Abstract
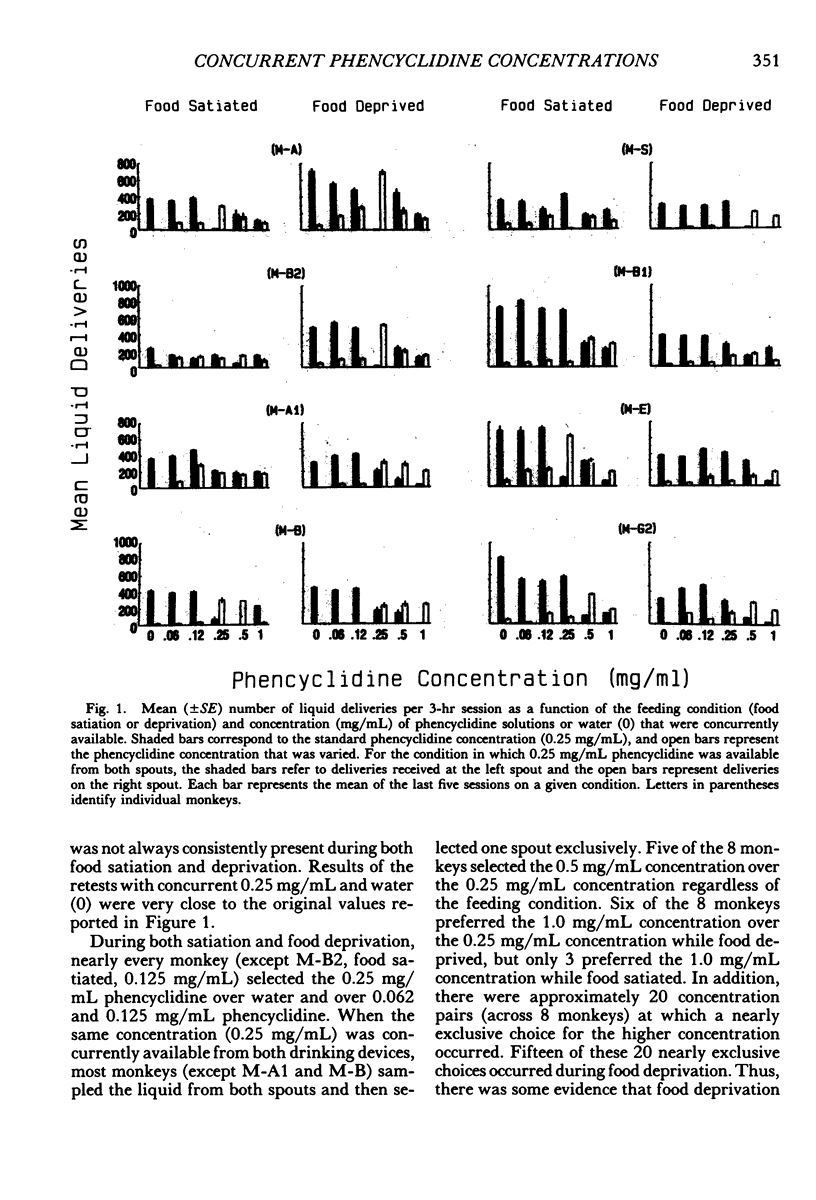
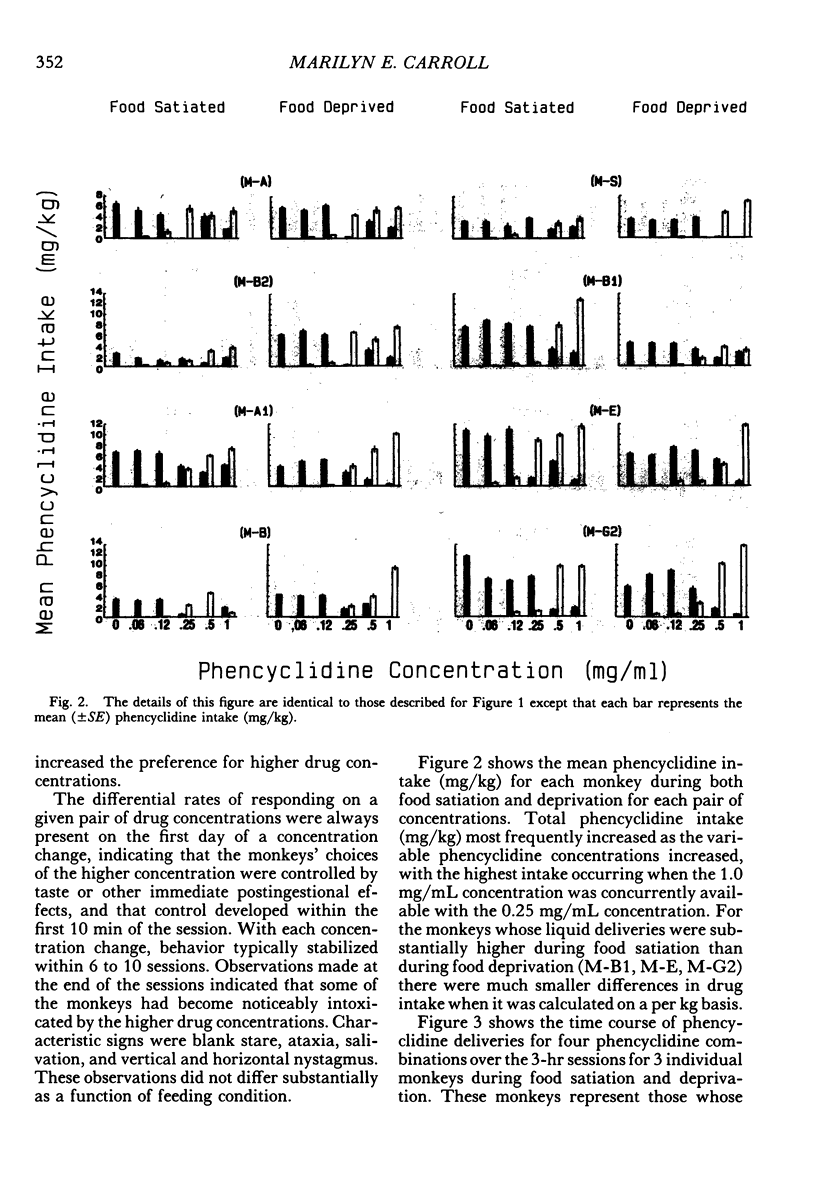
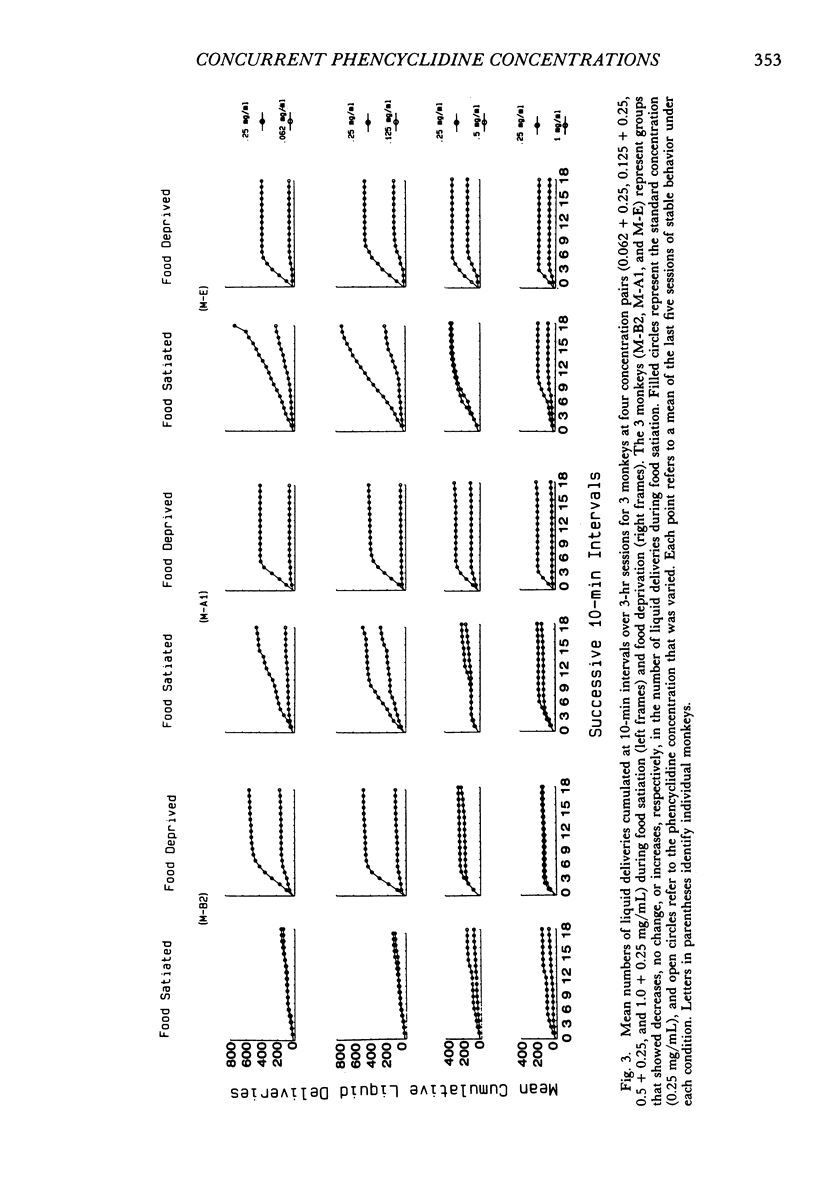
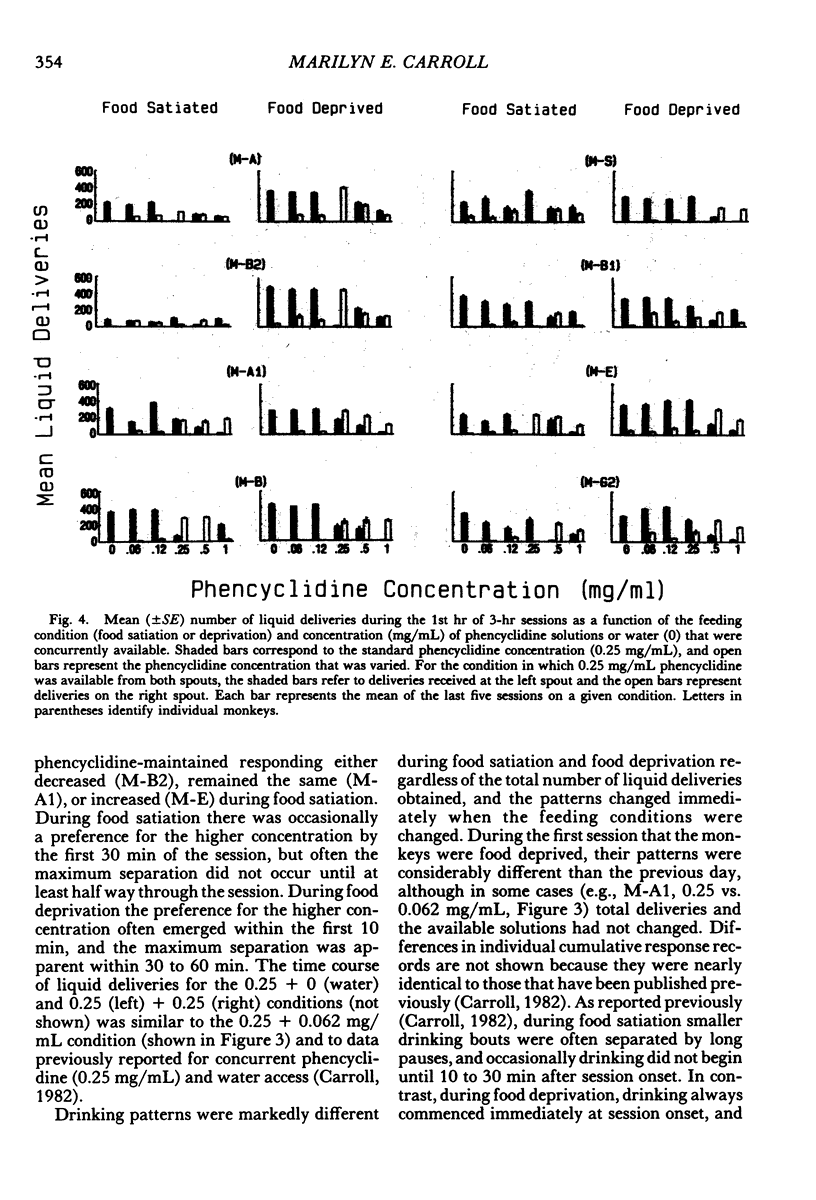
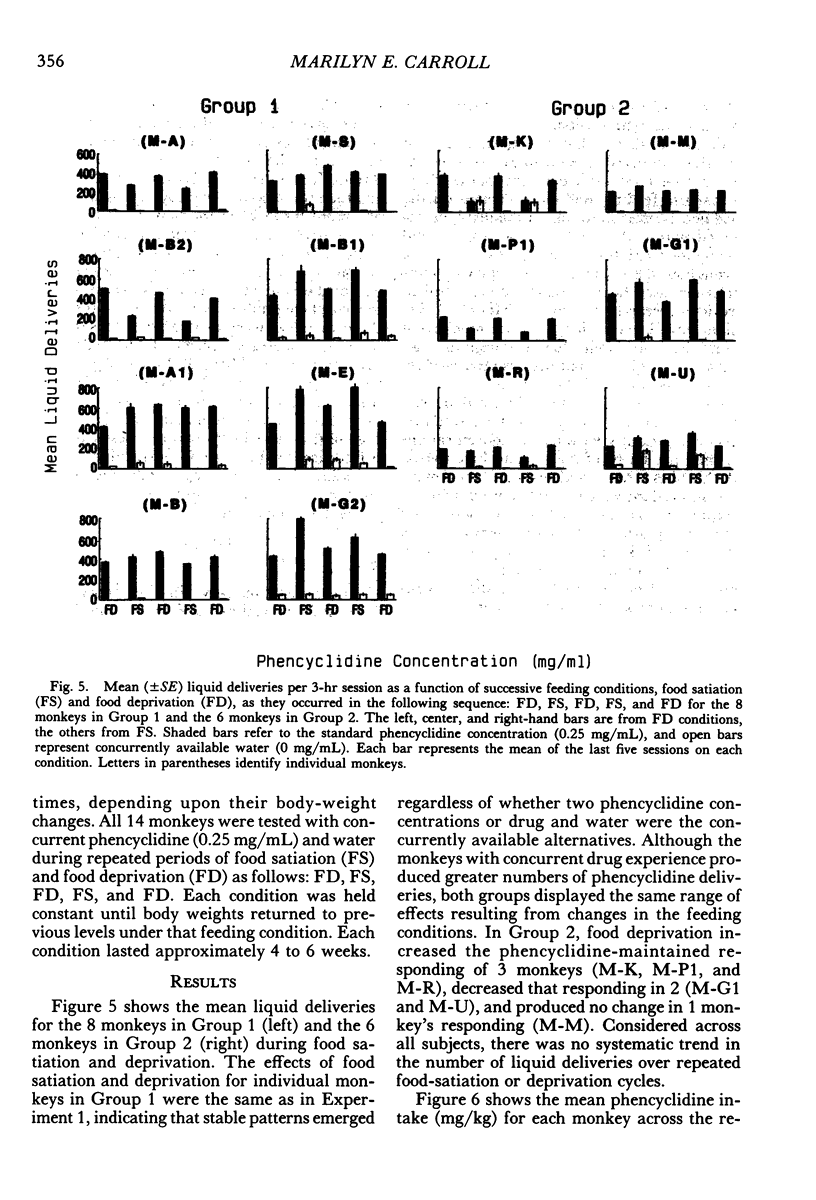
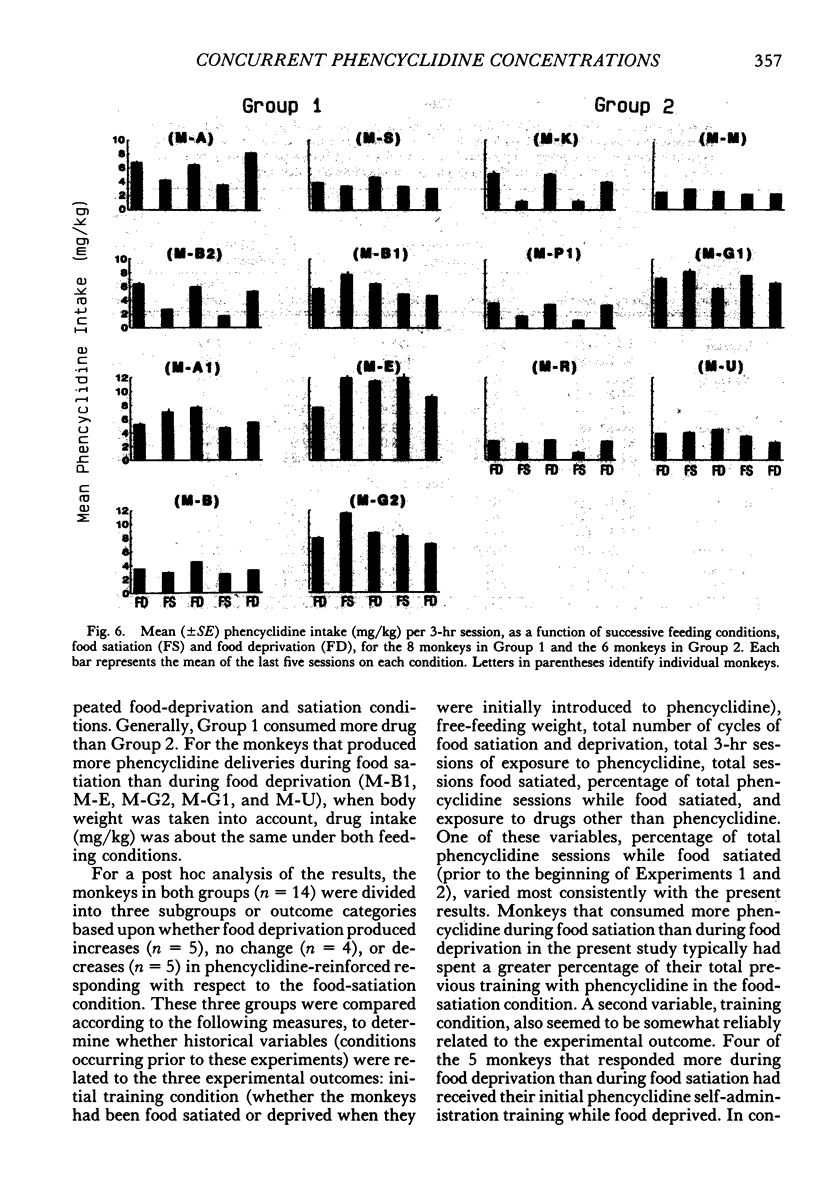
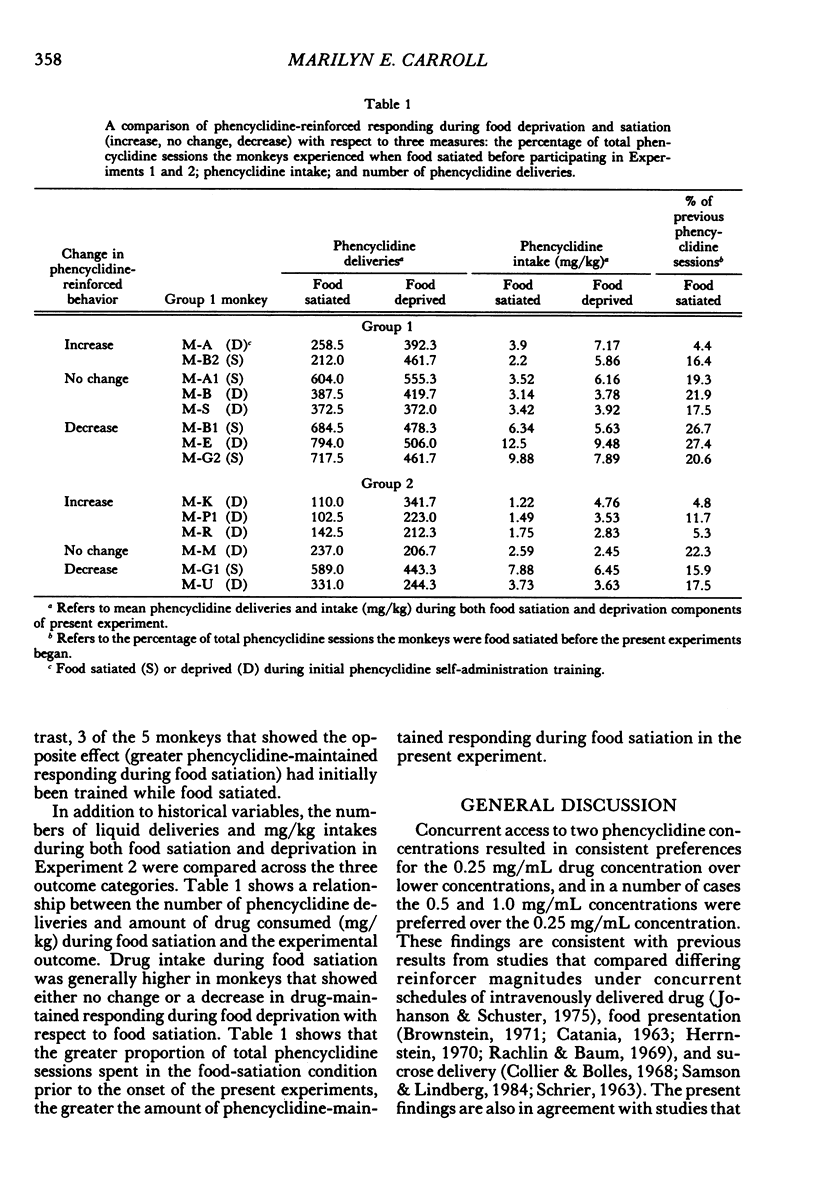
Two experiments addressed the effects of food satiation and deprivation on oral self-administration of two concurrently available phencyclidine concentrations. In the first experiment, 8 rhesus monkeys self-administered either of two concentrations of phencyclidine ("PCP, angel dust") and water under concurrent fixed-ratio 16 schedules. One concentration was always held constant (0.25 mg/mL) while a series of other phencyclidine concentrations, ranging from 0 (water) to 1.0 mg/mL, was presented in a nonsystematic order. Initially the monkeys were tested while food satiated, and the procedure was then repeated during food deprivation. The monkeys usually selected the higher concentration within the first few minutes of the session, indicating that taste and/or other immediate postingestional effects were important factors. Contrary to a number of previous reports, there were no consistent differences across subjects in the mean number of liquid deliveries or mean drug intake (mg/kg) during food satiation and deprivation. However, for all monkeys the within-session time course of responding during food satiation consistently differed from that during deprivation. A second experiment assessed whether the failure to find consistent differences in drug intake during food satiation and deprivation had been due to the history of concurrent access to different phencyclidine concentrations or to the extended experience with phencyclidine under food-satiation conditions. Six additional monkeys (Group 2) were exposed to the phencyclidine self-administration procedure (during food satiation and deprivation) for the same length of time as the monkeys in Experiment 1 (Group 1), except they received only concurrent access to phencyclidine (0.25 mg/mL) and water. Both groups then received concurrent access to phencyclidine and water during five repeated cycles of food deprivation and satiation. There were also marked individual differences in Group 2: During food satiation, 2 of the monkeys' responding increased, 1 showed no change, and 3 decreased. Examination of a number of historical variables indicated that the greater the percentage of total sessions spent during food satiation with phencyclidine available (before these experiments began), the greater the amounts of phencyclidine consumed during food satiation and the smaller the differences in phencyclidine intake when the two feeding conditions were compared.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstein A. J. Concurrent schedules of response-independent reinforcement: duration of a reinforcing stimulus. J Exp Anal Behav. 1971 Mar;15(2):211–214. doi: 10.1901/jeab.1971.15-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATANIA A. C. Concurrent performances: a baseline for the study of reinforcement magnitude. J Exp Anal Behav. 1963 Apr;6:299–300. doi: 10.1901/jeab.1963.6-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. E. Concurrent phencyclidine and saccharin access: presentation of an alternative reinforcer reduces drug intake. J Exp Anal Behav. 1985 Jan;43(1):131–144. doi: 10.1901/jeab.1985.43-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. E., Meisch R. A. Oral phencyclidine (PCP) self-administration in rhesus monkeys: effects of feeding conditions. J Pharmacol Exp Ther. 1980 Aug;214(2):339–346. [PubMed] [Google Scholar]
- Carroll M. E. Performance maintained by orally delivered phencyclidine under second-order, tandem and fixed-interval schedules in food-satiated and food-deprived rhesus monkeys. J Pharmacol Exp Ther. 1985 Feb;232(2):351–359. [PubMed] [Google Scholar]
- Carroll M. E. Rapid acquisition of oral phencyclidine self-administration in food-deprived and food-satiated rhesus monkeys: concurrent phencyclidine and water choice. Pharmacol Biochem Behav. 1982 Aug;17(2):341–346. doi: 10.1016/0091-3057(82)90089-2. [DOI] [PubMed] [Google Scholar]
- Carroll M. E., Santi P. A., Rudell R. L. A microcomputer system for the control of behavioral experiments. Pharmacol Biochem Behav. 1981 Mar;14(3):415–417. doi: 10.1016/0091-3057(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Carroll M. E., Stotz D. C. Increased phencyclidine self-administration due to food deprivation: interaction with concentration and training conditions. Psychopharmacology (Berl) 1984;84(3):299–303. doi: 10.1007/BF00555202. [DOI] [PubMed] [Google Scholar]
- Collier G., Bolles R. Some determinants of intake of sucrose solutions. J Comp Physiol Psychol. 1968 Jun;65(3):379–383. doi: 10.1037/h0025824. [DOI] [PubMed] [Google Scholar]
- Griffiths R. R., Bigelow G. E., Liebson I., Kaliszak J. E. Drug preference in humans: double-blind choice comparison of pentobarbital, diazepam and placebo. J Pharmacol Exp Ther. 1980 Dec;215(3):649–661. [PubMed] [Google Scholar]
- Griffiths R. R., Brady J. V., Snell J. D. Progressive-ratio performance maintained by drug infusions: comparison of cocaine, diethylpropion, chlorphentermine, and fenfluramine. Psychopharmacology (Berl) 1978 Jan 31;56(1):5–13. doi: 10.1007/BF00571401. [DOI] [PubMed] [Google Scholar]
- Healey M. L., Pickens R. W. Diazepam dose preference in humans. Pharmacol Biochem Behav. 1983 Mar;18(3):449–456. doi: 10.1016/0091-3057(83)90468-9. [DOI] [PubMed] [Google Scholar]
- Henningfield J. E., Meisch R. A. Drinking device for rhesus monkeys. Pharmacol Biochem Behav. 1976 May;4(5):609–610. doi: 10.1016/0091-3057(76)90204-5. [DOI] [PubMed] [Google Scholar]
- Herrnstein R. J. On the law of effect. J Exp Anal Behav. 1970 Mar;13(2):243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglauer C., Woods J. H. Concurrent performances: reinforcement by different doses of intravenous cocaine in rhesus monkeys. J Exp Anal Behav. 1974 Jul;22(1):179–196. doi: 10.1901/jeab.1974.22-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C. E. Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev. 1975 Sep;27(3):343–355. [PubMed] [Google Scholar]
- Johanson C. E., Schuster C. R. A choice procedure for drug reinforcers: cocaine and methylphenidate in the rhesus monkey. J Pharmacol Exp Ther. 1975 May;193(2):676–688. [PubMed] [Google Scholar]
- Johanson C. E., Uhlenhuth E. H. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71(3):269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- LESTER D., GREENBERG L. A. Nutrition and the etiology of alcoholism; the effect of sucrose, saccharin and fat on the self-selection of ethyl alcohol by rats. Q J Stud Alcohol. 1952 Dec;13(4):553–560. [PubMed] [Google Scholar]
- Lemaire G. A., Meisch R. A. Oral drug self-administration in rhesus monkeys: interactions between drug amount and fixed-ratio size. J Exp Anal Behav. 1985 Nov;44(3):377–389. doi: 10.1901/jeab.1985.44-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire G. A., Meisch R. A. Pentobarbital self-administration in rhesus monkeys: drug concentration and fixed-ratio size interactions. J Exp Anal Behav. 1984 Jul;42(1):37–49. doi: 10.1901/jeab.1984.42-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewellyn M. E., Iglauer C., Woods J. H. Relative reinforcer magnitude under a nonindependent concurrent schedule of cocaine reinforcement in rhesus monkeys. J Exp Anal Behav. 1976 Jan;25(1):81–91. doi: 10.1901/jeab.1976.25-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin J. A. Response strength in multiple schedules. J Exp Anal Behav. 1974 May;21(3):389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R., Cunningham M. R., Heston L. L., Eckert E., Gustafson L. K. Dose preference during pentobarbital self-administration by humans. J Pharmacol Exp Ther. 1977 Nov;203(2):310–318. [PubMed] [Google Scholar]
- Rachlin H., Baum W. M. Response rate as a function of amount of reinforcement for a signalled concurrent response. J Exp Anal Behav. 1969 Jan;12(1):11–16. doi: 10.1901/jeab.1969.12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHETTLEWORTH S., NEVIN J. A. RELATIVE RATE OF RESPONSE AND RELATIVE MAGNITUDE OF REINFORCEMENT IN MULTIPLE SCHEDULES. J Exp Anal Behav. 1965 Jul;8:199–202. doi: 10.1901/jeab.1965.8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson H. H., Falk J. L. Alteration of fluid preference in ethanol-dependent animals. J Pharmacol Exp Ther. 1974 Aug;190(2):365–376. [PubMed] [Google Scholar]
- Samson H. H., Lindberg K. Comparison of sucrose-sucrose to sucrose-ethanol concurrent responding in the rat: reinforcement schedule and fluid concentration effects. Pharmacol Biochem Behav. 1984 Jun;20(6):973–977. doi: 10.1016/0091-3057(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Samson H. H., Roehrs T. A., Tolliver G. A. Ethanol reinforced responding in the rat: a concurrent analysis using sucrose as the alternate choice. Pharmacol Biochem Behav. 1982 Aug;17(2):333–339. doi: 10.1016/0091-3057(82)90088-0. [DOI] [PubMed] [Google Scholar]
- Woolverton W. L., Johanson C. E. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. J Exp Anal Behav. 1984 Jan;41(1):35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


