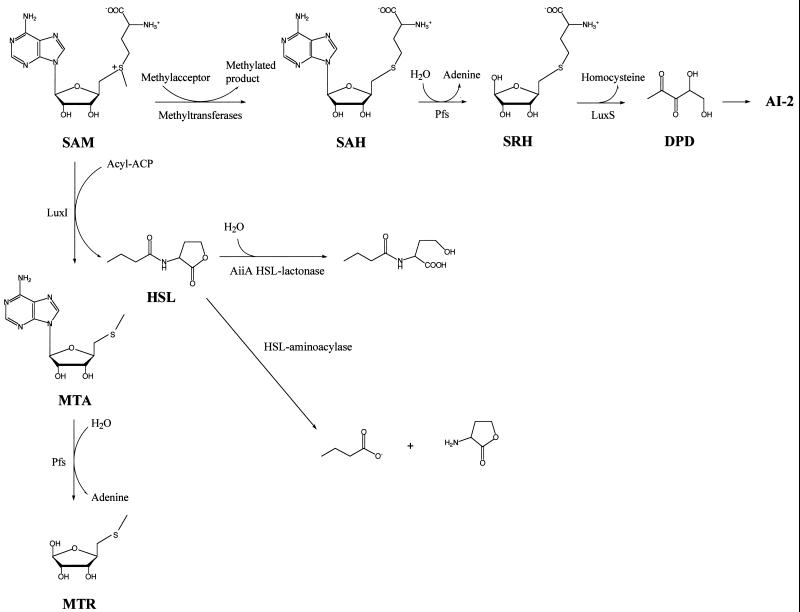
Several LuxI-like enzymes have been studied at the biochemical level. These enzymes obtain acyl groups from fatty acyl-acyl carrier proteins (fatty acyl-ACP) that are diverted from the fatty acid biosynthetic machinery. The HSL group is derived from S-adenosylmethionine (SAM). The reaction joining the HSL to the acyl side chain releases the ACP and also methylthioadenosine (MTA) as by-products (37, 69, 82, 103). The biosynthetic pathway for acyl-HSL production is shown in Fig. 2 . Acyl-HSL autoinducers can diffuse through the bacterial envelope, allowing them to increase in concentration in the external environment in conjunction with cell population growth (51). However, an active pump accelerates the efflux of at least one autoinducer, 3-oxo-C12-HSL, from Pseudomonas aeruginosa (28). The extracellular concentration of the autoinducers rises with increasing population density to the point where autoinducer efflux equals autoinducer influx. Subsequently, the intracellular concentration of autoinducer rises to the point where the LuxR-like proteins can bind their cognate autoinducers and then activate transcription of target DNA. The amino-terminal regions of the LuxR-like proteins are involved in autoinducer binding and oligomerization, while the carboxyl-terminal domains of the LuxR-like proteins are responsible for DNA binding and transcriptional activation (38).
FIG. 2.
Acyl-HSL and AI-2 biosynthesis and metabolism. Both acyl-HSL autoinducers and AI-2 are derived from SAM. In acyl-HSL biosynthesis (vertical reaction pathway), the LuxI-like enzymes catalyze the formation of the autoinducers (HSL) from SAM and specific acyl-ACP. MTA is formed as a product of AI-1 production. MTA is toxic, and the toxicity is relieved by the Pfs nucleosidase. Pfs hydrolyzes adenine from MTA to form methylthioribose (MTR). The further catabolism of MTR has not been defined. The HSL shown here is N-butyryl-l-HSL from P. aeruginosa. In AI-2 biosynthesis (top horizontal reaction pathway), methyl transfer from SAM to its various substrates results in the production of S-adenosyl homocysteine (SAH). SAH is toxic, and as in HSL biosynthesis, Pfs is responsible for relieving toxicity. In this case, the Pfs nucleosidase enzyme hydrolyzes adenine from SAH to form S-ribosylhomocysteine (SRH). LuxS acts on SRH to produce 4,5-dihydroxy-2,3-pentanedione (DPD) and homocysteine. DPD undergoes further rearrangements to produce the active AI-2 molecule. A Bacillus isolate and the soil bacterium Variovorax paradoxus produce autoinducer degradative activities. In the Bacillus isolate, the AiiA enzyme, an HSL lactonase, hydrolyzes the lactone ring of the autoinducer (middle horizontal reaction pathway). In the representative reaction shown, the P. aeruginosa autoinducer N-butyryl-l-homoserine lactone is converted to N-butyryl-l-homoserine. V. paradoxus produces an aminoacylase that cleaves HSL from the acyl side chain moiety (diagonal reaction pathway). Cleavage of the P. aeruginosa autoinducer is shown.
Acyl-HSL signaling in pathogenic bacteria.
A number of bacteria use LuxI/LuxR-type systems during colonization of plants and animals. Probably the best-studied animal pathogen is P. aeruginosa, which uses a complex circuit to regulate expression of various virulence factors (16, 81). Specifically, P. aeruginosa uses two LuxI/LuxR pairs (called LasI/LasR and RhlI/RhlR) that work in tandem to control a variety of genes (7, 83). The LasI/LasR system initiates the signaling cascade by inducing the transcription of virulence factors (13, 49, 83). Among the target genes of LasR is the rhlR gene (75). The RhlI/RhlR system further activates genes that are under LasI/LasR control, and RhlI/RhlR also activate another set of specific target genes (7, 39, 58, 81, 84, 88, 108). Regulation of RhlI/RhlR by LasI/LasR causes the two P. aeruginosa quorum-sensing circuits to initiate sequentially and in the proper order. A third LuxR homologue, named QscR, has recently been identified from the completed genome sequence of P. aeruginosa, although strangely, a corresponding LuxI-like autoinducer synthase was not identified. At the conference, QscR was described by Peter Greenberg as having an inhibitory effect on production of the LasI-dependent autoinducer (10). Apparently this hierarchical network ensures the precise timing of the expression of each of the quorum-sensing-controlled target genes in P. aeruginosa (E. P. Greenberg, Cell-Cell Communication in Bacteria meeting, abstr. S-1, 2001).
Signaling also plays an important role in the biology of the plant pathogen Agrobacterium tumefaciens, which causes crown gall tumors in plants. In A. tumefaciens, quorum-sensing controls the conjugation of a plasmid called the Ti plasmid that is required for A. tumefaciens pathogenicity (9, 89, 96). An autoinducer synthase, TraI, and an autoinducer receptor, TraR, control the expression of genes required for interbacterial conjugal transfer of the Ti plasmid (31, 89, 111). This circuit is also responsive to signals produced by the plant. Plant chemicals called opines that are produced in plant tumors initiate the quorum-sensing cascade by inducing the expression of traR. This step allows the characteristic LuxI/LuxR-type autoinduction circuit to be established only at the bacterium-plant interface.
Many other examples of gram-negative quorum-sensing circuits that regulate virulence (and other functions) exist that utilize a basic LuxI/LuxR quorum-sensing mechanism and incorporate additional regulators (68). These “designer” regulatory components enable a wide assortment of behaviors to be controlled by a common mechanism while precisely adapting the circuits to the specialized needs of an individual bacterial species residing in a unique niche. In most of these systems, the protein resembling LuxR acts as an autoinducer-dependent transcriptional activator, but in a few cases, the LuxR homologue acts as an autoinducer-independent repressor whose activity is inhibited by an autoinducer. The best characterized of these systems is the EsaR/EsaI system of Pantoea stewartii, which is a vascular pathogen of maize. Susanne von Bodman described her finding that EsaR represses its own transcription in the absence of the signal molecule produced by EsaI (3-oxo-C6-HSL) and that esaR is derepressed by the autoinducer. This result indicates that the signal inactivates the EsaR protein rather than activating it, which is fundamentally different from most LuxR-type proteins (S. B. von Bodman, T. D. Minogue, M. Wehland, and F. Bernhard, Cell-Cell Communication in Bacteria meeting, abstr. 22, 2001).
Acyl-HSL signaling in symbiotic bacteria.
The most extensively studied quorum-sensing system involved in bacterial symbiosis is that of the above-mentioned V. fischeri, in which LuxI/LuxR was first identified. Some strains of V. fischeri colonize the light organs of the squid Euprymna scolopes. Genetic aspects of this symbiosis are beginning to be revealed by the laboratories of Ned Ruby and Karen Visick. Ruby described a gene, litR, in which mutations delay lux gene expression but do not adversely affect symbiosis. The LitR protein can bind to the luxR promoter and is proposed to positively regulate luxR expression (E. G. Ruby, Cell-Cell Communication in Bacteria meeting, abstr. S-12, 2001). Visick described a gene, rscS, encoding a two-component kinase required for colonization. The RscS protein resembles the hybrid kinase-response regulator BvgS of Bordetella pertussis (K. L. Visick, Cell-Cell Communication in Bacteria meeting, abstr. 26, 2001). This system is hypothesized to detect a host-produced signal required for squid colonization (105). Colonization of the light organ causes profound developmental changes, including increased microvillar density along the crypt brush border and swelling of the epithelial cells, such that every bacterium directly contacts host tissues (104).
Allan Downie described quorum sensing in the legume symbiont Rhizobium leguminosarum (J. A. Downie, A. Wilkinson, J. K. Lithgow, B. Rodelas, F. Wisniewski-Dye, V. Danino, J. Jones, A. Zorreguieta, P. Williams, and A. Hardman, Cell-Cell Communication in Bacteria meeting, abstr. S-13, 2001). This organism contains no fewer than four LuxI-type proteins and six LuxR-type proteins (63). The CinI/CinR system synthesizes and detects 3-OH-C14:1-HSL. In addition to inducing cinI, CinR activates the plasmid-located raiI-raiR operon, encoding a second quorum-sensing system. RaiI synthesizes primarily 3-oxo-C8-HSL, which also activates raiI expression. The activation of this system also requires a second LuxR-type regulator, ExpR. This regulator activates the expression of a gene that was proposed to be involved in release of bacteria from biofilms. The symbiotic plasmid contains the genes encoding the third and fourth quorum-sensing systems (rhiI/rhiR, traI/triR, and also a gene called bisR). RhiI synthesizes primarily C6-HSL, and TraI synthesizes 3-oxo-C8-HSL. BisR detects 3-OH-C14:1-HSL and activates triR expression, while TriR detects 3-oxo-C8-HSL and activates the tra regulon of the plasmid. The tra functions control transfer of the plasmid required for symbiosis, while the rhi components influence nodulation.
Structural studies of LuxI/LuxR-type proteins.
Two structural biology presentations were given at the conference, one on the LuxR-type protein TraR of A. tumefaciens and the other on the LuxI-type protein EsaI of P. stewartii. Andrzej Joachimiak crystallized TraR in the presence of autoinducer and DNA (T. Pappas and S. C. Winans, Cell-Cell Communication in Bacteria meeting, abstr. S-4, 2001). The complex contained a dimer of protein per DNA binding site and one autoinducer per protein monomer. The autoinducer was completely engulfed by the protein, supporting biochemical studies showing that TraR uses its autoinducer as a scaffold for protein folding (112). The axis of dyad symmetry of the two N-terminal domains intersected the axis of symmetry of the C-terminal DNA binding domains at about 70°, creating a pronounced overall asymmetry to the protein. Conferee Stephen Farrand described two positive control mutants of a closely related TraR protein, one having a substitution at Asp10 and the other at Gly123. Asp10 of each protomer lies virtually adjacent to the Gly123 residue of the opposite protomer, suggesting that these amino acids could be located on a surface that contacts RNA polymerase (S. K. Farrand, Y. Qin, K. R. Piper, Z. Luo, and P. Oger, Cell-Cell Communication in Bacteria meeting, abstr. S-8, 2001). Conferee Mair Churchill and colleagues solved the structure of EsaI (W. T. Watson, F. V. Murphy, T. A. Gould, P. Jambeck, D. L. Val, J. E. Cronan, Jr., S. Beck von Bodman, and M. E. Churchill, Cell-Cell Communication in Bacteria meeting, abstr. 2, 2001). Churchill confirmed that the protein is monomeric and modeled one of the substrates (the fatty acyl group and phosphopantethiene moiety of acyl-ACP) into the active site. This modeling was partially based on a structural similarity between EsaI and a histone acetyltransferase and confirmed by the demonstration that a threonine residue predicted to bind the 3-oxo group of the fatty acid is required for wild-type substrate specificity.
SIGNALING IN MULTICELLULAR DEVELOPMENT AND SECONDARY METABOLISM
Quorum sensing in biofilms.
Quorum sensing was recently implicated in the maturation of biofilms formed by P. aeruginosa. Biofilms are highly ordered bacterial communities that allow bacteria to live adhered to surfaces. Biofilms can be made up of single or multiple species, and they possess aqueous channels for hydrating the cells, for distributing nutrients to the members of the community, and for removing waste products. Differential patterns of gene expression can be observed in distinct locations within a biofilm, suggesting that the individual members of the community have specific duties that, in combination, enhance the survival of the entire consortium (11, 12). P. aeruginosa biofilms exist in the lungs of cystic fibrosis (CF) sufferers, and an intact quorum-sensing circuit is necessary for proper biofilm formation by P. aeruginosa (13, 81). Autoinducers can be detected in the sputum of CF patients, according to conferees David Erickson (D. Erickson, R. Endersby, and D. G. Storey, Cell-Cell Communication in Bacteria meeting, abstr. 19, 2001) and Michael Surette (M. G. Surette, H. Rabin, C. Dammel, and J. Stein, Cell-Cell Communication in Bacteria meeting, abstr. 8, 2001). These findings imply that quorum sensing could be vital for the virulence of P. aeruginosa. Matthew Parsek described strains that express green fluorescent protein in response to the two autoinducers made by P. aeruginosa, and he used them to show that quorum-inducible genes are expressed preferentially at the basal layers of microcolonies (M. R. Parsek, M. Hentzer, M. Kiristis, R. Richards, and P. Stoodley, Cell-Cell Communication in Bacteria meeting, abstr. 9, 2001). These green fluorescent protein reporters were also used to study how signaling is affected by the flow of bulk medium across a biofilm.
Burkholderia cepacia is a newly recognized pathogen in CF infections (33). Usually, CF patients infected with B. cepacia are coinfected with P. aeruginosa (102). Conferee Michelle Visser and colleagues showed that the addition of the P. aeruginosa autoinducers to B. cepacia induces the expression of B. cepacia virulence factors (S. Lewenza, M. B. Visser, and P. A. Sokol, Cell-Cell Communication in Bacteria meeting, abstr. 30, 2001). It is hypothesized that interspecies communication may allow a mixed population of P. aeruginosa and B. cepacia to coordinately regulate virulence factors and influence the progression of lung disease during coinfections (61).
Development of multicellular fruiting bodies in Myxococcus xanthus.
M. xanthus is a soil bacterium that glides over solid surfaces and colonizes decaying plant material. These bacteria exhibit intricate social behaviors, such as hunting in swarms for nutrients. This behavior allows the individual bacterial cells to profit from the secreted hydrolytic enzymes produced by neighboring cells (21, 22). When M. xanthus experiences starvation at high cell density, it undergoes a series of developmental events that include rippling, aggregation to form fruiting bodies, and sporulation within these fruiting bodies. Bacteria inside the fruiting body undergo a differentiation process that leads to myxospore formation.
Several cell-cell signaling processes control spore formation. One cell-cell signal, called the A signal, is a mixture of amino acids that are produced as a consequence of the action of extracellular proteases (56, 57, 91). Genetic analysis of developmental mutants shows that three loci, designated asgA, asgB, and asgC, are required to produce A signal. AsgA is a two-component histidine kinase, AsgB is a DNA binding transcriptional regulator, and AsgC is the major housekeeping ςsgr; factor (14, 90, 92). These proteins, along with other, as-yet-unidentified factors, function in a pathway that regulates the expression of the genes encoding the secreted proteases. The proteases, in turn, function to produce the mixture of amino acids that comprise the A signal. The A signal is detected by another two-component system, composed of the SasS sensor kinase and the SasR response regulator pair (52, 109). SasR∼P interacts with the alternative ςsgr; factor ςsgr;54 to activate structural and regulatory genes involved in the myxospore differentiation process (32).
The A signal functions early in the M. xanthus developmental cycle. At later times in development, additional extracellular signals are required (36, 67). The best characterized of these other signals is the C signal, which is a cell contact signal. Dale Kaiser described how the C signal is a morphogen for the assembly of fruiting bodies and the differentiation of myxospores (D. Kaiser, Cell-Cell Communication in Bacteria meeting, abstr. S-5, 2001). The specific activity of this signal, which is encoded by the csgA gene and is displayed on the cell surface, increases about 15-fold from early development to 20 h when sporulation begins, due to a positive feedback loop. The rise in C-signal specific activity is important for Myxococcus development because rippling, aggregation, and sporulation each have different CsgA thresholds, and the C-signal increase organizes those stages appropriately. The increase in C signal is controlled by the four genes in the act operon. The actA and actB genes regulate the maximum level of CsgA production, and the actC and actD genes regulate its timing pattern (34). Rippling, the production of traveling ridge-like heaps of cells, is confined to the first 20 h of development in the wild type. However, in mutants without the act operon-directed positive feedback loop, rippling may persist for 4 days, consistent with prolonged low levels of the signal. C signal initiates aggregation via the frz (frizzy) phosphorelay. Mutations in a frz gene result in very abnormal aggregates (107). Although the frz genes encode proteins resembling enteric bacterial proteins that are required for chemotaxis, aggregation is not the result of chemotaxis. Rather, the C signal channeled through the frz phosphorelay has a chemokinetic effect, which apparently causes the cells to decrease their reversal frequency and increase their speed (45). This change in behavior leads cells to stream into aggregation centers.
Heterocyst development in Anabaena.
Signaling via peptides has also been documented in at least one member of the cyanobacteria. In the presence of fixed nitrogen, Anabaena spp. grow in long chains of phototrophic cells. Growth under nitrogen limiting conditions causes 10% of these cells to differentiate into nitrogen-fixing heterocysts. The heterocysts are arranged as single cells at regular intervals along the chains of vegetative cells (24). This heterocyst spatial pattern is established by the release of a peptide signal that is produced only in the differentiated heterocysts. The peptide signal inhibits heterocyst development in neighboring cells. A precursor of this peptide is encoded by the patS gene, which is highly expressed in heterocysts but not in vegetative cells, and addition of a synthetic peptide corresponding to the last five amino acids of PatS is sufficient to inhibit heterocyst development (110). The concentration of signal should be highest in the two cells that are immediately adjacent to the differentiated heterocyst, and signal concentration decreases in each successive cell along the chain. The PatS peptide signal is required to resolve clusters of nitrogen-starved cells so that only a single cell differentiates into a heterocyst (110). To determine whether PatS functions within the bacterial cell, conferee James Golden and colleagues constructed “minigene” alleles of patS that lack its signal sequence and contain only the last five to eight codons. These minigenes were functional in suppressing heterocysts, suggesting that PatS is functional intracellularly and may interact with a cytoplasmic receptor (J. W. Golden, H. Yoon, D. Liu, M. H. Lee, I. Khudyakov, and X. Wu, Cell-Cell Communication in Bacteria meeting, abstr. S-7, 2001).
Streptomyces signaling.
Sporulation and the production of secondary metabolites, including antibiotics, by members of the genus Streptomyces require cell-cell signaling. These filamentous bacteria synthesize and release a family of related γ-butyrolactones that stimulate production of antibiotics and other secondary metabolites, and they also stimulate the formation of aerial spores (43, 76, 77). These γ-butyrolactones possess a striking chemical similarity to acyl-HSLs in that both are composed of a polar ring and a short-chain fatty acid.
The A factor is 2-iso-capryloyl-3R-hydroxymethyl-γ-butyrolactone, and it serves as a cell-cell signaling molecule in Streptomyces griseus (53, 54). Synthesis of A factor requires the afsA gene, and expression of this gene in Escherichia coli results in the production of A factor. AfsA activity can be blocked by cerulenin, indicating that the AfsA enzyme consumes fatty acid biosynthetic intermediates. This biochemical mechanism is extremely similar to LuxI-type HSL synthases, which also consume intermediates in fatty acid biosynthesis. However, AfsA is unlike LuxI-type proteins in that it does not consume SAM. Rather, AfsA uses glycerol or a similar compound for biosynthesis of A factor. A factor binds with high affinity to the ArpA protein. Null mutations in the arpA gene cause constitutive expression of streptomycin and cause premature sporulation as well as the inability to sequester A factor, indicating that ArpA is a repressor that is inactivated upon binding of A factor (78, 79). Cloning and analysis of aprA show that AprA is a cytoplasmic protein with an N-terminal helix-turn-helix DNA binding domain (78).
Eriko Takano of the John Innes Center described a homologous system in the genetically well-characterized species Streptomyces coelicolor (E. Takano, T. Nihira, Y. Yamada, and M. J. Bibb, Cell-Cell Communication in Bacteria meeting, abstr. 15, 2001). This species releases a γ-butyrolactone similar to A factor that, when added exogenously, stimulates antibiotic production but does not affect sporulation. The scbA gene is required for production of this compound and resembles afsA of S. griseus, while the divergently transcribed scbR resembles arpA and encodes a regulatory protein that detects the signal molecule. ScbR binds to the region between these genes, activating scbA and repressing scbR. Binding is abolished by γ-butyrolactone. Signaling via γ-butyrolactones is not limited to gram-positive organisms, as they have also been implicated in signaling in at least one proteobacterium, Xanthomonas campestris (1).
MULTILINGUAL BACTERIA
All of the quorum-sensing systems we have described so far rely on the precise recognition of an autoinducer by its cognate detector. However, recent studies suggest that many bacteria possess interspecies signaling systems as well as species-specific systems. These findings imply that bacteria can assess their own population numbers and also the population density of other species of bacteria in the vicinity. Furthermore, distinct responses to the intraspecies and interspecies signals could allow a particular species of bacteria to properly modulate its behavior depending on whether it makes up a majority or a minority of any given consortium.
Vibrio harveyi and the LuxS language.
Research on interspecies bacterial communication originated with studies of the bioluminescent marine bacterium V. harveyi (3). V. harveyi, while related to V. fischeri, is not known to live in symbiotic associations with higher organisms. V. harveyi uses quorum sensing to control bioluminescence. However, V. harveyi uses two different autoinducer signals (4, 5), only one of which (AI-1) is a typical acyl-HSL (8). The second autoinducer (AI-2) is a novel signaling molecule that appears to have been designed for interspecies cell-cell communication (2, 3, 68, 94, 99).
V. harveyi does not employ a typical LuxI/LuxR-type quorum-sensing cascade (4-6). Instead, each signal is detected by a dedicated kinase (LuxN for AI-1 and LuxQ for AI-2) (4, 5). Additionally, a soluble periplasmic protein, LuxP, which resembles several ribose binding proteins, is required for detection of AI-2 (5). This homology seems appropriate, since AI-2 resembles ribose (95). Both LuxN and LuxQ are two-component hybrid sensor kinase-response regulator proteins that funnel phosphoryl groups via LuxU to LuxO, a ςsgr;54-dependent transcriptional activator protein that is hypothesized to control the expression of an unidentified repressor of the luciferase structural operon (luxCDABE) (5, 29, 62).
Stephan Schauder reported at the conference that AI-2 synthesis requires a protein designated LuxS (100), which, along with Pfs, makes up a pathway for the conversion of S-adenosyl homocysteine (SAH, which is generated from SAM) to homocysteine, adenine, and a compound that is proposed to spontaneously cyclize to form AI-2 (94, 95). Purified Pfs and LuxS enzymes from diverse bacteria were used to synthesize AI-2 from SAH in vitro. In every case, AI-2 with identical specific activity was produced, and the other product of the LuxS reaction was homocysteine (S. Schauder and B. L. Bassler, Cell-Cell Communication in Bacteria meeting, abstr. 3, 2001). These findings strongly suggest that AI-2 molecules from diverse species of bacteria are chemically identical (95). The pathway for AI-2 production is presented in Fig. 2. The figure shows that biosynthesis of acyl-HSL autoinducers and that of AI-2 require common components. Production of both classes of signaling molecules relies on the central metabolite SAM, and the nucleosidase enzyme Pfs is required in both pathways for the removal of the toxic by-products MTA (acyl-HSLs) and SAH (AI-2) (95).
The ability to synthesize AI-1 is largely restricted to V. harveyi. However, many genera of eubacteria produce AI-2, and luxS homologues have been identified in over 40 species of eubacteria (68, 100). Most of these bacteria have been shown to produce AI-2, and in every case tested, mutagenesis of luxS eliminated AI-2 production (15, 50, 65, 98, 101; S. Y. Kim, S. E. Lee, Y. R. Kim, J. H. Kim, P. Y. Ryu, S. S. Chung, and J. H. Rhee, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-248, 2000). These results show that LuxS defines a new bacterial signal, one that is released by diverse types of eubacteria.
It is of interest to know what genes are regulated by AI-2 in all the luxS-containing bacteria. To date, several reports indicate that pathogenicity factors are controlled by AI-2 in E. coli, Shigella flexneri, Streptococcus pyogenes, and Vibrio vulnificus (15, 65, 98; Kim et al., Abstr. 100th Gen. Meet. Am. Soc. Microbiol.). At the conference, Michiko Taga reported that an ABC-type transporter is controlled by AI-2 in Salmonella enterica serovar Typhimurium and that this transport apparatus is required for import of the AI-2 molecule from S. enterica serovar Typhimurium culture fluid (101; M. E. Taga and B. L. Bassler, Cell-Cell Communication in Bacteria meeting, abstr. S-14, 2001). Not all of the bacteria that possess a luxS gene are pathogens. It is suspected that a fundamental regulon of genes is controlled by AI-2 in many luxS-containing bacteria. In addition, niche-specific genes that differ for each LuxS-containing species of bacteria are probably also controlled by this newly identified signaling molecule.
INTRASPECIES, INTERSPECIES, AND INTERKINGDOM CELL-CELL COMMUNICATION
Regardless of the type of autoinducer signal used, intraspecies bacterial quorum sensing allows a population of bacteria to coordinate its behavior. Therefore, quorum sensing enables a group of bacteria to act in a concerted manner and thus acquire some of the characteristics of multicellular organisms. Likewise, interspecies bacterial communication can serve to synchronize the specialized functions of the species in the group. Appropriate exploitation of the diversity present in any given population could enhance the survival of the entire community. Furthermore, the productive interactions promoted by quorum sensing could be instrumental in the development of multispecies bacterial organizations such as biofilms as well as in the establishment of specific symbiotic associations with eukaryotic hosts.
At times it might be useful for one group of bacteria to disrupt the quorum-sensing circuit of a second competing group of bacteria. New research on this topic has revealed several examples of anti-quorum-sensing strategies in use by coexisting populations of bacteria. As mentioned, S. aureus groups use peptide quorum sensing to control their own agr virulence and also to inhibit virulence in other S. aureus groups (46, 66, 80). An alternative strategy for preventing quorum sensing by competitor bacteria has evolved in an isolate of Bacillus. Conferee Lian-Hui Zhang has found that this isolate produces an enzyme called AiiA that destroys acyl-HSL autoinducers (Y. H. Dong, L. Wang, J. L. Xu, H. B. Zhang, and L. Zhang, Cell-Cell Communication in Bacteria meeting, abstr. 32, 2001). The hypothesis that this enzyme could block signaling was tested using Erwinia carotovora, a plant pathogen that uses quorum sensing to synthesize pectinases and other hydrolytic enzymes and also to regulate antibiotic production. AiiA blocked the production of both classes of compounds (18). Presumably this strategy makes E. carotovora unable to compete effectively against this Bacillus isolate. Transgenic tobacco plants expressing AiiA were protected against E. carotovora infection (17), and P. aeruginosa expressing AiiA was deficient in biofilm formation and showed attenuated pathogenicity. Similarly, the soil bacterium Variovorax paradoxus can use acyl-HSLs as the sole source of carbon and nitrogen (60). This study suggests that, in its natural habitat, V. paradoxus might grow on acyl-HSLs as a means to thwart other quorum-sensing bacteria and gain a competitive edge in the environment. A different biochemical mechanism is used for degradation of acyl-HSL autoinducers by the AiiA containing a Bacillus isolate and V. paradoxus. The autoinducer degradation pathways are shown in Fig. 2 (17, 60). The AiiA enzyme destroys HSL autoinducers by hydrolyzing the lactone ring (Fig. 2) (17), while V. paradoxus apparently produces an aminoacylase that cleaves the intact lactone ring from its acyl side chain (Fig. 2) (60).
CONCLUSIONS
Studies of quorum-sensing systems demonstrate that bacteria have evolved multiple chemical languages for communicating within and between species. Intra- and interspecies cell-cell communication allows bacteria to coordinate various physiological activities and to behave like multicellular organisms. The biological functions regulated by quorum sensing are diverse and presumably reflect the specialized needs of bacterial communities inhabiting distinct niches. Competing bacteria and susceptible eukaryotic hosts have evolved natural strategies for obstructing quorum-sensing bacteria by destroying the chemical signal molecules or producing signal antagonists that interfere with recognition of the bona fide autoinducer molecule. Similarly, in symbiotic associations, bacteria and most likely their eukaryotic hosts have evolved tactics that enhance the quorum-sensing abilities of beneficial bacteria. These natural strategies that inhibit or enhance quorum sensing are being used as models in the design of analogous synthetic therapies intended to manipulate quorum-sensing systems. Significant biotechnological research is now focused on the design or identification of molecules that are structurally related to autoinducers. Such molecules have potential use as antimicrobial drugs designed to defeat bacteria that employ quorum-sensing circuits in the regulation of pathogenicity. Similarly, the biosynthetic enzymes involved in autoinducer production as well as the autoinducer detection apparatuses are viewed as potential targets for antibacterial drug design. Finally, biotechnological methods aimed at exploiting useful bacterial quorum-sensing processes have potential uses in the improvement of industrial-scale production of natural products such as antibiotics. With or without practical applications, continued study of bacterial quorum-sensing systems promises to give biologists new insights into novel mechanisms of intra- and intercellular signal transmission, intra- and interspecies communication, and the evolution of multicellular organisms. The ASM-sponsored conference at Snowbird in 2001 highlighted many new areas in the fascinating practical and theoretical aspects of how bacteria talk to each other.
Acknowledgments
We appreciate financial assistance for this conference from the National Institute of General Medical Sciences, the National Institutes of Dental and Craniofacial Research, the National Science Foundation, as well as Aurora Biosystems, Pfizer, Stovall Life Sciences, and Syngentia. Research in our laboratories is supported by NIGMS grant GM42893 and NSF grant MCB-9904917 to S.C.W. and by NSF grants MCB-0083160 and MCB-0094447 and ONR grant N00014-99-0767 to B.L.B.
We thank all conference participants for the scientific openness that characterizes this field.
REFERENCES
- 1.Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. Wilson, H. Slater, J. M. Dow, P. Williams, and M. J. Daniels. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555-566. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 3.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. [DOI] [PubMed] [Google Scholar]
- 5.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 6.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Sequence and function of LuxO, a negative regulator of luminescence in Vibrio harveyi. Mol. Microbiol. 12:403-412. [DOI] [PubMed] [Google Scholar]
- 7.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, J. G., and E. A. Meighen. 1989. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J. Biol. Chem. 264:21670-21676. [PubMed] [Google Scholar]
- 9.Christie, P. J. 1997. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J. Bacteriol. 179:3085-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chugani, S. A., M. Whiteley, K. M. Lee, D. D'Argenio, C. Manoil, and E. P. Greenberg. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 98:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 12.Costerton, J. W., Z. Lewandowski, D. DeBeer, D. Caldwell, D. Korber, and G. James. 1994. Biofilms, the customized microniche. J. Bacteriol. 176:2137-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 14.Davis, J. M., J. Mayor, and L. Plamann. 1995. A missense mutation in rpoD results in an A-signalling defect in Myxococcus xanthus. Mol. Microbiol. 18:943-952. [DOI] [PubMed] [Google Scholar]
- 15.Day, W. A., Jr., and A. T. Maurelli. 2001. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect. Immun. 69:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 18.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunny, G. M., R. A. Craig, R. L. Carron, and D. B. Clewell. 1979. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid 2:454-465. [DOI] [PubMed] [Google Scholar]
- 20.Dunny, G. M., and S. C. Winans. 1999. Cell-cell communication in bacteria. American Society for Microbiology, Washington, D.C.
- 21.Dworkin, M. 1973. Cell-cell interactions in the myxobacteria. Symp. Soc. Gen. Microbiol. 23:125-147. [Google Scholar]
- 22.Dworkin, M., and D. Kaiser. 1985. Cell interactions in myxobacterial growth and development. Science 230:18-24. [DOI] [PubMed] [Google Scholar]
- 23.Egland, R., G. Hobbs, N. J. Bainton, and D. M. Roberts. 1999. Microbial signalling and communication. Cambridge University Press, Cambridge, United Kingdom.
- 24.Elhai, J., and C. P. Wolk. 1990. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 9:3379-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 26.Engebrecht, J., and M. Silverman. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA 81:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engebrecht, J., and M. Silverman. 1987. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic Acids Res. 15:10455-10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans, K., L. Passador, R. Srikumar, E. Tsang, J. Nezezon, and K. Poole. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 180:5443-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman, J. A., and B. L. Bassler. 1999. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 181:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 31.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorski, L., T. Gronewold, and D. Kaiser. 2000. A ςsgr;54 activator protein necessary for spore differentiation within the fruiting body of Myxococcus xanthus. J. Bacteriol. 182:2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996. Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 34.Gronewold, T. M., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for Myxococcus xanthus development. Mol. Microbiol. 40:744-756. [DOI] [PubMed] [Google Scholar]
- 35.Grossman, A. D., and R. Losick. 1988. Extracellular control of spore formation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 85:4369-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 37.Hanzelka, B. L., and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J. Bacteriol. 178:5291-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanzelka, B. L., A. M. Stevens, M. R. Parsek, T. J. Crone, and E. P. Greenberg. 1997. Mutational analysis of the Vibrio fischeri LuxI polypeptide: critical regions of an autoinducer synthase. J. Bacteriol. 179:4882-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassett, D. J., J. F. Ma, J. G. Elkins, T. R. McDermott, U. A. Ochsner, S. E. West, C. T. Huang, J. Fredericks, S. Burnett, P. S. Stewart, G. McFeters, L. Passador, and B. H. Iglewski. 1999. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol. Microbiol. 34:1082-1093. [DOI] [PubMed] [Google Scholar]
- 40.Hastings, J. W., and K. H. Nealson. 1977. Bacterial bioluminescence. Annu. Rev. Microbiol. 31:549-595. [DOI] [PubMed] [Google Scholar]
- 41.Havarstein, L. S., G. Coomaraswamy, and D. A. Morrison. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 92:11140-11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havarstein, L. S., R. Hakenbeck, and P. Gaustad. 1997. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J. Bacteriol. 179:6589-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horinouchi, S. 1999. γ-Butyrolactones that control secondary metabolism and cell differentiation in Streptomyces, p. 193-207. In G. M. Dunny and S. C. Winans (ed.), Cell-cell communication in bacteria. American Society for Microbiology, Washington, D.C.
- 44.Hui, F. M., and D. A. Morrison. 1991. Genetic transformation in Streptococcus pneumoniae: nucleotide sequence analysis shows comA, a gene required for competence induction, to be a member of the bacterial ATP-dependent transport protein family. J. Bacteriol. 173:372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jelsbak, L., and L. Sogaard-Andersen. 1999. The cell surface-associated intercellular C-signal induces behavioral changes in individual Myxococcus xanthus cells during fruiting body morphogenesis. Proc. Natl. Acad. Sci. USA 96:5031-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 47.Ji, G., R. C. Beavis, and R. P. Novick. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92:12055-12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang, M., W. Shao, M. Perego, and J. A. Hoch. 2000. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol. 38:535-542. [DOI] [PubMed] [Google Scholar]
- 49.Jones, S., B. Yu, N. J. Bainton, M. Birdsall, B. W. Bycroft, S. R. Chhabra, A. J. Cox, P. Golby, P. J. Reeves, S. Stephens, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joyce, E. A., B. L. Bassler, and A. Wright. 2000. Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded autoinducer. J. Bacteriol. 182:3638-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan, H. B., A. Kuspa, and D. Kaiser. 1991. Suppressors that permit A-signal-independent developmental gene expression in Myxococcus xanthus. J. Bacteriol. 173:1460-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khokhlov, A. S. 1988. Results and perspectives of actinomycete autoregulators studies, p. 338-345. In Y. Okami, T. Beppu, and H. Ogawara (ed.), Biology of actinomycetes '88. Japan Scientific Societies Press, Tokyo, Japan.
- 54.Khokhlov, A. S., I. I. Tovarova, L. N. Borisova, S. A. Pliner, L. N. Shevchenko, E. I. Kornitskaia, N. S. Ivkina, and I. A. Rapoport. 1967. The A-factor, responsible for streptomycin biosynthesis by mutant strains of Actinomyces streptomycini. Dokl. Akad. Nauk. SSSR 177:232-235. [PubMed] [Google Scholar]
- 55.Kolibachuk, D., and E. P. Greenberg. 1993. The Vibrio fischeri luminescence gene activator LuxR is a membrane-associated protein. J. Bacteriol. 175:7307-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhiR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 59.Lazazzera, B. A., T. Palmer, J. Quisel, and A. D. Grossman. 1999. Cell density control of gene expression in Bacillus subtilis, p. 27-46. In G. M. Dunny and S. C. Winans (ed.), Cell-cell communication in bacteria. American Society for Microbiology, Washington, D.C.
- 60.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lilley, B. N., and B. L. Bassler. 2000. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36:940-954. [DOI] [PubMed] [Google Scholar]
- 63.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dye, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 64.Lyon, G. J., P. Mayville, T. W. Muir, and R. P. Novick. 2000. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc. Natl. Acad. Sci. USA 97:13330-13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyon, W. R., J. C. Madden, J. C. Levin, J. Stein, and M. G. Caparon. 2001. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 42:145-157. [DOI] [PubMed] [Google Scholar]
- 66.Mayville, P., G. Ji, R. Beavis, H. Yang, M. Goger, R. P. Novick, and T. W. Muir. 1999. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl. Acad. Sci. USA 96:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McVittie, A., F. Messik, and S. A. Zahler. 1962. Developmental biology of Myxococcus. J. Bacteriol. 84:546-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 69.More, M. I., L. D. Finger, J. L. Stryker, C. Fuqua, A. Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 70.Morfeldt, E., L. Janzon, S. Arvidson, and S. Lofdahl. 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211:435-440. [DOI] [PubMed] [Google Scholar]
- 71.Nakayama, J., Y. Abe, Y. Ono, A. Isogai, and A. Suzuki. 1995. Isolation and structure of the Enterococcus faecalis sex pheromone, cOB1, that induces conjugal transfer of the hemolysin/bacteriocin plasmids, pOB1 and pYI1. Biosci. Biotechnol. Biochem. 59:703-705. [DOI] [PubMed] [Google Scholar]
- 72.Nakayama, J., K. Yoshida, H. Kobayashi, A. Isogai, D. B. Clewell, and A. Suzuki. 1995. Cloning and characterization of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J. Bacteriol. 177:5567-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nealson, K. H., and J. W. Hastings. 1979. Bacterial bioluminescence: its control and ecological significance. Microbiol. Rev. 43:496-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novick, R. P. 1999. Regulation of pathogenicity in Staphylococcus aureus by a peptide-based density-sensing system, p. 129-146. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 75.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohnishi, Y., and S. Horinouchi. 1999. Regulation of secondary metabolism and morphological differentiation by a microbial hormone in Streptomyces. Tanpakushitsu Kakusan Koso 44:1552-1561. [PubMed] [Google Scholar]
- 77.Ohnishi, Y., S. Kameyama, H. Onaka, and S. Horinouchi. 1999. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: identification of a target gene of the A-factor receptor. Mol. Microbiol. 34:102-111. [DOI] [PubMed] [Google Scholar]
- 78.Onaka, H., N. Ando, T. Nihira, Y. Yamada, T. Beppu, and S. Horinouchi. 1995. Cloning and characterization of the A-factor receptor gene from Streptomyces griseus. J. Bacteriol. 177:6083-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Onaka, H., and S. Horinouchi. 1997. DNA-binding activity of the A-factor receptor protein and its recognition DNA sequences. Mol. Microbiol. 24:991-1000. [DOI] [PubMed] [Google Scholar]
- 80.Otto, M., R. Sussmuth, C. Vuong, G. Jung, and F. Gotz. 1999. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 450:257-262. [DOI] [PubMed] [Google Scholar]
- 81.Parsek, M. R., and E. P. Greenberg. 1999. Quorum sensing signals in development of Pseudomonas aeruginosa biofilms. Methods Enzymol. 310:43-55. [DOI] [PubMed] [Google Scholar]
- 82.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 84.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perego, M. 1997. A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc. Natl. Acad. Sci. USA 94:8612-8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perego, M. 1999. Self-signaling by Phr peptides modulates Bacillus subtilis development, p. 243-258. In G. M. Dunny and S. C. Winans (ed.), Cell-cell communication in bacteria. American Society for Microbiology, Washington, D.C.
- 87.Perego, M., and J. A. Hoch. 1996. Cell-cell communication regulates the effects of protein aspartate phosphatases on the phosphorelay controlling development in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:1549-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pesci, E. C., J. P. Pearson, P. C. Seed, and B. H. Iglewski. 1997. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 179:3127-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piper, K. R., S. B. von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448-450. [DOI] [PubMed] [Google Scholar]
- 90.Plamann, L., J. M. Davis, B. Cantwell, and J. Mayor. 1994. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J. Bacteriol. 176:2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174:3311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plamann, L., Y. Li, B. Cantwell, and J. Mayor. 1995. The Myxococcus xanthus asgA gene encodes a novel signal transduction protein required for multicellular development. J. Bacteriol. 177:2014-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandburg, C. 1916. Chicago poems. Henry Holt and Company, New York, N.Y.
- 94.Schauder, S., and B. L. Bassler. 2001. The languages of bacteria. Genes Dev. 15:1468-1480. [DOI] [PubMed] [Google Scholar]
- 95.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 96.Sheng, J., and V. Citovsky. 1996. Agrobacterium-plant cell DNA transport: have virulence proteins, will travel. Plant Cell 8:1699-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10:2014-2024. [DOI] [PubMed] [Google Scholar]
- 98.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 96:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taga, M. E., J. L. Semmelhack, and B. L. Bassler. 2001. The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol. Microbiol. 42:777-794. [DOI] [PubMed] [Google Scholar]
- 102.Taylor, R. F., H. Gaya, and M. E. Hodson. 1993. Pseudomonas cepacia: pulmonary infection in patients with cystic fibrosis. Respir. Med. 87:187-192. [DOI] [PubMed] [Google Scholar]
- 103.Val, D. L., and J. E. Cronan, Jr. 1998. In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the LuxI family of autoinducer synthases. J. Bacteriol. 180:2644-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Visick, K. L., and L. M. Skoufos. 2001. Two-component sensor required for normal symbiotic colonization of Euprymna scolopes by Vibrio fischeri. J. Bacteriol. 183:835-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waldburger, C., D. Gonzalez, and G. H. Chambliss. 1993. Characterization of a new sporulation factor in Bacillus subtilis. J. Bacteriol. 175:6321-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ward, M. J., and D. R. Zusman. 1999. Motility in Myxococcus xanthus and its role in developmental aggregation. Curr. Opin. Microbiol. 2:624-629. [DOI] [PubMed] [Google Scholar]
- 108.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang, C., and H. B. Kaplan. 1997. Myxococcus xanthus sasS encodes a sensor histidine kinase required for early developmental gene expression. J. Bacteriol. 179:7759-7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoon, H. S., and J. W. Golden. 1998. Heterocyst pattern formation controlled by a diffusible peptide. Science 282:935-938. [DOI] [PubMed] [Google Scholar]
- 111.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]
- 112.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 98:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]