Abstract
The enzyme CwlJ is involved in the depolymerization of cortex peptidoglycan during germination of spores of Bacillus subtilis. CwlJ with a C-terminal His tag was functional and was extracted from spores by procedures that remove spore coat proteins. However, this CwlJ was not extracted from disrupted spores by dilute buffer, high salt concentrations, Triton X-100, Ca2+-dipicolinic acid, dithiothreitol, or peptidoglycan digestion, disappeared during spore germination, and was not present in cotE spores in which the spore coat is aberrant. These findings indicate the following: (i) the reason decoated and cotE spores germinate poorly with dipicolinic acid is the absence of CwlJ from these spores; and (ii) CwlJ is located in the spore coat, presumably tightly associated with one or more other coat proteins.
Spores of various Bacillus and Clostridium species can remain in the dormant state for long periods of time, but they respond rapidly to nutrients in the environment and return to life through the process of spore germination (18). Among the crucial events in spore germination is the depolymerization of the spore's thick peptidoglycan cortex, as this process allows the germinating spore to take up sufficient water to initiate metabolic reactions (18, 20). In Bacillus subtilis two proteins, CwlJ and SleB, are involved in cortex degradation; either protein alone is sufficient for cortex degradation, but cwlJ sleB spores do not degrade their cortex and are extremely inefficient in giving rise to viable cells (9, 11, 20). SleB is likely a lytic transglycosylase and acts only on cortical peptidoglycan (3). The sleB gene is transcribed primarily in the forespore compartment of the sporulating cell, and the protein has been localized by immunoelectron microscopy to the boundary region between the spore's cortex and coats (12). In contrast to SleB, CwlJ is transcribed primarily in the mother cell compartment of the sporulating cell (9). However, while the sequence of CwlJ is consistent with its being a corticolytic enzyme (9), the specificity of this protein has not yet been determined; the location of CwlJ in the spore has also not been determined.
Recent work has indicated that CwlJ is activated during spore germination by the release of the spore core's large depot of pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) (14). CwlJ action, and thus spore germination, can also be triggered by exogenous DPA complexed with Ca2+ (14). In contrast, SleB does not respond to either endogenous or exogenous Ca2+-DPA (14). The ability of B. subtilis spores to initiate spore germination in response to exogenous Ca2+-DPA was abolished or greatly reduced when spore coats were either removed chemically or were aberrant due to the coat assembly mutation cotE (4, 5, 14). While there are several explanations for the latter findings, a simple hypothesis is that CwlJ is in the spore's external layers, perhaps in the spore coats, is removed by coat extraction, and does not assemble properly on the coats of cotE spores.
Generation of a cwlJ gene encoding a His-tagged product.
In order to determine the validity of the hypothesis that CwlJ is in the spore coats, we undertook to localize CwlJ in spores of B. subtilis. To do this we first created an epitope-tagged version of CwlJ using the plasmid pET23a (Novagen) to construct a plasmid for integration into the B. subtilis chromosome of a version of cwlJ encoding a C-terminally hexahistidine-tagged (His tag) product. A cassette providing resistance to erythromycin (ermC) in B. subtilis was excised from the plasmid pE194 (7) by digestion with ClaI and XbaI and cloned into plasmid pET23a between the PvuII and XbaI sites, resulting in plasmid pET23a::ermC. A fragment containing 162 bp up to the 3" end of the cwlJ open reading frame (ORF) but not including the stop codon was amplified by PCR using the following primers: cwlJ-His5", 5"-GAAGATCTAACTAGTTGAATGGTGAAAGGCGCTGG; and cwlJ-His3", 5"-CCGCTCGAGAAATGTGTTATATACATTTTCAC. The underlined nucleotides are complementary to the cwlJ ORF with the C-terminal codon being complementary to the three boldfaced nucleotides in primer cwlJ-His3". The additional nucleotides in the primers are primarily restriction sites (in cwlJ-His3" and cwlJ-His5"), as well as stop codons in all reading frames in front of the PCR fragment (in cwlJ-His5") to ensure that this fragment alone will not be translated when inserted into B. subtilis. The PCR product was cloned into plasmid pCR2.1 (Invitrogen), resulting in plasmid pIB644, and the insert in this plasmid was sequenced to confirm the fidelity of the original PCR. This plasmid was digested with EcoRI (site in plasmid pCR2.1) and XhoI (site in primer cwlJ-His3"). The EcoRI/XhoI fragment was cloned between the EcoRI and XhoI sites of plasmid pET23a::ermC, creating an in-frame fusion of the His tag to the 3" end of the cwlJ ORF as well as adding two extra amino acids (Leu and Glu) between the normal C terminus and the His tag. The resulting plasmid, pIB647, was used to transform B. subtilis strain PS832 (a laboratory derivative of strain 168) to resistance to erythromycin (1 μg/ml) and lincomycin (25 μg/ml) as described previously (1, 14). Southern blot analysis (data not shown) confirmed that in the resulting strain, IB650 (with a genotype termed cwlJ-His-tag), the plasmid pIB647 had integrated by a single crossover event at the cwlJ locus and contained, as the only full-length copy of cwlJ, a version of cwlJ encoding the His tag fused at the C terminus of CwlJ.
Assessment of the function of the His-tagged CwlJ (cwlJ-His-tag) protein
To prove that the presence of the two extra C-terminal amino acids and the His tag do not interfere with CwlJ function, we tested the Ca2+-DPA-induced germination of the cwlJ-His-tag spores. For this experiment we first used chromosomal DNA from strain IB650 to transfer the cwlJ-His-tag construct into a strain lacking the spore's three functional germinant receptors (strain FB104; termed ger3 [16]) and thus unable to germinate with nutrients. Spores of the resultant strain, IB652 (as well as all other spores), were prepared on plates, cleaned, and stored as described previously (13, 15). The cleaned spores were suspended at an optical density at 600 nm (OD600) of 1 in 60 mM Ca2+-DPA at pH 8.3 as described previously (14, 16) and incubated for 1 h at room temperature, and serial dilutions were plated on Luria-Bertani medium (19) plates that were incubated for ∼20 h at 37°C prior to enumeration of colonies. Previous work has shown this to be a good measure of the germination of these spores in Ca2+-DPA (14). We also tested ger3 spores as a positive control and ger3 cwlJ spores (strain FB115 [14]) as a negative control, since the latter spores germinate much less efficiently in Ca2+-DPA. As hoped, the ger3 cwlJ-His-tag spores exhibited germination in Ca2+-DPA equivalent to that of ger3 spores containing a wild-type copy of cwlJ (Table 1). To further prove that CwlJ-His-tag functions normally during nutrient-induced spore germination, the cwlJ-His-tag construct was placed in a sleB background by transformation of strain FB110 (sleB [14]) with chromosomal DNA from strain IB650, giving strain IB664 (cwlJ-His-tag sleB). In spores of this strain, CwlJ-His-tag is the only cortex hydrolase present, and if the CwlJ derivative is not active, germination of these spores will be extremely poor (9, 14). Germination of cwlJ-His-tag sleB spores on Luria-Bertani plates at 37°C was compared to that of cwlJ sleB (strain FB113) and sleB (strain FB110) spores (14), and again the germination of cwlJ-His-tag sleB spores was identical to that of sleB spores (Table 1). These experiments show that CwlJ containing the C-terminal His tag is fully functional, and thus, spores containing this epitope-tagged version of CwlJ can be used with confidence to localize CwlJ using antibodies against the His tag.
TABLE 1.
Ca2+-DPA- and nutrient-induced germination of spores of various strainsa
| Inducer | Strain (genotype) | Amt of spores at an OD600 of 1 (CFU/ml) |
|---|---|---|
| Ca2+-DPA | FB104 (ger3) | 1.9 × 108 |
| IB652 (ger3 cwlJ-His-tag) | 2.0 × 108 | |
| FB115 (ger3 cwlJ) | 7.0 × 106 | |
| Nutrients | FB110 (sleB) | 1.2 × 108 |
| IB664 (sleB cwlJ-His-tag) | 1.0 × 108 | |
| FB113 (sleB cwlJ) | <105 |
Spore germination with Ca2+-DPA or nutrients was assessed as described in the text. Note that wild-type (strain PS832) spores routinely give 1×108 to 2×108 CFU in these assays (14, 16).
Methods for localization of the CwlJ-His-tag protein in spores
In experiments to localize CwlJ, we examined protein in various fractions from spores carrying the cwlJ-His-tag construct by Western blot analysis and carried out these analyses in parallel on fractions isolated from spores of strain PS832, which does not contain a His tag. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% gels (17) was used for separation of proteins followed by transfer of the proteins to an Immobilon-P membrane (Millipore). His-tag monoclonal antibodies were purchased from Novagen, and Western blot analysis to detect His-tagged proteins was performed essentially as described previously (6, 17) using goat anti-mouse alkaline phosphatase-conjugated secondary antibody (Sigma) and CSPD chemiluminescent substrate for alkaline phosphatase (Roche Diagnostics), with chemiluminescence detected using X-ray film.
Spores were decoated as described previously (14). Briefly, ∼6 mg of dry spores was suspended in 1 ml of 0.1 M NaOH-0.1 M NaCl-0.1 M dithiothreitol (DTT)-0.5% SDS and incubated for 30 min at 70°C (alkaline decoating), followed by centrifugation in a microcentrifuge for 1 min. The supernatant fluid was dialyzed against deionized water overnight in the cold room and lyophilized, and the dry powder was dissolved in 100 μl of 1× SDS sample buffer (62.5 mM Tris-HCl (pH 6.8)-10% glycerol-2% SDS-0.003% bromophenol blue-1% 2-mercaptoethanol) and boiled for 5 min. The decoated spores were washed as described previously (14) and lyophilized. In one experiment the decoating was done by the SDS-urea-Tris-DTT method at pH 8 (13) (urea decoating), and the coat extract was processed as described above.
For routine preparation of spore extracts, spores at an OD600 of 100 (all values denote spores in 1 ml at the given OD600) (dry weight, 12 mg) were lyophilized and dry ruptured (pulsed 18 times for 30 s each, with a 30-s cooling period between pulses) with 100 mg of glass beads in a Dental amalgamator (Wig-L-Bug). In a few experiments, washed decoated spores were disrupted. The resultant dry powder was extracted with 200 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM phenylmethylsulfonylfluoride (PMSF) for 30 min on ice, followed by a 5-min centrifugation at top speed in a microcentrifuge in the cold room. The supernatant fluid (termed the soluble fraction) was mixed with an equal volume of 2× SDS sample buffer and boiled for 5 min. The pellet (termed the insoluble fraction) was suspended in 200 μl of 1× SDS sample buffer, incubated at 70°C for 15 min with occasional vortexing, boiled for 5 min, and spun in a microcentrifuge at room temperature to remove particulate matter, and aliquots of the supernatant fluid were analyzed by Western blotting as described above. In some cases the insoluble fraction was extracted for 20 min on ice with 200 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM PMSF-1% Triton X-100 or 200 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM PMSF-1 M NaCl and centrifuged in the cold as described above. In other experiments the insoluble fraction was subjected to the following: (i) treatment with 200 μg of lysozyme/ml in 200 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM PMSF for 1 h at 37°C; (ii) treatment with 200 μl of 50 mM HEPES (pH 7.5)-5 mM EDTA-1 mM PMSF-1 M NaCl-0.1 M DTT for 20 min at room temperature; or (iii) a wash with 200 μl of 50 mM HEPES (pH 7.5)-1 mM PMSF, centrifugation, and suspension of the pellet fraction in 400 μl of 150 mM Tris-HCl (pH 8.3)-60 mM Ca2+-DPA-1 mM PMSF for 30 min at room temperature. In all cases the incubations were centrifuged in the cold as described above, the soluble fractions were mixed with an equal volume of 2× SDS sample buffer and boiled, and the insoluble fractions were suspended in 1× SDS sample buffer, heated for 15 min at 70°C, and boiled and centrifuged prior to subsequent analyses.
Localization and amount of the CwlJ-His-tag protein in dormant spores
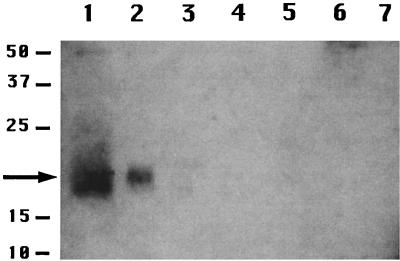
Western blot analysis of protein extracted from intact spores by either the alkaline procedure (Fig. 1, lane 1), the urea procedure (data not shown), or the alkaline procedure without DTT in the extraction solution (data not shown) gave a strong band at ∼18 kDa, the size expected for the CwlJ-His-tag protein (17.4 kDa). This was the only prominent immunoreactive band seen in these coat extracts, and this band was not seen with coat extracts from wild-type spores (Fig. 1, lane 3), which gave only a very faint band at ∼50 kDa, also seen in extracts from cwlJ-His-tag spores (Fig. 1, lanes 1 to 4). These data strongly suggest that the 18-kDa band detected in coat extracts is CwlJ. In disrupted cwlJ-His-tag spores that had not been decoated, the 18-kDa band was present exclusively in the insoluble fraction (Fig. 1, lanes 2 and 6), but this band was absent from the insoluble fraction of decoated spores (Fig. 1, lane 5). Again there was no 18-kDa signal in any fractions from wild-type spores (Fig. 1, lanes 3, 4, and 7). The CwlJ band was consistently more prominent in the coat extract than in the insoluble fraction from the same amount of spores (Fig. 1, lanes 1, and 2). This difference in band intensity was found to be ∼2-fold when different amounts of the two samples were run in parallel (data not shown). While the reason for this twofold difference is not clear, possibilities include physical losses in the dry rupture procedure as well as a slight loss of antigenicity in this procedure due to its rather drastic nature. Note, however, that the CwlJ protein did not simply relocate to the insoluble fraction upon the dry rupture procedure, as the protein was removed from intact spores by coat extraction.
FIG. 1.
Localization of CwlJ in spore fractions. Samples were prepared from spores of strains PS832 (wild-type) and IB650 (cwlJ-His-tag) and run on an SDS-polyacrylamide gel, proteins were transferred to an Immobilon-P membrane, and His-tagged proteins were detected as described in the text. The samples run in the various lanes are as follows: lanes 1 and 3, protein from cwlJ-His-tag or wild-type spores, respectively, extracted at an OD600 of 2.5 by the alkaline decoating procedure; lanes 2 and 4, insoluble fraction of disrupted cwlJ-His-tag or wild-type spores, respectively, at an OD600 of 2.5; lane 5, insoluble fraction from disrupted decoated cwlJ-His-tag spores at an OD600 of 2.5; lanes 6 and 7, soluble fractions of cwlJ-His-tag and wild-type spores, respectively, at an OD600 of 2.5. The numbers to the left of the figure denote the migration positions of molecular size markers in kDa; the arrow denotes the position of the CwlJ-His-tag protein. This figure, as well as all others, was processed in Adobe Photoshop, and all adjacent lanes are from a single SDS-PAGE experiment.
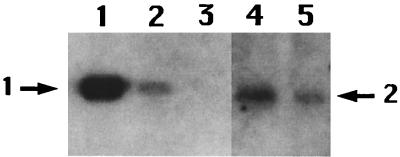
In order to estimate the number of CwlJ-His-tag molecules per spore, the CwlJ-His-tag signal in the insoluble fraction from several amounts of spores was compared to the signal obtained from a known amount of purified P16-His-tag protein (22) (provided by Li Luo). The P16-His-tag protein has six histidine residues at the N terminus and is about the same size as CwlJ (22). The signal at an OD600 of 2.5 of cwlJ-His-tag spores appeared similar in intensity to the signal generated by 4 × 1012 molecules of P16-His-tag (Fig. 2). Since there are about 5 × 108 spores at an OD600 of 2.5, there are ∼8 × 103 molecules of CwlJ per spore (4 × 1012/5 × 108). This number is much higher than the 24 to 40 molecules of germination receptor protein GerBA per spore (17). These relative amounts of germinant receptors and CwlJ are consistent with a signal transduction cascade in which a relatively few germinant receptors activate a much larger number of enzymes whose action on a major spore component results in later events in spore germination.
FIG. 2.
Level of CwlJ in spores. Various amounts of the P16-His-tag protein (22) or the insoluble fraction from cwlJ-His-tag spores were run on SDS-polyacrylamide gels, and His-tagged proteins were detected as described in the text. The samples run in the various lanes are the following: lanes 1, 2, and 3, 1 μg, 100 ng, and 10 ng, respectively, of the P16-His-tag protein, corresponding to 4 × 1013, 4 × 1012, and 4 × 1011 molecules, respectively; lanes 4 and 5, insoluble fractions of spores at OD600s of 5 and 2.5, respectively. The arrow labeled 1 denotes the migration position of the P16-His-tag protein; the arrow labeled 2 denotes the position of the CwlJ-His-tag protein. In this figure some intervening lanes on the original Western blot have been removed.
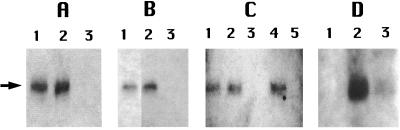
The removal of CwlJ from spores by decoating procedures is consistent with CwlJ being associated with the spore coat (4). However, coat extraction also solubilizes proteins from the outer spore membrane (2) and might also solubilize proteins bound loosely in the spore cortex. CwlJ likely acts on the spore cortex and has a pI of 9.4, so it could well be associated with some negatively charged component such as peptidoglycan present in the outer layers of the spore. To examine if CwlJ is associated with the spore cortex, we treated the insoluble fraction of cwlJ-His-tag spores with lysozyme to digest peptidoglycan in this fraction, but this treatment did not release CwlJ (Fig. 3A) and also released almost no protein (data not shown). Note that the insoluble fraction was isolated from mechanically disrupted spores in which significant amounts of cortex are present (8) and that the cortical material in this fraction is accessible to lysozyme even though the spores had not first been decoated. Even extraction of the lysozyme-treated insoluble fraction with 1 M NaCl did not solubilize CwlJ (Fig. 3B). These data suggest that CwlJ is unlikely to be localized in the cortex. Another possible location for CwlJ is the outer spore membrane. Although the integrity of the outer membrane in spores is questionable, this membrane is adjacent to the spore cortex, presumably the site of CwlJ action (5, 18). However, CwlJ does not have any transmembrane domains and so is unlikely to be an integral membrane protein, and its overall amino acid sequence does not suggest that it is a membrane protein. Indeed, CwlJ was not removed from the insoluble fraction by extraction with 1% Triton X-100 either before or after lysozyme treatment (Fig. 3C). CwlJ was also not removed from the insoluble fraction by treatment with DTT in high salt as described above (data not shown), suggesting that CwlJ is not held in the insoluble fraction by disulfide bonds alone. Another possibility, given the dependence of CwlJ function on Ca2+-DPA (14), was that CwlJ might be solubilized by this agent. However, incubation of the insoluble fraction with Ca2+-DPA as described above again did not solubilize CwlJ (data not shown).
FIG. 3.
Lack of solubilization of CwlJ by cortex digestion, high-salt conditions, or Triton X-100, and disappearance of CwlJ upon spore germination. (A, B, and C) Spores of strain IB650 (cwlJ-His-tag) were disrupted, and the insoluble fraction was isolated and incubated with lysozyme (A), lysozyme and then 1 M NaCl (B), or 1% Triton X-100 (C) with or without prior lysozyme treatment as described in the text. After incubation, the samples were centrifuged, aliquots of the soluble and insoluble fraction were run on an SDS-polyacrylamide gel, and CwlJ was detected by Western blot analysis as described in the text. Note that in panel C insoluble protein was isolated and suspended in buffer containing 1% Triton X-100 prior to being mixed with sample buffer and boiled, since Triton X-100 interfered slightly with the Western blot analysis. (D) Spores of strain IB652 (cwlJ-His-tag ger3) were germinated with Ca2+-DPA and washed as described in the text, these spores as well as dormant spores were disrupted, and soluble and insoluble fractions were isolated. Aliquots equivalent to original dormant spores of various strains at an OD600 of 5 were loaded in each well, and the samples run on the various lanes are the following. (A) Lane 1, insoluble fraction prior to lysozyme treatment; lane 2, insoluble fraction after treatment with lysozyme; lane 3, protein solubilized by treatment of the insoluble fraction with lysozyme. (B) Lane 1, insoluble fraction after treatment with lysozyme; lane 2, insoluble fraction after treatment with lysozyme and then extraction with 1 M NaCl; lane 3, protein solubilized by extraction of the lysozyme-treated insoluble fraction with 1 M NaCl. (C) Lane 1, insoluble fraction prior to extraction with 1% Triton X-100; lane 2, insoluble fraction after extraction with Triton X-100; lane 3, protein solubilized from the insoluble fraction by Triton X-100; lane 4, insoluble fraction after digestion with lysozyme followed by extraction with Triton X-100; lane 5, protein solubilized by extraction of the lysozyme-treated insoluble fraction with Triton X-100. (D) Lane 1, soluble fraction from germinated spores; lane 2, insoluble fraction from dormant spores; lane 3, insoluble fraction from germinated spores. The arrow denotes the position of the 18-kDa CwlJ-His-tag protein. In panel B, some intervening lanes on the original Western blot have been removed.
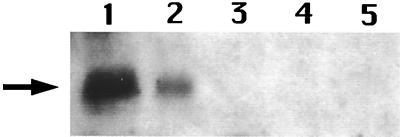
The data given above are most consistent with CwlJ in dormant spores being located in the spore coat. This is consistent with recent work (14) indicating that an intact spore coat is necessary for Ca2+-DPA-induced germination. One piece of evidence on which the latter conclusion is based is that a cotE mutation that alters spore coat assembly greatly reduced Ca2+-DPA-induced spore germination. A simple prediction is then that CwlJ is not present in cotE spores (14). To test this prediction we generated a cotE cwlJ-His-tag strain by transformation of strain PS3328 (cotE [14]) with chromosomal DNA from strain IB650 (cwlJ-His-tag). Analysis of CwlJ in the spores of this strain (IB673) showed that the amount of CwlJ in the insoluble or soluble fractions was below the detection limit of the Western blot analysis and well below the level in IB650 spores (Fig. 4, lanes 1 to 4). We also could not detect the CwlJ-associated signal in the coat fraction extracted from cotE cwlJ-His-tag spores by the alkaline procedure (data not shown). When the Ca2+-DPA-induced germination of the cotE cwlJ-His-tag spores was examined as described previously (14, 16), only ∼15% of these spores germinated, while ≥99% of the cwlJ-His-tag spores germinated in this assay (data not shown). These data are thus consistent with the absence of the great majority of CwlJ from the cotE spores (14).
FIG. 4.
Level of CwlJ in cotE spores. Spores of strains IB650 (cwlJ-His-tag) and IB673 (cotE cwlJ-His-tag) were disrupted, the soluble and insoluble fractions were isolated, aliquots were run on an SDS-polyacrylamide gel, and CwlJ-His-tag proteins were identified by Western blot analysis as described in the text. The samples run in the various lanes are the following: lanes 1 and 2, insoluble fractions of cwlJ-His-tag spores at OD600s of 5 and 2.5, respectively; lanes 3 and 4, insoluble fractions of cotE cwlJ-His-tag spores at OD600s of 5 and 10, respectively; lane 5, soluble fraction of cotE cwlJ-His-tag spores at an OD600 of 7.5. The arrow denotes the position of the 18-kDa CwlJ-His-tag protein.
Fate of the CwlJ-His-tag protein upon spore germination.
Given the role of CwlJ in spore germination, an obvious question is the fate of CwlJ upon spore germination. To examine this question, spores of strain IB652 (cwlJ-His-tag ger3) (an OD600 of 100) were germinated at an OD600 of 1 for 1 h at room temperature in 60 mM Ca2+-DPA as described previously (14). More than 95% of the spores were germinated by this procedure as determined by observation in the phase-contrast microscope. The germinated spores were washed four times with water, three times with 50 mM Tris-HCl (pH 8), and three times with water to remove precipitated Ca2+-DPA. The washed spores were lyophilized and dry ruptured, and the soluble and insoluble fractions were isolated as described above. SDS-PAGE and Western blot analysis of the latter fractions showed that the CwlJ-His-tag had largely disappeared from the insoluble fraction (Fig. 3D, lanes 2 and 3) but was not present in the soluble fraction (Fig. 3D, lane 1). While this experiment clearly indicates that there is some change in CwlJ upon spore germination, it is not yet clear if this is due to the excretion or degradation of CwlJ or simply the loss of its C-terminal His tag.
Conclusions.
It seems clear from the results in this report that the 18-kDa immunoreactive protein detected with anti-His-tag antiserum in cwlJ-His-tag spores is CwlJ. This is indicated by the appropriate molecular weight of this antigen and its absence from wild-type spores. While it was possible that the C-terminal His tag might have inactivated CwlJ, this was not the case. Spore germination dependent on CwlJ function was identical in spores with either wild-type CwlJ or the CwlJ-His-tag. We cannot, of course be sure that all the CwlJ-His-tag protein is functional, but this seems likely. It was also possible that the presence of the His tag might have altered CwlJ localization in the spore. Again this seems unlikely, as the CwlJ-His-tag protein and the wild-type CwlJ activity behaved identically in both their removal by decoating procedures and their greatly reduced presence in cotE spores.
The latter two findings strongly suggest that CwlJ is a spore coat protein. Indeed, CwlJ is synthesized in the mother cell at the same time as many of the spore coat proteins, including CotE, and is also synthesized in the mother cell compartment of the sporulating cell, the site of synthesis of spore coat proteins (4, 5, 9). CwlJ was also not extracted from disrupted spores by lysozyme digestion, high salt, or Triton X-100, indicating that this protein is not in the cortex or the spore's outer membrane. The B. subtilis spore coat is a multilamellar structure composed primarily of an outer and inner coat (4, 5). One would expect, a priori, that CwlJ would be located close to the site of its action, the cortex, and would thus be in the inner coat. Indeed, it is in this approximate region that SleB is located (12), and mutations in genes encoding inner coat proteins often also cause spore germination defects (4, 21). Since the outer coat is not present in cotE spores (4), the absence of CwlJ from these spores might suggest that this protein is in the outer coat. However, there are also obvious defects in the inner coat of cotE spores, and the cortex of cotE spores is accessible to lysozyme (4). Proteins in the inner coat of cotE spores are also likely exposed to sporulation proteases and could be degraded (4). We would thus suggest that CwlJ is located in the spore's inner coat, likely near the spore cortex, and is presumably tightly associated with one or more coat proteins, which is the reason for the insolubility of CwlJ in disrupted spores. Interestingly, SleB is also not soluble in extracts of disrupted spores, although this protein is not removed or inactivated by decoating regimens (12, 14).
CwlJ has four cysteine residues that are conserved among the CwlJ proteins encoded by all Bacillus species whose genomes have been sequenced. While these may form two internal disulfide bonds in the protein, it is formally possible that the cysteine residues are involved in disulfide bond formation with other coat proteins and that this is the reason for the apparent insolubility of CwlJ in disrupted spores. However, CwlJ was not extracted from the insoluble fraction by DTT treatment alone and was extracted from intact spores by the alkaline procedure without DTT present, suggesting although by no means proving that the latter scenario is not correct.
If CwlJ is indeed in the inner coat, the obvious question is how this protein acts to facilitate cortex depolymerization from this location. One possibility is that the cortex extends to this area of the spore and that cortex hydrolysis begins from the outer surface of the cortex. This is obviously consistent with the location of both CwlJ and SleB in this area. There is evidence that the outermost layer of the cortex has a different structure than the inner layer (10), and perhaps this facilitates the initiation of cortex hydrolysis in this area. A second possibility is that there is some redistribution of CwlJ upon initiation of spore germination, in particular when the spore's large depot of Ca2+-DPA that is essential for full activation of CwlJ is released from the spore core (14, 18). While Ca2+-DPA alone did not solubilize CwlJ from disrupted dormant spores, this protein disappeared from spores upon germination. However, we do not yet know whether this is due to excretion or degradation of CwlJ or only loss of the antigenic His tag. If the disappearance of CwlJ accompanying spore germination is due to loss of the whole protein, perhaps this is a reflection of a change in the proteins with which CwlJ associates in dormant and germinated spores. Indeed, there has yet been no demonstration of CwlJ activity in vitro, suggesting that one or more additional proteins are required for the CwlJ activity. This possibility, as well as the cause of the disappearance of CwlJ upon spore germination, are currently under investigation.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM19698).
We are grateful to Li Luo for supplying the purified P16-His-tag protein.
REFERENCES
- 1.Anagnostopoulos, C., and J. Spizizen. 1961. Requirements for transformation in Bacillus subtilis. J. Bacteriol. 81:74-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan, C. E., and S. L. Neyman. 1986. Correlation of penicillin-binding protein composition with different functions of two membranes in Bacillus subtilis forespores. J. Bacteriol. 165:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland, F. M., A. Atrih, H. Chirakkal, S. J. Foster, and A. Moir. 2000. Complete spore-cortex hydrolysis during germination of Bacillus subtilis 168 requires SleB and YpeB. Microbiology 146:57-64. [DOI] [PubMed] [Google Scholar]
- 4.Driks, A. 1999. The Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driks, A., and P. Setlow. 1999. Morphogenesis and properties of the bacterial spore, p. 191-218. In Y. V. Brun and L. J. Shimkets (ed.), Prokaryotic development. American Society for Microbiology, Washington, D.C.
- 6.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 7.Horinouchi, S., and B. Weisblum. 1982. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide and streptogramin type B antibiotics. J. Bacteriol. 150:804-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa, S., K. Yamane, and J. Sekiguchi. 1998. Regulation and characterization of a newly deduced cell wall hydrolase gene (cwlJ) which affects germination of Bacillus subtilis spores. J. Bacteriol. 180:1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meador-Parton, J., and D. L. Popham. 2000. Structural analysis of Bacillus subtilis spore peptidoglycan during sporulation. J. Bacteriol. 182:4491-4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moriyama, R., S. Miyata, S. Kudoh, A. Hattori, and S. Makino. 1996. A gene (sleB) encoding a spore cortex-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine mediated germination. J. Bacteriol. 178:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriyama, R., H. Fukuoka, S. Miyata, S. Kudoh, A. Hattori, S. Kozuka, Y. Yosuda, K. Tochikubo, and S. Makino. 1999. Expression of a germination-specific amidase, SleB, of bacilli in the forespore compartment of sporulating cells and its localization on the exterior surface of the cortex in dormant spores. J. Bacteriol. 181:2373-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England.
- 14.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paidhungat, M., and P. Setlow. 2000. Role of Ger-proteins in nutrient and non-nutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant-receptor protein (GerBA) to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paidhungat, M., and P. Setlow. 2001. Spore germination and outgrowth, p. 537-548. In A. L. Sonenshein, J. Hoch, and R. Losick (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 19.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage in spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seyler, R. W., Jr., A. O. Henriques, A. J. Ozin, and C. P. Moran, Jr. 1997. Assembly and interactions of cotJ-encoded proteins, constituents of the inner layers of the Bacillus subtilis spore coat. Mol. Microbiol. 25:955-966. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, B., and Z.-Y. Peng. 1996. Defective folding of mutant p16INK4 proteins encoded by tumor-derived alleles. J. Biol. Chem. 271:28734-28737. [PubMed] [Google Scholar]