Abstract
Many microorganisms have sets of parallel pathways for ATP production in respiration and for ATP utilization in glutamate synthesis. The alternatives differ in efficiency of ATP production and utilization. The choice among these parallel pathways has been hypothesized to control the speed and efficiency of growth. Thus, the organism should be able to alleviate (or exaggerate) deficiency in one pathway by deleting another. I show here that in Escherichia coli the effect of lack of the glutamate-synthesizing enzyme glutamate dehydrogenase on glucose-limited growth is altered predictably by ndh, cyo, and cyd mutations affecting parallel pathways leading to ATP synthesis in respiration.
In a classic study (6), Bauchop and Elsden showed that the apparent energy cost of biosynthesis (ATP equivalents) by a variety of microbes using different fermentation pathways was similar. In contrast, the energy cost during respiration appears to vary. In both fermentation and respiration it seems much greater than needed to fuel synthesis of the cell (29, 37). Recent work suggests that many microbes have sets of parallel pathways for ATP production in respiration and for ATP utilization in biosynthesis. The alternative paths differ in efficiency. Pathway choice within each set is thought to dictate the speed and efficiency of growth; the organism uses the fastest growth consistent with energy availability (8, 22). Thus, the energy cost of building an organism may be more when unlimited for energy than when limited for energy.
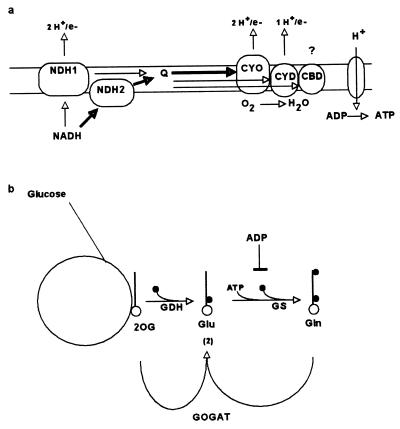
In Escherichia coli two sets of parallel pathways for proton extrusion leading to ATP synthesis occur in the respiratory chain (Fig. 1a) (15). Two NADH dehydrogenases transfer electrons to quinones to form quinoles. One (NDH1, nuoA-N genes) is thought to export two protons per electron, while the other (NDH2, ndh) exports none. Three terminal oxidases transfer electrons from the quinoles to oxygen. The bo-type oxidase CYO (cyoABCDE genes) exports two protons per electron; the bd-oxidase CYD (cydAB genes) exports one. A poorly understood third oxidase CBD (appBC [11], cyxAB [3], or cbdAB [36]) genes homologous with cydAB) is active during anaerobiosis, on entry into stationary phase, and at low phosphate or carbon concentrations (4), although its role is very minor during unlimited aerobic growth. Since proton reentry through ATP synthase is coupled to ATP production, the choices of dehydrogenase and oxidase control the efficiency of ATP synthesis. By choosing the appropriate member of each set of respiratory enzymes the potential ATP yield per glucose respired ranges from 12 to 33, assuming one ATP per three protons (8) and one to two protons per electron for the CBD oxidase.
FIG. 1.
Parallel pathways for respiration and for biosynthesis. (a) Parallel pathways in respiration and ATP synthase in the cell membrane of E. coli. Q represents the mobile quinones and/or quinols that accept electrons from the dehydrogenases and transfer them to the terminal oxidases. Heavy arrows represent the major pathway during aerobic growth with substrates in excess, but all components shown play a role in electron transfer under these conditions (8, 11, 15). (b) Two pathways for glutamate synthesis. The solid circles (•) represent nitrogen as ammonia, amino, and amide. 2OG is the citric acid cycle intermediate, 2-oxoglutarate. Glu, glutamate; Gln, glutamine; GS, glutamine synthetase; GOGAT, glutamate synthase. GOGAT transfers the amide group of Gln to 2OG to give two glutamates. Glutamate is formed in each pathway by reductive amination with NADPH (not shown).
E. coli has two ways of incorporating ammonium to form glutamate (Fig. 1b). These differ in that one (glutamate dehydrogenase, or GDH pathway) is energetically efficient (no direct requirement for ATP) but has a poorer affinity for the substrates ammonium and 2-oxoglutarate than does the GOGAT pathway (glutamine synthetase plus glutamate synthase), which uses ATP (32). Use of the latter pathway is estimated to boost the total ATP cost of biosynthesis by 10 to 20% (22).
Thus, by choosing appropriately from sets of enzymes in ATP synthesis and in glutamate synthesis, essentially the same cell can be built efficiently but slowly or rapidly but expensively (8, 22, 23). If the organism can optimize growth through choice among parallel pathways, it should be possible to alleviate (or exaggerate) deficiency in one pathway by deleting another. I show here that in E. coli the effect on growth of deficiency in glutamate dehydrogenase is altered predictably by ndh, cyo, or cyd mutations affecting the parallel pathways in respiratory metabolism. This may reflect global control over growth exerted through the ATP level (2).
MATERIALS AND METHODS
Strains.
Strains RBH830 and RBH828 are gdhA+ and gdhA1, respectively, but are otherwise isogenic strains of E. coli K-12 (22, 23). The gdhA1 mutant lacks glutamate dehydrogenase activity as the result of replacement of a single essential amino acid at the active site. Mutations of the ndh, cyo, and cyd genes were transferred by transduction from the following donor strains into RBH830 and RBH828 with selection for the associated antibiotic resistance and testing to ensure that they were free of the viruses P1 and lambda. Strain RBH1237 is strain GO104 (8) and contains the deletion Δ(cyoA-E)456::kan. Strain RBH1880 is strain MWC215 (8) cured of a P1 prophage (16). It contains a Cmr gene inserted within codon 285 of the 434-amino-acid codon ndh gene (7). Strain RBH1890 is strain GO103 (8) and contains the deletion Δ(cydAB")455 and the adjacent insertion zbg-2200::kan (30). Strains GO103, GO104, and MWC215 were received from P. Tsatsos and R. Gennis.
Arabinose-resistant derivatives of the arabinose-sensitive starting strains were obtained by selection on arabinose-containing broth plates (25). The resistance served to mark one of the two competing strains in each experiment.
Culture conditions and competition experiments.
The procedures were as described fully elsewhere (22, 24, 25). In brief, each organism was grown overnight in 100 ml of minimal glucose (0.05%) medium, transferred to a continuous culture apparatus (chemostat) with a 190-ml working volume and grown overnight at 30°C with glucose added to 0.0125% (0.694 mM) at a dilution rate of ca. 0.2 h−1 (cell generation time of 3.5 h). Cultures were mixed in equal volumes, and growth continued under the same conditions; appropriate dilutions were plated periodically on minimal glycerol plates, and the resulting colonies were replicated onto glycerol-arabinose plates to determine the frequencies of each strain. For each competition the growth rate of the gdhA1 mutant relative to that of the wild type (relative fitness) was determined for 10 to 35 generations after mixing (22, 25). Differences in growth rates of the two strains were calculated in the standard way by least-squares regression of the slope of the natural logarithm of the ratios of strain frequencies versus generations of growth (24). Experiments were repeated with the arabinose markers reversed in order to correct for any selective effect (22). Each point represents the average of at least four competition experiments.
Growth experiments.
Growth rates during unlimited growth on standard medium and subsequent growth yields were determined in shaking flasks with glucose at 0.025% and other conditions as described above. Growth was monitored at A550 in a Zeiss spectrophometer. Yields during glucose-limited continuous culture were determined by using a chemostat culture as for the competition experiments (see above) except that they were determined with glucose concentrations of both 0.0125 and 0.025%. These yields represent the average of repeated samples taken during growth over about generations 12 to 30 after initiation. In each case the wild-type strain was grown in parallel under the same conditions and results are reported (Table 1) as the ratios of the mutant to the wild type. Detailed conditions and results are given elsewhere (25) for a strain closely related to the wild-type strain used here and that showed no apparent difference in growth behavior. Results represent the average of at least two (unlimited growth) or four (limited growth) independent experiments.
TABLE 1.
Growth parameters of GDH+ and GDH− strainsa
| Genotype | GDH+ strains
|
Genotype | GDH− strains
|
|||||
|---|---|---|---|---|---|---|---|---|
| μmax (SD) | YU (SD) | YL (SD) | μmax (SD) | YU (SD) | YL (SD) | |||
| Wild type | 1.00 | 1.00 | 1.00 | |||||
| ndh | 0.49 (0.03) | 0.76 (0.02) | 1.01 (0.03) | |||||
| cyo | 0.90 (0.01) | 0.91 (0.02) | 0.87 (0.01) | |||||
| cyd | 0.97 (0.02) | 0.92 (0.01) | 0.89 (0.02) | |||||
| gdhAcyd | 1.00 | 1.00 | 0.96 (0.01) | |||||
| ndh gdhA | 0.47 (0.03) | 0.76 (0.02) | 0.98 (0.02) | |||||
| cyo gdhA | 0.91 (0.02) | 0.91 (0.02) | 0.86 (0.02) | |||||
| cyd gdhA | 0.97 (0.02) | 0.92 (0.01) | 0.85 (0.01) | |||||
μmax is the maximum specific growth rate relative to that of the wild type (unlimited growth). YU is the growth yield after unlimited growth relative to that of the wild type. YL is the relative growth yield after glucose-limited growth. The wild type and the gdhA mutant did not differ detectably during unlimited growth (22).
RESULTS
Competition experiments.
The most direct way of determining the role of a gene in a cell is to compare the growth of a mutant deficient in that gene with that of the wild-type in competition. A gdhA mutant lacking GDH does not differ from the wild type in growth on minimal medium with unlimited glucose (22).
A more stringent test is to compare two strains in direct competition during carbon- and energy-limited growth in continuous culture (25). In order to do so, I grew the competing strains separately in continuous culture and then mixed them; I then sampled them periodically to determine the frequency of each strain over time. From this I calculated the relative growth rate of the mutant (see Materials and Methods). Growth rates of the mutant strains are defined as fractions of those of the wild-type reference strain and are denoted relative fitnesses, as is conventional (i.e., the relative fitness of the gdhA+ reference strain is 1.0 by definition [24]). The selection coefficient (growth detriment) is (1 − the relative fitness).
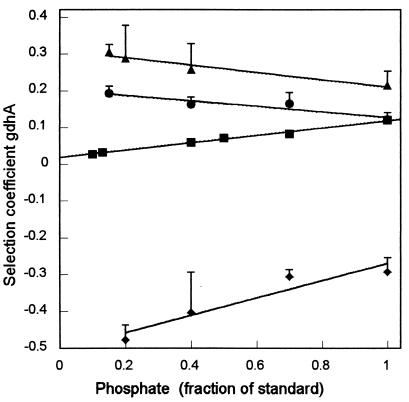
During glucose-limited growth in standard medium, the gdhA mutant shows a growth disadvantage relative to the wild type (selection coefficient of 0.12) (Fig. 2) (22). The growth disadvantage of the mutant presumably results because it is stressed for energy (lowering the ATP pool) but forced to use the energy-demanding GOGAT pathway to synthesize glutamate under conditions in which the wild-type organism would use GDH. The disadvantage is small at a low phosphate concentration but increases as the concentration is raised. Why phosphate affects the growth is unclear and has been discussed elsewhere (22, 23).
FIG. 2.
Effect of lack of a component of the respiratory chain on the relative fitness of a gdhA mutant during glucose-limited growth as a function of phosphate concentration. Symbols: ▪, competition between a gdhA1 mutant and the wild type (22); ⧫, a similar competition but both strains are deficient in the NDH2 dehydrogenase; ▴, a similar competition but both strains are deficient in the CYO oxidase; •, a similar competition but both strains are deficient in the CYD oxidase. The vertical bars represent the standard error.
If the mutant organism is stressed for energy, it should be able to compensate by reducing flux through one of the less-efficient pathways in respiration, thereby increasing the yield of ATP (Fig. 1a). I constructed derivatives of the gdhA+ and gdhA1 strains that were devoid of NDH2 as the result of the mutation of ndh and put the derivatives in competition during glucose-limited growth. NDH2 is the major dehydrogenase during unlimited growth with aeration, it but can be replaced by NDH1 in the mutant (15). As predicted, the mutant lacking GDH for glutamate synthesis no longer showed the growth disadvantage (Fig. 2). Instead, the gdhA+ organism was at a severe disadvantage relative to its mutant counterpart when both were lacking the non-ATP-yielding NDH2. (On unlimited glucose, the absence of GDH had little affect on the growth rate or yield of any of the strains used in the results reported here [Table 1].) The response to phosphate concentration appeared to be unchanged in the ndh mutant background (similar slope), suggesting that control by phosphate is not exerted through NADH dehydrogenase.
In a similar way one can predict that deletion of the cyoABCDE genes for efficient quinol oxidase CYO would exacerbate the growth detriment of the gdhA1 mutant. The results are consistent with the expectation (Fig. 2). However, the slope of the relative growth detriment of the gdhA1 mutant as a function of the phosphate concentration differs from those of the previous curves. One interpretation of this difference is that the phosphate concentration controls growth through its effect on terminal oxidase activity.
The terminal oxidase CYD constitutes a minor proportion of the terminal oxidase activity during unlimited aerobic growth on standard medium (15). If this is true during glucose-limited growth as well (8), deletion of cydAB should have a smaller effect on the growth of the gdhA1 mutant than should the deletion of cyo genes. Again, the results are consistent with the hypothesis (Fig. 2). The cyd deletion had little effect at the standard phosphate concentration. The overall response to phosphate was similar to that of the cyo deletion, however (compare the slopes in Fig. 2), again suggesting that the role of phosphate in determining the pathway for glutamate synthesis might be at the level of the terminal oxidases.
Growth rates and yields.
The lack of GDH affected the growth rate of strains in competition during glucose-limited culture, as shown above. If this reflected a substantially lower ATP pool (2), the growth yields might also be altered. Therefore, I determined the growth rates and yields during unlimited growth (batch culture) and yields during glucose-limited growth of the wild-type and of the respiratory mutants with or without GDH. As noted earlier, the gdhA mutation had no apparent affect on either the rate (maximum specific growth rate) or the yield in any strain when growth was unlimited (22) (Table 1). (The growth rate of ndh strains in unlimited culture could be improved by adding serine, as anticipated [27].) During limited growth, the yield of the gdh mutant was lower than that of the wild type (Table 1). Under the same conditions, the lack of functional GDH had no significant effect on the yields of the respiratory mutants, suggesting that, under the conditions employed, the growth rate (Fig. 2) is more sensitive to change in ATP pool size than is the growth yield (Table 1).
DISCUSSION
Growth in substrate-limited culture may be described by the following equation: μ = μmax s/(Ks + s), where μ is the growth rate, μmax is the maximum specific growth rate, s is the concentration of the limiting substrate within the chemostat, and Ks is the saturation coefficient (i.e., the substrate concentration at a half-maximum growth rate) (13, 22, 28). According to this equation, the growth rate is a function of two parameters intrinsic to the organism: μmax and Ks. If two strains compete for a limiting nutrient, the organism that has values for these parameters that result in the lower s should win, and this prediction has been validated (21).
The maximum specific growth rate (μmax) appears to be little affected by the gdhA mutation in any strain (Table 1). Thus, it is likely that the effects on growth rate observed during competition (Fig. 2) reflect an alteration in the Ks value, i.e., the saturation coefficient. This value is a measure of the ability of the organism to scavenge the low quantity of glucose present in the chemostat environment. Under the conditions used, glucose is expected to be imported mainly by the high-affinity, energy-intensive Mgl system rather than by the lower-affinity and less-costly glucose phosphotransferase system (12, 26). It is reasonable to hypothesize that the gdhA strains have an altered Ks and altered Mgl-mediated transport because they have a lower ATP pool.
According to this reasoning, a gdhA mutant demonstrates poorer glucose transport than the wild-type because of its lower ATP pool, and the pool is lowered further when it is also deficient in the high-efficiency CYO terminal oxidase.
However, the situation is complex. A mutant lacking NDH2 may have a higher ATP pool than the wild-type (because it uses the high-efficiency NDH1 exclusively as its NADH dehydrogenase). An ndh mutant competes better for limiting glucose if it is also deficient in GDH (Fig. 2), so an ATP pool that is too high may also be detrimental to capturing glucose, and the relative fitness may reflect any deviation from an optimum. (Of course, it may be a derivative of the ATP pool that is sensed as, for example, the adenylate energy charge [2].) To further complicate matters, mutations affecting the respiratory enzymes have multiple effects in addition to a direct one on ATP generation (see, for example, references 1, 5, 9, 17, 18, 27, 31, 34, and 39), genes for the respiratory enzymes are subject to complex controls at the transcriptional levels (15, 19, 20, 38), and the levels of expression of these genes during substrate-limited growth have not been determined. Although the overall results of competition with ndh, cyo, and cyd mutants were essentially as anticipated, a full explanation of the kinetics in all strains will not be possible until the functional levels of each of the respiratory enzymes are determined across the range of phosphate concentrations.
The results show that an ndh mutation increasing the yield of ATP alleviates the deficiency in GDH, while a cyo mutation decreasing the yield exaggerates the detrimental effect of the GDH deficiency on growth rate. How does the wild-type organism optimize pathway choice in glutamate synthesis? High ATP energizes the first step in the GOGAT path (glutamine formation). The enzymes in this pathway have substantially higher affinities for the substrates ammonium and 2OG and so compete effectively with GDH. On the other hand, lowering the ATP pool not only reduces a substrate but increases its competitive inhibitor for glutamine synthetase, ADP, and the decrease in the ATP/ADP ratio is expected to reduce flux into the GOGAT pathway (40). Activation of the GDH path is likely to depend on an increased pool of the substrate, 2OG, to match the relatively poor affinity of GDH for 2OG. Increased 2OG may result directly from reducing flux through GS (see Fig. 1b), but the increase is probably aided by a greater flow through glycolysis and into the citric acid cycle (10, 14). Of course, at a low ammonium concentration the organism remains essentially restricted to the GOGAT pathway anyway because of the low affinity of GDH for ammonium.
Are there other instances of parallel pathways that might play a role in optimizing the ATP pool? Several sets of parallel pathways are known in anaerobic respiration. The case most closely similar to those reported here is the multiple nitrate reductases transferring electrons to nitrate as terminal electron acceptors (15). It is also possible that the efficiency of ATP synthase (proton influx per ATP produced) is controlled (33, 35). The organism may be able to adjust the speed of growth in a variety of ways to match the potential energy available and gain an advantage over competitors.
Acknowledgments
I thank J. R. Guest and M. M. Attwood of the University of Sheffield, where initial studies were done, for their hospitality, and I thank the NIH for support as a John E. Fogarty Senior International Fellow. I thank R. Bender for strains and other support, R. Gennis and P. Tsatsos for strains, and D. Hicks and P. Belin for information.
This work was supported in part by NIH grant GM47156.
REFERENCES
- 1.Alexandre, G., and I. B. Zhulin. 2001. More than one way to sense chemicals. J. Bacteriol. 183:4681-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, K. B., and K. von Meyenburg. 1977. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J. Biol. Chem. 252:4151-4156. [PubMed] [Google Scholar]
- 3.Atlung, T., and L. Brondsted. 1994. Role of the transcriptional activator AppY in regulation of the cyx appA operon of Escherichia coli by anaerobiosis, phosphate starvation, and growth phase. J. Bacteriol. 176:5414-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlung, T., K. Knudsen, L. Heerfordt, and L. Brondsted. 1997. Effects of ςsgr;S and the transcriptional activator AppY on induction of the Escherichia coli hya and cbdAB-appA operons in response to carbon and phosphate starvation. J. Bacteriol. 179:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader, M., W. Muse, D. P. Ballou, C. Gassner, and J. C. A. Bardwell. 1999. Oxidative protein folding is driven by the electron transport system. Cell 98:217-227. [DOI] [PubMed] [Google Scholar]
- 6.Bauchop, T., and S. R. Elsden. 1960. The growth of micro-organisms in relation to their energy supply. J. Gen. Microbiol. 23:457-469. [DOI] [PubMed] [Google Scholar]
- 7.Calhoun, M. W., and R. B. Gennis. 1993. Demonstration of separate genetic loci encoding distinct membrane-bound respiratory NADH dehydrogenases in Escherichia coli. J. Bacteriol. 175:3013-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calhoun, M. W., K. L. Oden, R. B. Gennis, M. J. Teixeira de Mattos, and O. M. Neijssel. 1993. Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J. Bacteriol. 175:3020-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, G. M., C. Loder, B. Soballe, G. P. Stafford, J. Membrillo-Hernandez, and R. K. Poole. 1998. A factor produced by Escherichia coli K-12 inhibits the growth of E. coli mutants defective in the cytochrome bd quinol oxidase comples: enterochelin rediscovered. Microbiology 144:3297-3308. [DOI] [PubMed] [Google Scholar]
- 10.Cronan, J. E., Jr., and D. LaPorte. 1996. Tricarboxylic acid cycle and glyoxylate bypass, p. 206-216. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 11.Dassa, J., H. Fsihi, C. Marck, M. Dion, M. Kieffer-Bontemps, and P. L. Boquet. 1991. A new oxygen-regulated operon in Escherichia coli comprises the genes for a putative third cytochrome oxidase and for pH 2.5 acid phosphatase (appA). Mol. Gen. Genet. 229:341-352. [DOI] [PubMed] [Google Scholar]
- 12.Death, A., and T. Ferenci. 1993. The importance of the binding-protein-dependent Mgl system to the transport of glucose in Escherichia coli growing on low sugar concentrations. Res. Microbiol. 144:529-537. [DOI] [PubMed] [Google Scholar]
- 13.Ferenci, T. 1999. Regulation by nutrient limitation. Curr. Opin. Microbiol. 2:208-213. [DOI] [PubMed] [Google Scholar]
- 14.Fraenkel, D. G. 1996. Glycolysis, pentose phosphate pathway, and Entner-Doudoroff pathway, p. 189-198. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 15.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 16.Goldberg, R. B., R. A. Bender, and S. L. Streicher. 1974. Direct selection for P1-sensitive mutants of enteric bacteria. J. Bacteriol. 118:810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman, B. S., K. K. Gabbert, and R. G. Kranz. 1996. The temperature-sensitive growth and survival phenotypes of Escherichia coli cydDC and cydAB strains are due to deficiencies in cytochrome bd and are corrected by exogenous catalase and reducing agents. J. Bacteriol. 178:6348-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Flecha, B., and B. Demple. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270:13681-13687. [DOI] [PubMed] [Google Scholar]
- 19.Govantes, F., A. V. Orjalo, and R. P. Gunsalus. 2000. Interplay between three global regulatory proteins mediates oxygen regulation of the Escherichia coli cytochrome d oxidase. Mol. Microbiol. 38:1061-1073. [DOI] [PubMed] [Google Scholar]
- 20.Green, J., M. F. Anjum, and J. R. Guest. 1997. Regulation of the ndh gene of Escherichia coli by integration host factor and a novel regulator, Arr. Microbiology 143:2865-2875. [DOI] [PubMed] [Google Scholar]
- 21.Hansen, S. R., and S. P. Hubbell. 1980. Single-nutrient microbial competition: qualitative agreement between experimental and theoretically forecast outcomes. Science 207:1491-1493. [DOI] [PubMed] [Google Scholar]
- 22.Helling, R. B. 1994. Why does Escherichia coli have two primary pathways for synthesis of glutamate? J. Bacteriol. 176:4664-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helling, R. B. 1998. Pathway choice in glutamate synthesis in Escherichia coli. J. Bacteriol. 180:4571-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helling, R. B., T. Kinney, and J. Adams. 1981. The maintenance of plasmid-containing organisms in populations of Escherichia coli. J. Gen. Microbiol. 123:129-141. [DOI] [PubMed] [Google Scholar]
- 25.Helling, R. B., C. N. Vargas, and J. Adams. 1987. Evolution of Escherichia coli during growth in a constant environment. Genetics 116:349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manche, K., L. Notley-McRobb, and T. Ferenci. 1999. Mutational adaptation of Escherichia coli to glucose limitation involves distinct evolutionary pathways in aerobic and oxygen-limited environments. Genetics 153:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miesel, L., T. R. Weisbrod, J. A. Marcinkeviciene, R. Bittman, and W. R. Jacobs, Jr. 1998. NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J. Bacteriol. 180:2459-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monod, J. 1942. Recherches sur la croissance des cultures bacteriennes. Prentice-Hall, Englewood Cliffs, N.J.
- 29.Neijssel, O. M., and M. J. Teixeira do Mattos. 1994. The energetics of bacterial growth: a reassessment. Mol. Microbiol. 13:179-182. [DOI] [PubMed] [Google Scholar]
- 30.Oden, K. L., L. C. DeVeaux, C. R. T. Vibat, J. E. Cronan, Jr., and R. B. Gennis. 1990. Genomic replacement in Escherichia coli K-12 using covalently closed circular plasmid DNA. Gene 96:29-36. [DOI] [PubMed] [Google Scholar]
- 31.Rapisarda, V. A., L. L. Montelongo, R. N. Farias, and E. M. Massa. 1999. Characterization of an NADH-linked cupric reductase activity from the Escherichia coli respiratory chain. Arch. Biochem. Biophys. 370:143-150. [DOI] [PubMed] [Google Scholar]
- 32.Reitzer, L. J. 1996. Ammonia assimilation and the biosynthesis of glutamine, glutamate, asparatate, asparagine, l-alanine, and d-alanine, p. 391-407. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 33.Schemidt, R. A., J. Qu, J. R. Williams, and W. S. Brusilow. 1998. Effects of carbon source on expression of F0 genes and on the stoichiometry of the c subunit in the F1F0 ATPase of Escherichia coli. J. Bacteriol. 180:3205-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steuber, J., C. Schmid, M. Rufibach, and P. Dimroth. 2000. Na+ translocation by complex I (NADH:quinone oxidoreductase) of Escherichia coli. Mol. Microbiol. 35:428-434. [DOI] [PubMed] [Google Scholar]
- 35.Stock, D., A. G. W. Leslie, and J. E. Walker. 1999. Molecular architecture of the rotary motor in ATP synthase. Science 286:1700-1705. [DOI] [PubMed] [Google Scholar]
- 36.Sturr, T. A. M. G. Krulwich, and D. B. Hicks. 1996. Purification of a cytochrome bd terminal oxidase encoded by the Escherichia coli app locus from a Δcyo Δcyd strain complemented by genes from Bacillus firmus OF4. J. Bacteriol. 178:1742-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tempest, D. W. 1978. The biochemical significance of microbial yields: a reassessment. Trends Biochem. Sci. 3:180-184. [Google Scholar]
- 38.Wackwitz, B., J. Bongaerts, S. D. Goodman, and G. Unden. 1999. Growth phase-dependent regulation of nuoA-N expression in Escherichia coli K-12 by the Fis protein: upstream binding sites and bioenergetic significance. Mol. Gen. Genet. 262:876-883. [DOI] [PubMed] [Google Scholar]
- 39.Way, S. S., S. Sallustio, R. S. Magliozzo, and M. B. Goldberg. 1999. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woolfolk, C. A., and E. R. Stadtman. 1967. Regulation of glutamine synthetase. III. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch. Biochem. Biophys. 118:736-755. [DOI] [PubMed] [Google Scholar]