Abstract
Subgenomic DNA microarrays were employed to evaluate the expression of the accessory gene regulator (agr locus) as well as multiple virulence-associated genes in Staphylococcus aureus. Gene expression was examined during growth of S. aureus in vitro in standard laboratory medium and rabbit serum and in vivo in subcutaneous chambers implanted in either nonimmune rabbits or rabbits immunized with staphylococcal enterotoxin B. Expression of RNAIII, the effector molecule of the agr locus, was dramatically repressed in serum and in vivo, despite the increased expression of secreted virulence factors sufficient to cause toxic shock syndrome (TSS) in the animals. Statistical analysis and clustering of virulence genes based on their expression profiles in the various experimental conditions demonstrated no positive correlation between the expression of agr and any staphylococcal virulence factors examined. Disruption of the agr locus had only a minimal effect on the expression in vivo of the virulence factors examined. An effect of immunization on the expression of agr and virulence factors was also observed. These results suggest that agr activation is not necessary for development of staphylococcal TSS and that regulatory circuits responding to the in vivo environment override agr activity.
A leading cause of nosocomial infections worldwide, Staphylococcus aureus is the etiologic agent of numerous diseases, ranging from relatively benign skin infections to life-threatening illnesses, such as toxic shock syndrome (TSS), septicemia, and osteomyelitis (reviewed in reference 25). Its ability to cause this range of disease is due, in part, to the expression of a wide array of secreted and cell surface-associated virulence factors. The expression of most of these virulence factors has been described as being regulated by the quorum-dependent accessory gene regulator (agr locus). The present model of agr activity and its effect on virulence factor expression is well reviewed elsewhere (15, 19). Briefly, the agr locus expresses two primary, divergent transcripts. RNAII (agrBDCA) encodes a two-component system (AgrA-AgrC), recognizing the agrD-encoded secreted autoinducing octapeptide (AIP), and AgrB, which is thought to act in the posttranslational processing and secretion of the AIP. The second transcript, RNAIII, acts as the effector molecule of the agr locus. A third, short transcript, RNAI, has been described as encoding AgrA. Upon accumulation in the growth medium of sufficient quantities of the AIP, usually during the transition from the exponential to the stationary phase of growth, signaling via AgrA-AgrC acts to increase transcription of both RNAII and RNAIII. In vitro, this results in the increased expression of secreted virulence factors, including the pyrogenic toxin superantigens and hemolysins, and the repression of numerous surface-associated virulence factors, typified by protein A, which binds the Fc component of immunoglobulin G.
Since the identification of agr (20), models of staphylococcal virulence have assigned it a central role in the organism's ability to cause disease (15, 19). Numerous studies using agr mutants have implicated this system in infections ranging from murine arthritis to endocarditis in rabbits (reviewed in reference 3). It has also been proposed that ligand-based inhibition of agr activity might form the basis of antistaphylococcal chemotherapy (11). However, very few studies have examined the expression of agr in vivo. In this study, we employed subgenomic DNA microarrays to describe the expression of agr, together with numerous associated virulence factors thought to be regulated by this system. Gene expression was examined during growth of S. aureus in serum and in vivo using a subcutaneous infection model in staphylococcal enterotoxin B (SEB)-immune and nonimmune rabbits. We found that agr activation was not necessary for the development of TSS and hypothesize that signals generated by the in vivo environment act to override agr activity and increase expression of secreted virulence factor genes by S. aureus.
MATERIALS AND METHODS
Bacterial strains.
S. aureus MN NJ is an isolate from a recent case of nonmenstrual TSS and produces SEB and two novel superantigens, SEK (16) and SEQ (P. M. Orwin, D. Y. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert, unpublished data). S. aureus MN8, used as a source of genomic template for amplification of virulence gene probes for the DNA microarrays, was isolated before 1980 from a case of menstrual TSS (23). S. aureus RN4282 and RN4256 are isogenic strains in which expression of the agr locus in RN4256 has been disrupted by insertion of the erythromycin resistance transposon Tn551 into agrA (13, 18, 20).
Growth of S. aureus in vitro.
Fifty-milliliter cultures of S. aureus MN NJ were grown aerobically with shaking at 37°C in either Todd-Hewitt (TH) medium (Becton Dickinson, Sparks, Md.) or rabbit serum (Gibco BRL, Carlsbad, Calif.). TH medium contains (per liter) 9 g of beef heart digest, 11 g of pancreatic digest of casein, 3 g of soybean peptone, 2 g of dextrose, 2.5 g of Na2CO3, 0.5 g of NaH2PO4, and 2 g of NaCl. Two independent cultures in each medium were grown in parallel, and samples were removed at the exponential, postexponential, and stationary phases of growth (2, 3, and 8 h, respectively, after inoculation with an initial optical density at 600 nm of 0.1). Expression of agr and associated virulence factors was quantified using DNA microarrays.
Immunization of Dutch-belted rabbits.
The use of animals in this study complied with all relevant federal and institutional guidelines. Two Dutch-belted rabbits were immunized with SEB, which is made in high concentrations (>10 μg/ml) by MN NJ grown in vitro. Rabbits were immunized by three subcutaneous injections at 2-week intervals, with each injection containing 25 μg of purified SEB resuspended in 0.5 ml of phosphate-buffered saline and emulsified in 0.5 ml of incomplete Freund's adjuvant. Development of antibody to SEB was determined by enzyme-linked immunosorbent assay of serum samples taken 1 week after the final immunization. The two rabbits developed anti-SEB (immunoglobulin G) titers of 1:5,120 and 1:10,240, respectively, compared to preimmune titers of <1:20.
Growth of S. aureus in vivo.
Sterilized perforated hollow polyethylene golf balls were implanted subcutaneously in four Dutch-belted rabbits (24). Implantation of the polyethylene balls and subsequent healing created transudate-filled cavities in the rabbits with volumes of approximately 15 ml. Six weeks after implantation of the polyethylene balls, ∼1010 CFU of S. aureus grown in TH medium was collected by centrifugation from the late exponential phase of growth, resuspended into 2 ml of phosphate-buffered saline, and injected into the implanted polyethylene balls. Two milliliters of transudate containing S. aureus was then removed from the infection chambers at the indicated times after inoculation, using a sterile syringe, S. aureus was enumerated by plating, and expression of agr and associated virulence factors was quantified using DNA microarrays.
RNA preparation and DNA microarrays.
Analysis of staphylococcal gene expression in vitro and in vivo using DNA microarrays was performed as described elsewhere (http://www.agac.umn.edu/microarray/protocols/protocols.htm). In brief, a library of targets representing 68 genes from S. aureus MN NJ and MN8 was constructed with primers designed to amplify fragments of ∼300 bp of each gene open reading frame from genomic DNA. Two successive rounds of PCR were performed to minimize genomic DNA contamination in the amplification products, and the final 100-μl reaction mixtures were checked for quality on agarose gels and purified with the QIAquick PCR Purification Kit (Qiagen, Valencia, Calif.). The purified products were printed in triplicate using a Total Array System robot (BioRobotics, Boston, Mass.). Cell pellets from centrifuged samples of S. aureus cultures were flash-frozen in liquid nitrogen. Total RNA was prepared using the RNeasy Mini Kit (Qiagen) according to the manufacturer's directions. DNA was removed from the RNA preparations using the RNase-Free DNase Set (Qiagen) according to the manufacturer's directions. cDNA prepared from 10 μg of RNA from S. aureus cultures to be compared was labeled with either Cy3 or Cy5 fluorescent dye (Amersham Pharmacia Biotech, Piscataway, N.J.) and competitively hybridized with the printed microarrays. Images of the hybridized arrays were obtained with a Scanarray 5000 microarray scanner (GSI Lumonics, Watertown, Mass.). One independent hybridization (in triplicate) was conducted for each of two independent experiments. Fluorescence intensities for individual spots were normalized based on the total intensity of fluorescence in the Cy3 and Cy5 channels. Fluorescence intensity was determined as the average intensity of the triplicate spots for each gene. Total fluorescence for each gene was normalized between arrays for independent experiments, the data were combined from both experiments, and statistical significance was determined using the Student t test to compare expression data from the two growth conditions of interest. To account for possible bias in labeling of cDNA by either Cy3 or Cy5, dye labeling was reversed in the second independent experiment for each of several experimental conditions. No dye bias was detected. A link to additional results for the microarray studies is posted (http://www.micab.umn.edu/faculty/Schlievert.html).
One-step real-time RT-PCR.
Real-time reverse transcription-PCR (RT-PCR) analysis of the RNA samples used in DNA microarrays assays was used to confirm relative expression changes in RNAIII, hla, and spa. RT-PCRs were performed using the SYBR Green PCR reagents and TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) according to the manufacturer's directions with an ABI Prism 7700 Sequence Detection System. Briefly, 25 ng of total RNA from the same preparations as used for the microarray analyses was added to each of 25-μl PCR mixtures containing 400 nM concentrations of forward and reverse primers (RNAIII, 5"-GATGTTGTTTACGATAGCT-3" and 5"-TTCAATGGCACAAGATATC-3"; hla, 5"-GCAAATGTTTCGATTGGT-3" and 5"-TGTTTGTTGTTTGGATGC-3"; spa, 5"-AGAACAACGCAATGGTTT-3" and 5"-GGCTTGTTGTTGTCTTCC-3"; 16S rRNA, 5"-CTGTGCACATCTTGACGGTA-3" and 5"-TCAGCGTCAGTTACAGACCA-3"). Reaction mixtures were incubated for 30 min at 48°C, followed by an incubation step for 10 min at 95°C and then by 30 cycles of 30 s at 95°C, 30 s at 54°C, and 1 min at 72°C. The increase in fluorescence between extension steps was used to monitor the increase in the amount of amplified product and to determine a fractional cycle number (CT) required to achieve a set threshold of amplification. CT values for the genes of interest were normalized using the CT of 16S rRNA for the corresponding sample.
RESULTS
To model staphylococcal virulence factor expression in serum and in vivo, we chose S. aureus strain MN NJ, an isolate from a case of nonmenstrual TSS. Our laboratory has sequenced a novel staphylococcal pathogenicity island (SaPI3) (J. M. Yarwood, J. K. McCormick, M. L. Paustian, P. M. Orwin, V. Kapur, and P. M. Schlievert, submitted for publication) in this strain that encodes SEB and two novel superantigenic enterotoxins, SEK (16) and SEQ (Orwin et al., unpublished data). Expression of virulence factors by this strain when grown in standard laboratory medium is as predicted by the present model of agr regulation (Table 1). Levels of RNAIII and exotoxins increased in the stationary phase of growth compared to the late exponential phase of growth, while the expression of the surface-associated protein A was repressed.
TABLE 1.
Comparison of gene expression by S. aureus MN NJ in stationary-phase growth versus late-exponential-phase growth in TH mediuma
| Gene | Description or product | Fold expression changeb |
|---|---|---|
| RNAII | agrBDCA | 2.2 ± 0.2 |
| RNAIII | Effector molecule of agr locus | 5.8 ± 1.3 |
| spa | Protein A | −33.6 ± 6.3 |
| hla | Alpha-hemolysin | 6.6 ± 0.9 |
| seb | Enterotoxin B | 8.2 ± 1.1 |
| hlgA | Gamma-hemolysin, a-component | 2.7 ± 0.5 |
Strain MN NJ was grown aerobically at 37°C with shaking, cells were harvested 2 and 8 h after inoculation (initial optical density at 600 nm of 0.1), and gene expression was determined using DNA microarrays as described in the text.
Values represent the fold change of gene expression ± standard error of the mean in the stationary-phase cultures compared to the late-exponential-phase cultures. Positive values indicate genes with increased expression in stationary-phase growth; negative values indicate genes repressed in stationary-phase growth. The results represent averages of data for two independent experiments, each of which was evaluated by arrays conducted in triplicate. All results were significant (P < 0.01 by Student's t test).
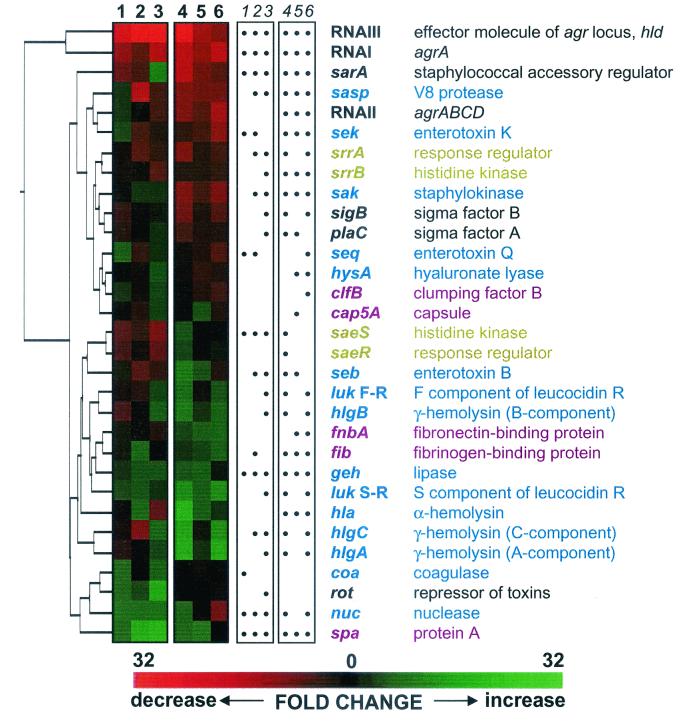
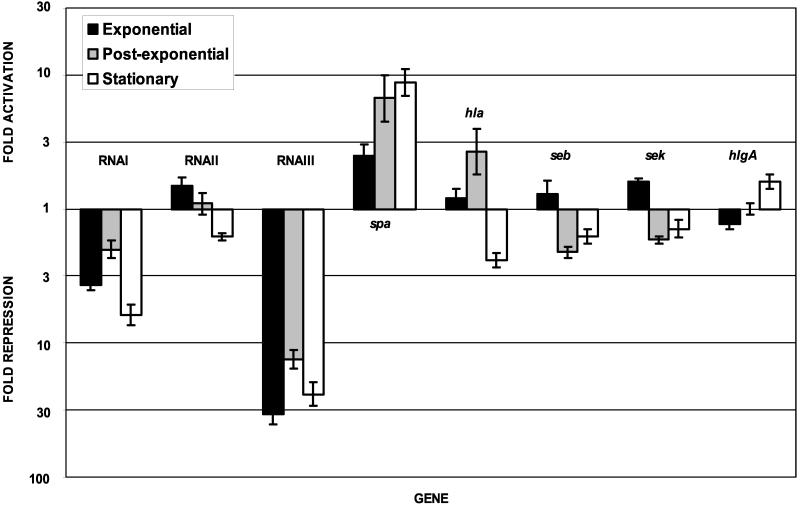
To approximate the expression of virulence factors in vivo and determine those genes whose expression is enhanced or repressed by growth in a standard laboratory medium, cultures of S. aureus were grown in either TH medium or rabbit serum. Expression profiles for virulence-associated genes reflecting the fold increase or decrease induced by growth in rabbit serum versus TH medium are shown in Fig. 1 (lanes 1 to 3), and a summary of results for selected genes is shown in Fig. 2. The most dramatic effect of growth in serum was the repression of agr, as indicated by the 34-, 13-, and 24-fold repression of RNAIII in the exponential, postexponential, and stationary phases of growth, respectively. As would be predicted by the present model of agr regulation, the level of spa transcript, encoding the surface molecule protein A, was increased during growth in serum. However, levels of hla transcript, encoding the secreted alpha-hemolysin, which would be predicted to decrease in response to repression of agr, were not significantly affected (P > 0.05 by Student's t test). Furthermore, the expression of other exotoxins was somewhat inconsistent with the repression of agr. Although RNAIII was strongly repressed during all phases of growth in serum, sek and seq transcript levels were increased during exponential-phase growth, while expression of hlgA was increased in the stationary phase of growth. (Unlike seb, sek and seq are constitutively expressed throughout the growth phases of S. aureus in vitro [unpublished data].) Transcription of RNAI was also repressed by growth in serum during the postexponential and stationary phases of growth, although it was not nearly as repressed as the divergent RNAIII transcript. RNAII levels were unaffected by growth in serum. The agr regulon is thought to operate in a positive-feedback mechanism to increase transcriptional activity from both the agr P2 (RNAII) and P3 (RNAIII) promoters. If this model is correct, these data indicating differential regulation of transcriptional activity from the P2 and P3 promoters suggest that the AIP is secreted and recognized by the AgrA-AgrC two-component system in serum as it is in TH medium and that a secondary, unknown factor (or factors) acts to repress transcriptional activity from the P3 promoter. This factor is unlikely to be SarA, whose expression did not correlate with that of agr (Fig. 1 [cluster analysis]) and which is generally thought to activate transcription of the agr locus (19).
FIG. 1.
Expression profiles and hierarchical clustering of 31 staphylococcal virulence-associated genes and significance of results. The red and green colors of the expression profiles (lane numbers in boldface) represent fold decreases and increases, respectively, in gene expression in response to growth in serum compared to TH medium (lanes 1 to 3) or growth in vivo compared to the inoculum (lanes 4 to 6). Results are shown for cultures in the exponential (lane 1), postexponential (lane 2), and stationary (lane 3) phases of growth in TH medium versus serum. Results are also shown for S. aureus cultures removed from the subcutaneous infection chambers in nonimmune rabbits at 2 h after inoculation (lane 4) and in SEB-immune rabbits at 2 h (lane 5) and 8 h (lane 6) after inoculation. Dots (lane numbers in italic) indicate those fold changes that were found to be significant (Student's t test; P < 0.05) in the corresponding lane of the expression profiles. Corresponding gene names and descriptions are shown as follows: black type, gene regulators; blue type, secreted proteins; magenta type, surface-associated molecules; and gold type, two-component systems. Clustering based on similarity of expression profiles and visualization was performed using the software program Spotfire DecisionSite 6.1 (http://www.spotfire.com). Similarities between expression profiles of individual genes in all six experimental conditions were determined using the “city-block distance” method (http://www.spotfire.com).
FIG. 2.
Activation or repression of selected S. aureus genes by growth of the bacterium in serum compared to growth in TH medium in the exponential, postexponential, and stationary phases of growth. Data are plotted against a logarithmic scale. Values are averages of data from two independent experiments, each of which was evaluated using triplicate DNA microarrays. Standard errors of the means are indicated.
To evaluate expression of S. aureus virulence factors in a subcutaneous infection model of TSS, sterilized perforated hollow polyethylene golf balls were implanted subcutaneously in four Dutch-belted rabbits (24). The rabbit model was chosen over a mouse model because rabbits develop symptoms of TSS more consistent with those described for humans (17). This is due in part to the relative resistance of mice to the lethal effects of staphylococcal superantigens without coadministration of a hepatotoxin, while in humans and rabbits, superantigens alone are lethal (5, 6). Two of the four rabbits were immunized with SEB.
All four rabbits developed symptoms consistent with TSS. These included hypotension, respiratory distress, erythema, and obvious discomfort in the vicinity of the infection chamber. However, the SEB-immunized rabbits experienced less severe and delayed onset of symptoms and were euthanized at 22 h after inoculation, while the nonimmune rabbits died from the infection several hours after inoculation. This indicates that immunity against SEB provided only partial protection against staphylococcal TSS, likely due to the fact that S. aureus MN NJ produces multiple superantigens. Cell densities from the recovered abscess samples did not vary more than 0.22 log unit from the inoculum cell density (Table 2), indicating that the observed effects on gene expression were not due to alterations in cell density but rather were due to a response of the organism to the in vivo environment.
TABLE 2.
CFU recovered from subcutaneous infection chambers 2 and 8 h after inoculationa
| Rabbit | Immunizedb | CFU/ml (108) at
|
|
|---|---|---|---|
| 2 h | 8 h | ||
| 1 | No | 8.8 | |
| 2 | No | 7.2 | |
| 3 | Yes | 4.4 | 4.2 |
| 4 | Yes | 4.6 | 4.0 |
The cell density of cultures used for inoculum prior to concentration for injection was 6.7 × 108 CFU/ml.
Rabbits were either nonimmune or immunized against SEB.
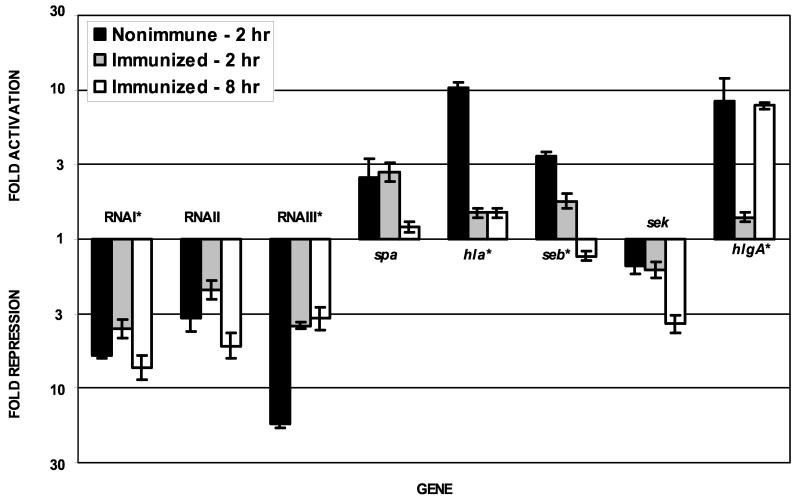
Expression profiles for virulence-associated genes reflecting fold increases or decreases during incubation in vivo compared to the inoculum are shown in Fig. 1 (lanes 4 to 6), and a summary of results for selected genes is shown in Fig. 3. As observed during growth in serum, transcription of RNAII and RNAIII was repressed by growth in vivo in both the nonimmune and immunized animals. Interestingly, a significant (P < 0.05 by Student's t test) effect of immunization on agr activity was observed, with RNAIII being much less repressed in the immune animals than in the nonimmune animals (4- versus 17-fold, respectively, at 2 h). This effect of immunization was much less dramatic for RNAI and RNAII transcription. An effect of immunization on the expression of alpha-toxin (hla), SEB (seb), and the A component of gamma-hemolysin (hlgA), all of whose fold activation was much less at 2 h in the immunized rabbits than the nonimmune rabbits, was also observed.
FIG. 3.
Activation or repression of selected genes in S. aureus removed from subcutaneous infection chambers at the indicated times compared to gene expression by S. aureus in the inoculum. Expression values are plotted for S. aureus removed from the nonimmmune rabbits 2 h after inoculation and from the SEB-immune rabbits 2 and 8 h after inoculation. Data are plotted against a logarithmic scale. Values are averages of data from experiments conducted with four rabbits (two immune and two nonimmune), each of which was evaluated using triplicate DNA microarrays. Standard errors of the means are indicated. Those genes whose expression was significantly affected (P < 0.05 by Student's t test) at 2 h after inoculation by immunization against SEB are indicated by an asterisk.
No positive correlation between RNAIII levels and the expression of exotoxins in vivo was observed. The present model of agr activity would have predicted that transcription of the alpha-hemolysin gene (hla), which has been shown to be strongly and positively regulated by agr in vitro (15), would be repressed in response to decreased RNAIII levels. Instead, hla expression was increased by 10-fold in the nonimmune rabbits, while RNAIII transcription levels were reduced 17-fold. The expression of SEB (seb) and gamma-hemolysin (hlgA) was increased in these animals as well. While RNAIII levels were somewhat restored in the SEB-immune animals, the fold activation of hla, seb, and hlgA transcription was reduced. Other virulence factor genes, such as those for capsule (cap5A) and coagulase (coa), were generally not affected by growth in vivo and the observed agr repression, despite having been reported as being regulated by agr (4, 14). Meanwhile, transcription of other virulence genes behaved as predicted from repression of agr. Expression of fibronectin-binding protein (fnbA) and protein A (spa), both surface-associated virulence factors, was increased in vivo, while that of V8 protease (sasp) and staphylokinase (sak) was repressed in vivo.
To further determine whether a functional agr locus was necessary for the expression of virulence factors in vivo, isogenic S. aureus strains RN4282 and RN4256 (agrA::Tn551) were each used to inoculate the subcutaneous infection chambers of two nonimmune rabbits. All four animals developed symptoms consistent with TSS and died within 16 h after inoculation. As expected, insertion of the transposon resulted in repression of RNAIII transcription (Table 3). However, the disruption of agr expression had only a minimal effect (fold change of <1.6) on the expression of representative surface-associated and secreted virulence factors both 2 and 8 h after inoculation of the infection chambers.
TABLE 3.
Comparison of virulence gene expression by S. aureus strains RN4256 (agrA::Tn551) and RN4282 (agr+) recovered from subcutaneous infection chambers at 2 and 8 h after inoculation
| Gene | Description or product | Fold expression changea at:
|
|
|---|---|---|---|
| 2 h | 8 h | ||
| RNAIIb | agrBDCA | −2.9 ± 0.5 | −1.6 ± 0.4 |
| RNAIII | Effector molecule of agr locus | −15.1 ± 3.4 | −7.1 ± 1.0 |
| spa | Protein A | 1.6 ± 0.2 | 1.4 ± 0.2 |
| hla | Alpha-hemolysin | 1.3 ± 0.3 | 1.3 ± 0.1 |
| tstH | TSS toxin 1 | −1.5 ± 0.5 | 1.5 ± 0.2 |
| hlgA | Gamma-hemolysin, a-component | −1.4 ± 0.2 | −1.5 ± 0.1 |
Values represent the fold change in gene expression ± standard error of the mean in strain RN4256 compared to strain RN4282 as determined using triplicate DNA microarrays. Results are averages of data obtained using four rabbits, two infected with RN4256 and two infected with RN4282.
Insertion of Tn551 did not interfere with the ability to detect RNAII, as the microarray probe for RNAII was specific for a region upstream of agrA.
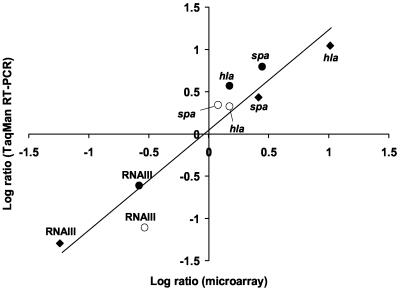
The relative changes in expression for RNAIII, hla, and spa in vivo versus the inoculum were confirmed using TaqMan RT-PCR with the same RNA samples as prepared for microarray analysis as templates (Fig. 4). The results demonstrated a strong positive correlation (r = 0.95) between the data obtained using the two different techniques.
FIG. 4.
Correlation of microarray and Taqman RT-PCR assays. The fold difference in the number of cDNA molecules present in vivo compared to the inoculum as determined by both microarrays and RT-PCR was log transformed, and values were plotted. Closed diamonds represent samples removed 2 h after inoculation of nonimmune rabbits, and closed and open circles represent samples removed 2 and 8 h, respectively, after inoculation of SEB-immune rabbits. The line of best fit is shown (r = 0.95).
DISCUSSION
Taken together, the data from both the serum and in vivo experiments suggest that expression of the agr locus, particularly its effector molecule RNAIII, is not critical for the expression of exotoxins important in the development of staphylococcal TSS associated with an abscess or bacteremia. Despite dramatic repression of agr both in serum and in vivo, the expression of alpha-hemolysin, SEB, and gamma-hemolysin was increased or unaffected. A cluster analysis of genes based on the similarity of their expression profiles under the various experimental conditions confirmed that expression of agr did not correlate with the expression of staphylococcal exotoxins (Fig. 1). Indeed, RNAI and RNAIII comprised an expression profile group separate from any of the virulence factors examined. Furthermore, disruption of the agr locus had only a minimal effect on the expression of both surface-associated and secreted virulence factors in vivo (Table 3).
Although contradictory to present models of staphylococcal virulence, our results are not entirely unprecedented, as some previous studies have suggested a less critical role for agr. In one of the few studies to quantify RNAIII levels in human infections, Goerke et al. directly quantified RNAIII transcript levels in sputum samples from cystic fibrosis patients infected with S. aureus (9). Those authors found that RNAIII was poorly expressed in vivo and concluded that agr activity was nonessential for S. aureus infection of the cystic fibrosis lung. More recently, the same group determined that expression of alpha-hemolysin in a guinea pig model of device-related infection was unaffected by disruption of agr expression (10). Furthermore, Cheung et al. were unable to demonstrate significantly attenuated virulence for an agr mutant in a rabbit model of endocarditis, although a sar agr double mutant was diminished in both infectivity and intravegetation bacterial densities (2). Additional studies have found that although agr mutants are somewhat attenuated in virulence, they remain clearly capable of causing infection in a rabbit model of osteomyelitis (8) and a rat model of endophthalmitis (7). These studies, combined with the data presented here, suggest a much more complicated picture of staphylococcal virulence than that modeled with agr as a global regulator of virulence factors.
The ability of staphylococci to sense and respond to the in vivo environment now seems to be of paramount importance in the regulation of virulence factor expression. In part, our data indicate that unknown regulatory elements act to repress transcription of RNAIII in vivo. The results also suggest the use by S. aureus of environmental sensing mechanisms that regulate virulence factors in response to multiple in vivo signals. These signals appear to override agr activity (perhaps even acting in its repression) to increase expression of secreted virulence factors. Indeed, numerous environmental conditions, consistent both in vitro and in vivo, have been shown to be necessary for exotoxin production (1, 12, 15, 21-23, 26, 27). For example, even in the presence of a functional agr system, decreased oxygen and carbon dioxide levels will result in repression of exotoxin production (21, 26, 27). However, there likely remains to be identified an additional in vivo environmental signal, or combination of signals, to which staphylococci respond in their regulation of virulence factors.
It is not yet clear why the rabbit immune status affects the expression of staphylococcal virulence genes (Fig. 3). The primary difference between the in vivo environments of the immune and nonimmune animals may be due to the superantigenic activity of SEB. SEB activity in the nonimmune animals would be expected to result in fairly rapid activation of and cytokine release by antigen-presenting cells, which was reflected in the visible erythema surrounding the infection chamber upon autopsy of the animals. On the other hand, neutralization of SEB by host antibody in the immunized animals likely eliminated its superantigenic activity. Thus, the immune response in the SEB-immune animals would more closely resemble the very early stages of a natural secondary immune response to conventional antigens. It is also possible that the presence of high antibody titers to SEB might interfere with secretion of SEB, and perhaps other proteins, by S. aureus. The ability of S. aureus to sense these various immune responses either directly or indirectly may be responsible for the observed changes in virulence gene expression.
In light of the findings presented here and elsewhere (9, 10), the proposed use of therapeutic agents to inhibit signaling by agr (11) may be ineffective and irrelevant, as the expression of agr appears to have little or no effect on the expression of exotoxins in vivo. Instead, considering the demonstrated importance of environmental conditions in the expression of staphylococcal virulence factors, more effective therapeutic strategies might seek to interfere with the ability of S. aureus to sense and respond to the in vivo environment.
Acknowledgments
This research was funded in part by the Procter & Gamble Co. In vivo experiments were supported by NHLBI grant HL36611 to P.M.S. J.M.Y. was supported by a Howard Hughes Medical Institute predoctoral fellowship.
We thank G. M. Dunny for his helpful review of the manuscript, T. K. Leonard for assistance in preparation of figures, B. J. May for assistance with RT-PCR, and J. Rucker for technical assistance.
REFERENCES
- 1.Chan, P. F., and S. J. Foster. 1998. The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325-4. Microbiology 144:2469-2479. [DOI] [PubMed] [Google Scholar]
- 2.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins, L. V., and A. Tarkowski. 2000. Animal models of experimental Staphylococcus aureus infection, p. 422-430. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 4.Dassy, B., T. Hogan, T. J. Foster, and J. M. Fournier. 1993. Involvement of the accessory gene regulator (agr) in expression of type 5 capsular polysaccharide by Staphylococcus aureus. J. Gen. Microbiol. 139:1301-1306. [DOI] [PubMed] [Google Scholar]
- 5.Dinges, M. M., J. Jessurun, and P. M. Schlievert. 1998. Comparisons of mouse and rabbit models of toxic shock syndrome. Int. Congr. Symp. Ser. 229:167-168. [Google Scholar]
- 6.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giese, M. J., J. A. Berliner, A. Riesner, E. A. Wagar, and B. J. Mondino. 1999. A comparison of the early inflammatory effects of an agr−/sar− versus a wild type strain of Staphylococcus aureus in a rat model of endophthalmitis. Curr. Eye Res. 18:177-185. [DOI] [PubMed] [Google Scholar]
- 8.Gillaspy, A., S. Hickmon, R. Skinner, J. Thomas, C. Nelson, and M. Smeltzer. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 63:3373-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goerke, C., S. Campana, M. G. Bayer, G. Doring, K. Botzenhart, and C. Wolz. 2000. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect. Immun. 68:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goerke, C., U. Fluckiger, A. Steinhuber, W. Zimmerli, and C. Wolz. 2001. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alpha-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol. Microbiol. 40:1439-1447. [DOI] [PubMed] [Google Scholar]
- 11.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 12.Kass, E. H., M. I. Kendrick, Y. C. Tsai, and J. Parsonnet. 1987. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J. Infect. Dis. 155:812-815. [DOI] [PubMed] [Google Scholar]
- 13.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 14.Lebeau, C., F. Vandenesch, T. Greenland, R. P. Novick, and J. Etienne. 1994. Coagulase expression in Staphylococcus aureus is positively and negatively modulated by an agr-dependent mechanism. J. Bacteriol. 176:5534-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 16.Orwin, P. M., D. Y. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsonnet, J., Z. A. Gillis, A. G. Richter, and G. B. Pier. 1987. A rabbit model of toxic shock syndrome that uses a constant, subcutaneous infusion of toxic shock syndrome toxin 1. Infect. Immun. 55:1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Projan, S. J., and R. P. Novick. 1997. The molecular basis of pathogenicity, p. 55-81. In K. B. Crossley and G. L. Archer (ed.), The staphylococci in human disease. Churchill Livingstone Inc., New York, N.Y.
- 20.Recsei, P., B. Kreiswirth, M. O'Reilly, P. Schlievert, A. Gruss, and R. P. Novick. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol. Gen. Genet. 202:58-61. [DOI] [PubMed] [Google Scholar]
- 21.Ross, R. A., and A. B. Onderdonk. 2000. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus requires both oxygen and carbon dioxide. Infect. Immun. 68:5205-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarafian, S. K., and S. A. Morse. 1987. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J. Med. Microbiol. 24:75-81. [DOI] [PubMed] [Google Scholar]
- 23.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 24.Scott, D. F., J. M. Kling, J. J. Kirkland, and G. K. Best. 1983. Characterization of Staphylococcus aureus isolates from patients with toxic shock syndrome, using polyethylene infection chambers in rabbits. Infect. Immun. 39:383-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenover, F. C., and R. P. Gaynes. 2000. The epidemiology of Staphylococcus aureus infections, p. 414-421. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 26.Todd, J. K., B. H. Todd, A. Franco-Buff, C. M. Smith, and D. W. Lawellin. 1987. Influence of focal growth conditions on the pathogenesis of toxic shock syndrome. J. Infect. Dis. 155:673-681. [DOI] [PubMed] [Google Scholar]
- 27.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]