Abstract
1. The rate of efflux of lactate from isolated frog sartorius muscles is measured with a superfusion technique. Efflux curves are followed after raising the internal lactate level of the muscles by repetitive electrical stimulation over a 200 sec period.
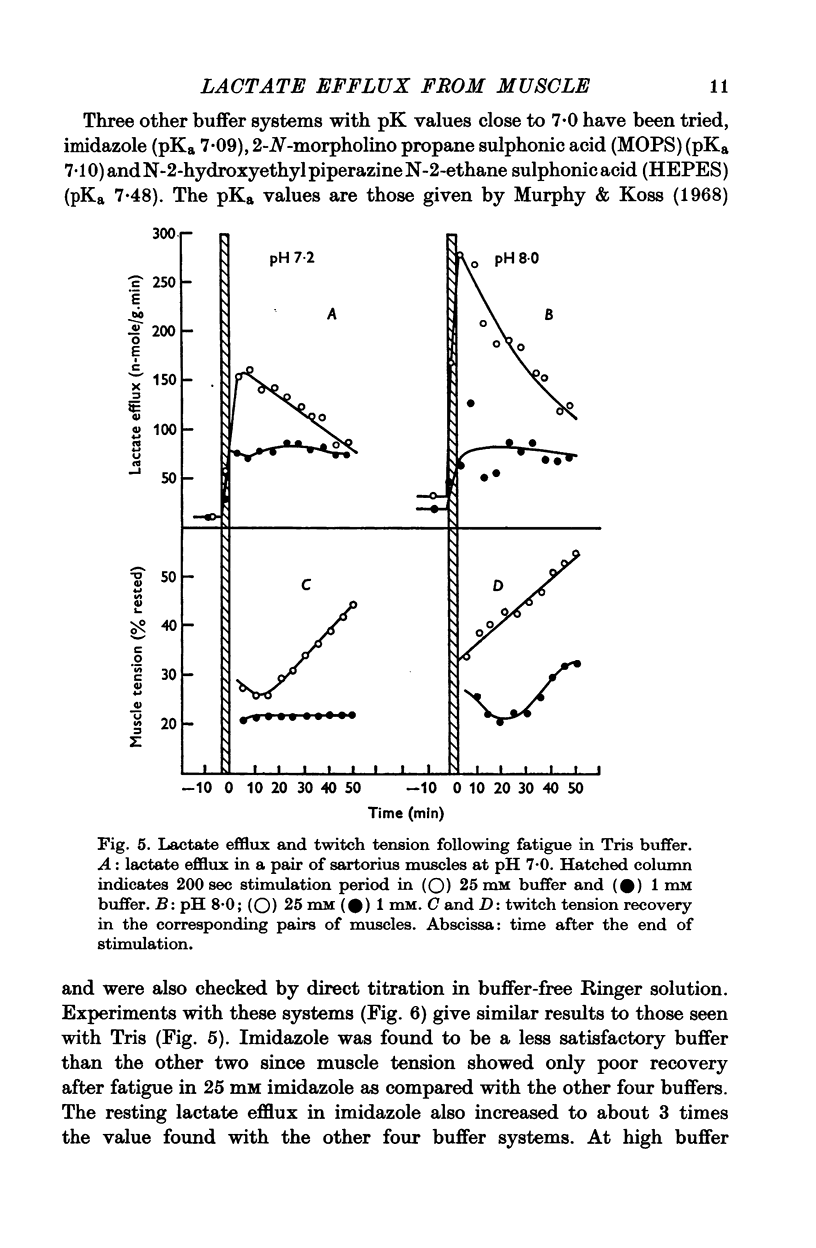
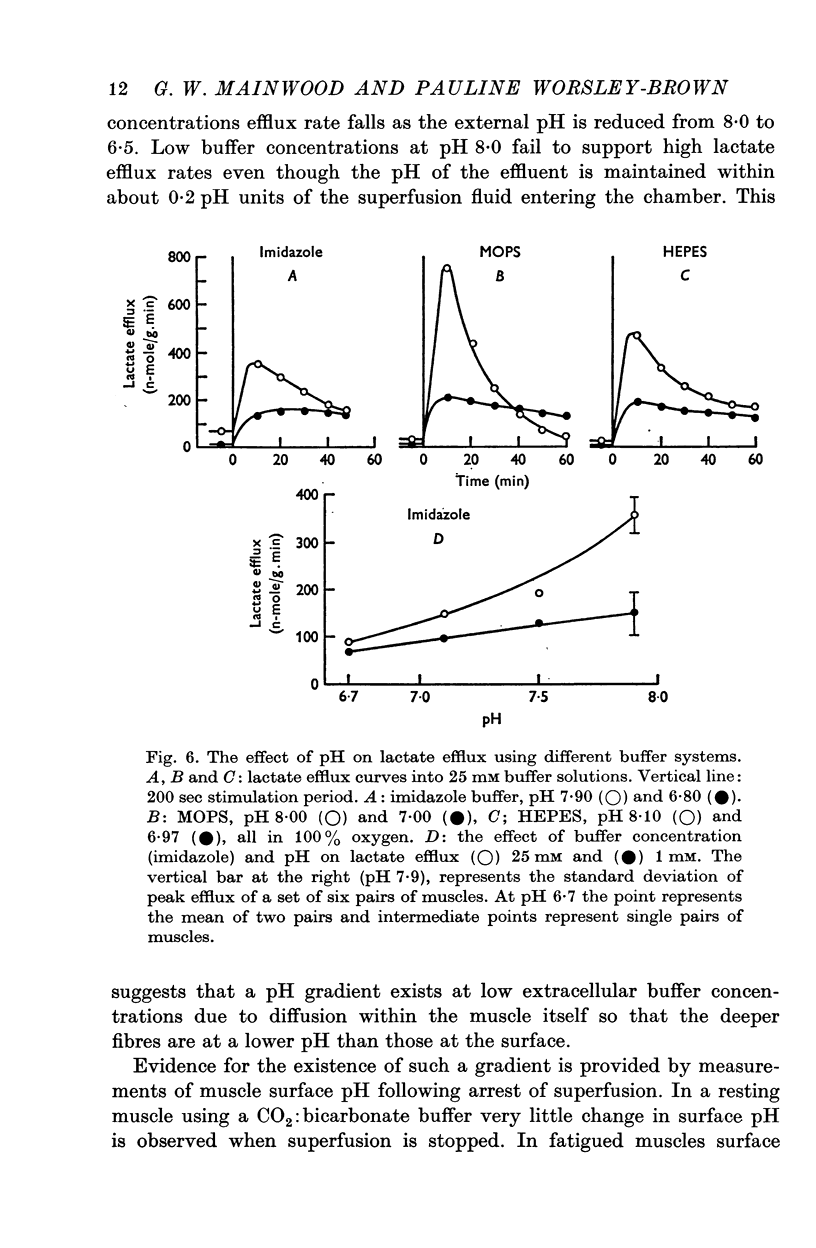
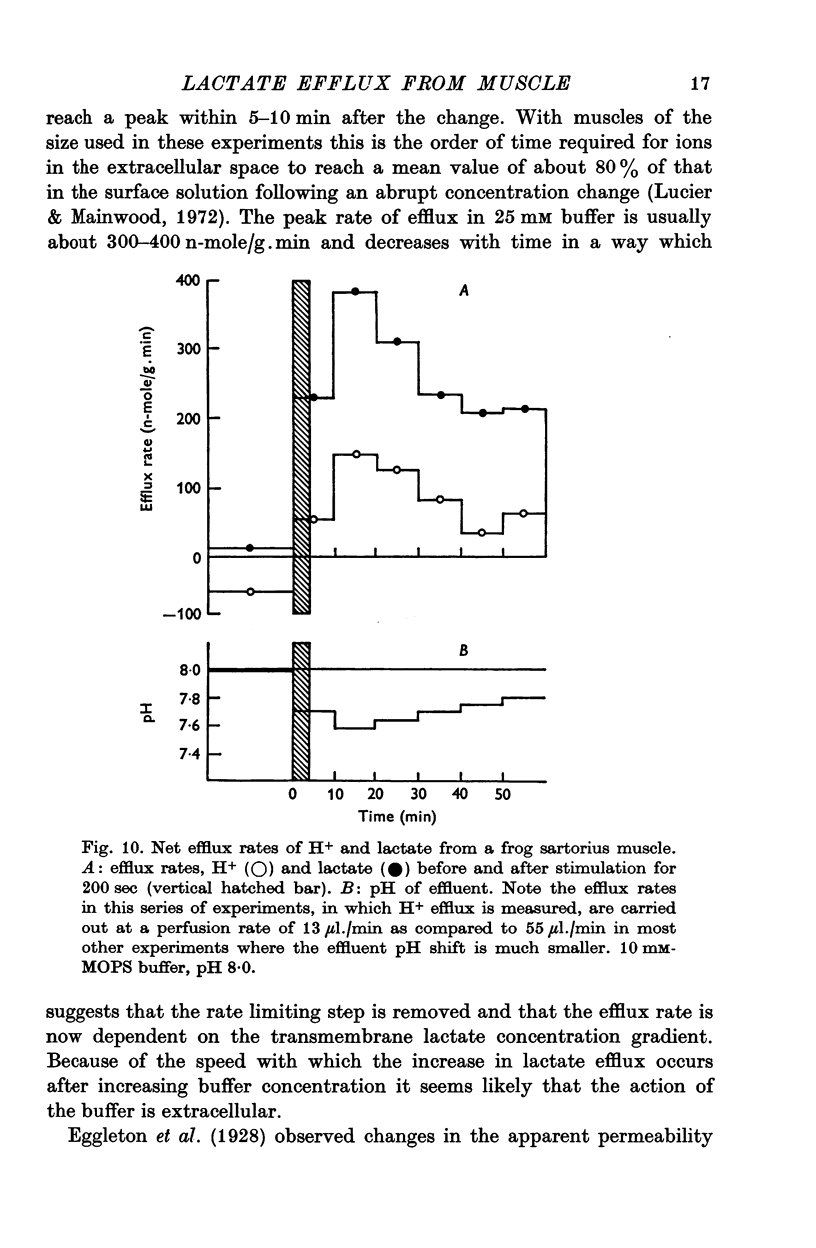
2. With an external pH of 7·0 or below the measured efflux rates following stimulation reach 100-150 n-mole/g.min. Increasing the pH of the superfusion fluid to 8·0 results in a two or threefold increase in the peak efflux rate. The effect is independent of the buffer system used and occurs fairly rapidly when the pH of the superfusion fluid is changed. This suggests that the effect of pH on lactate efflux is extracellular.
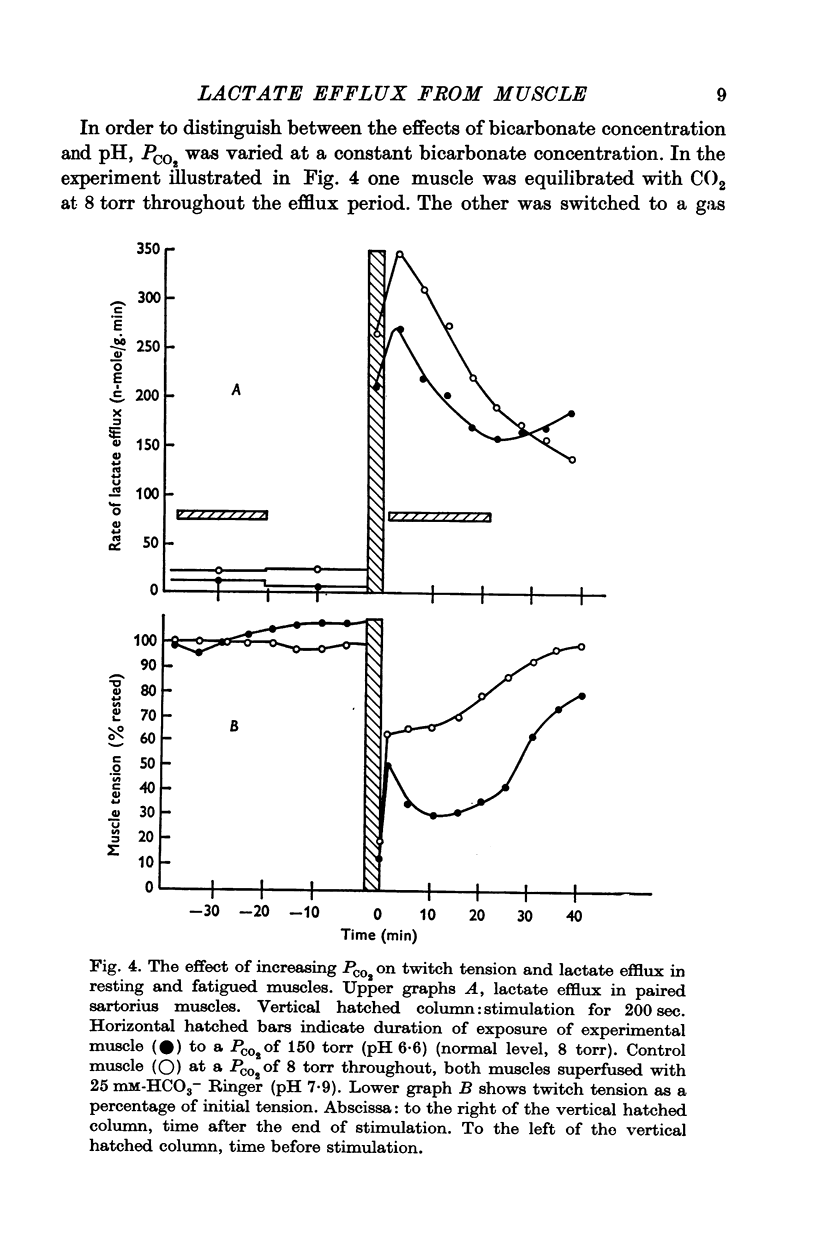
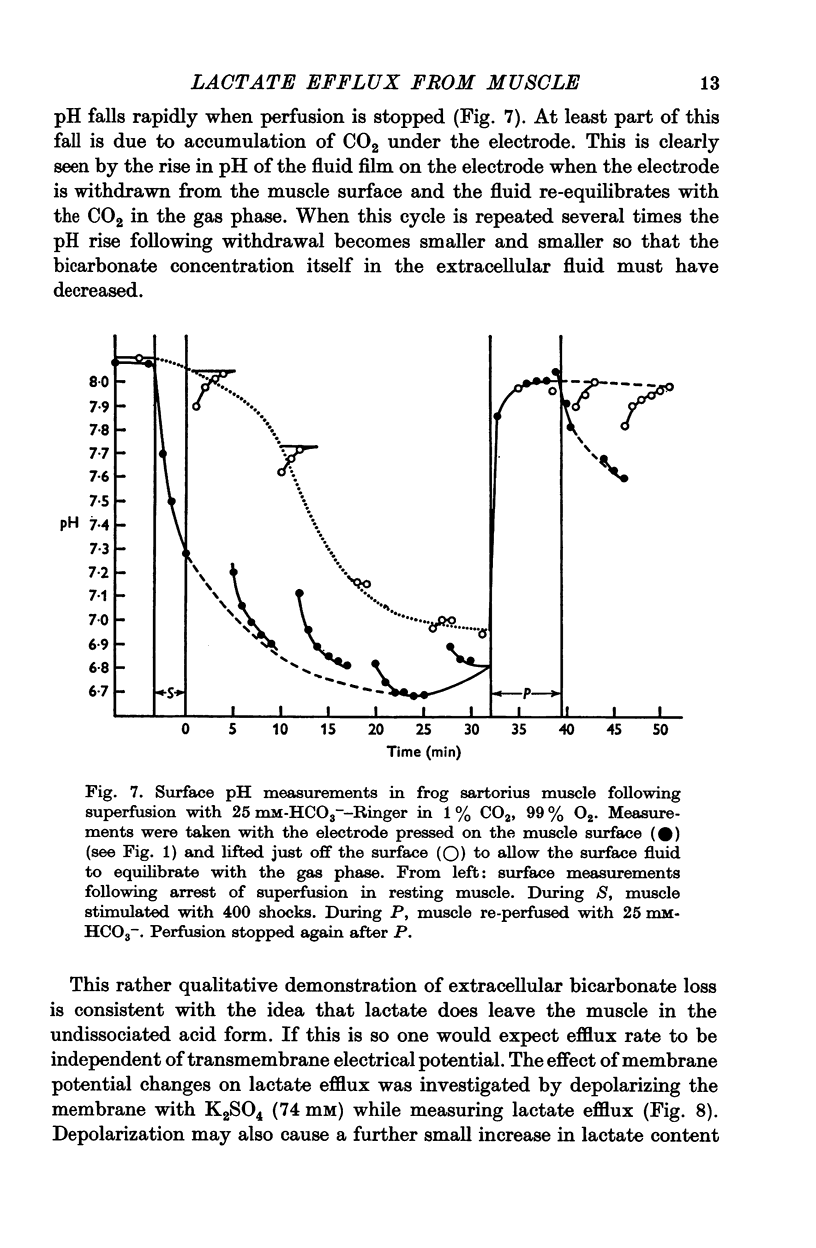
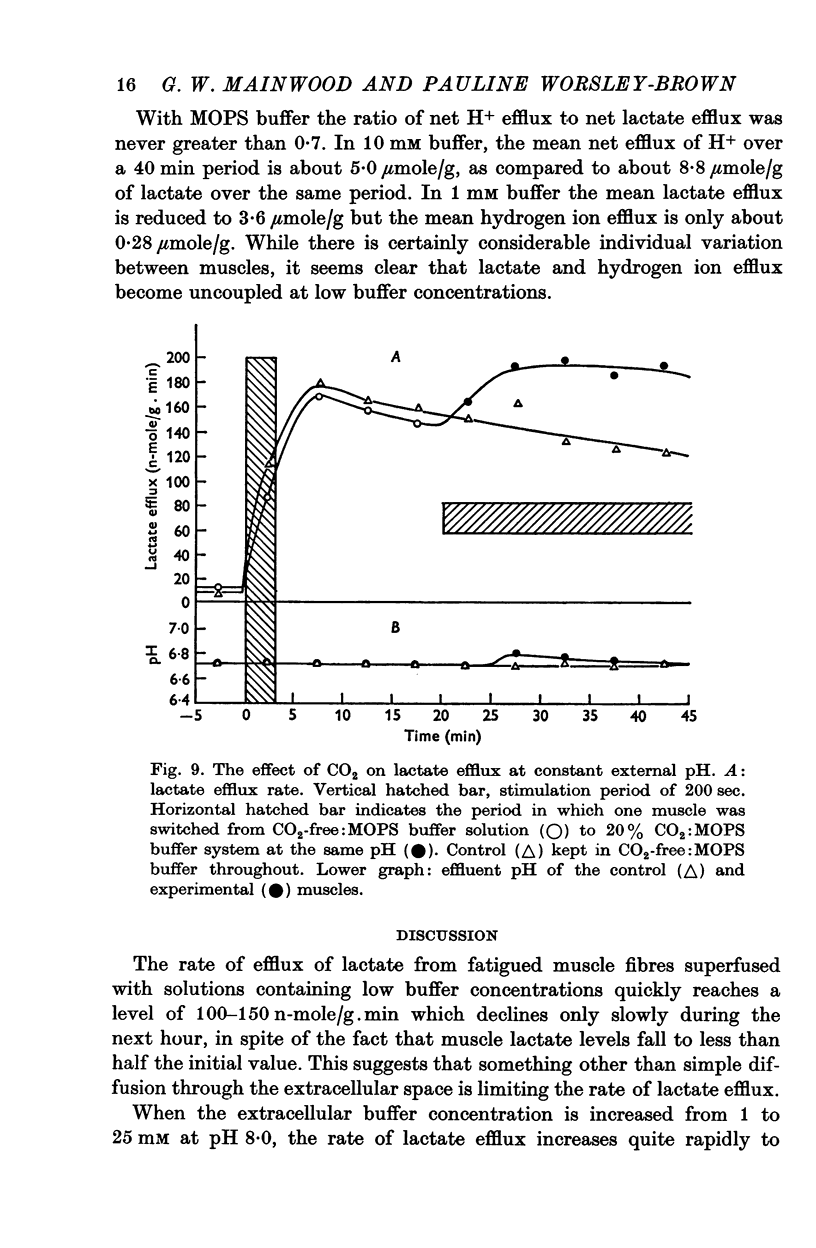
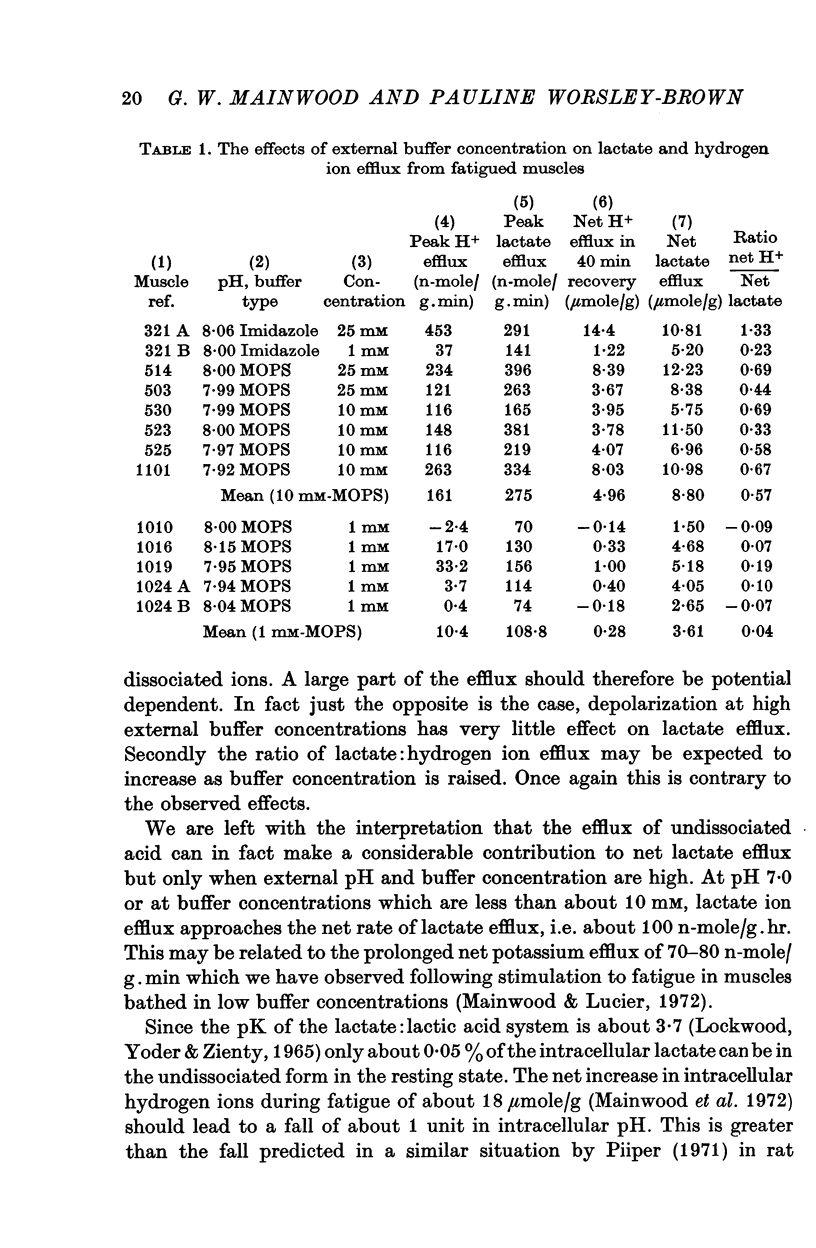
3. The increase in efflux rate due to an increase in pH is dependent on buffer concentration. This fact together with measurements of surface pH changes in muscles following arrest of superfusion indicates that a pH gradient exists through the muscle thickness during lactate efflux.
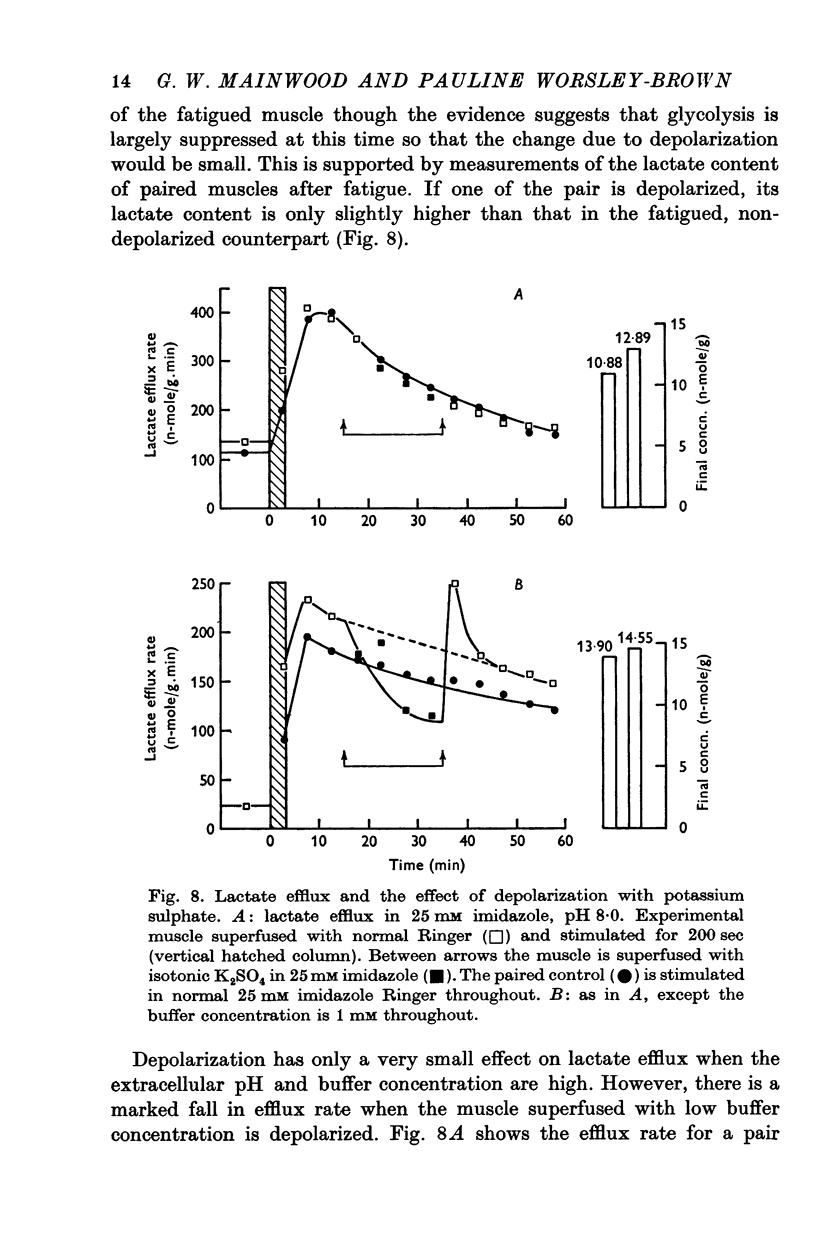
4. The low lactate efflux rate seen at a low buffer concentration (1 mM) is reduced to an even lower level by depolarization with potassium sulphate suggesting a membrane potential dependent component. At pH 8·0 with a high buffer concentration (25 mM) potassium sulphate only reduces efflux rate slightly.
The observations are interpreted as indicating that a fraction of lactate lost is in the form of undissociated acid and that this fraction increases with increasing external pH.
5. Conditions which favour loss of hydrogen ions and lactate from muscle are also associated with improved recovery of twitch tension.
Full text
PDF


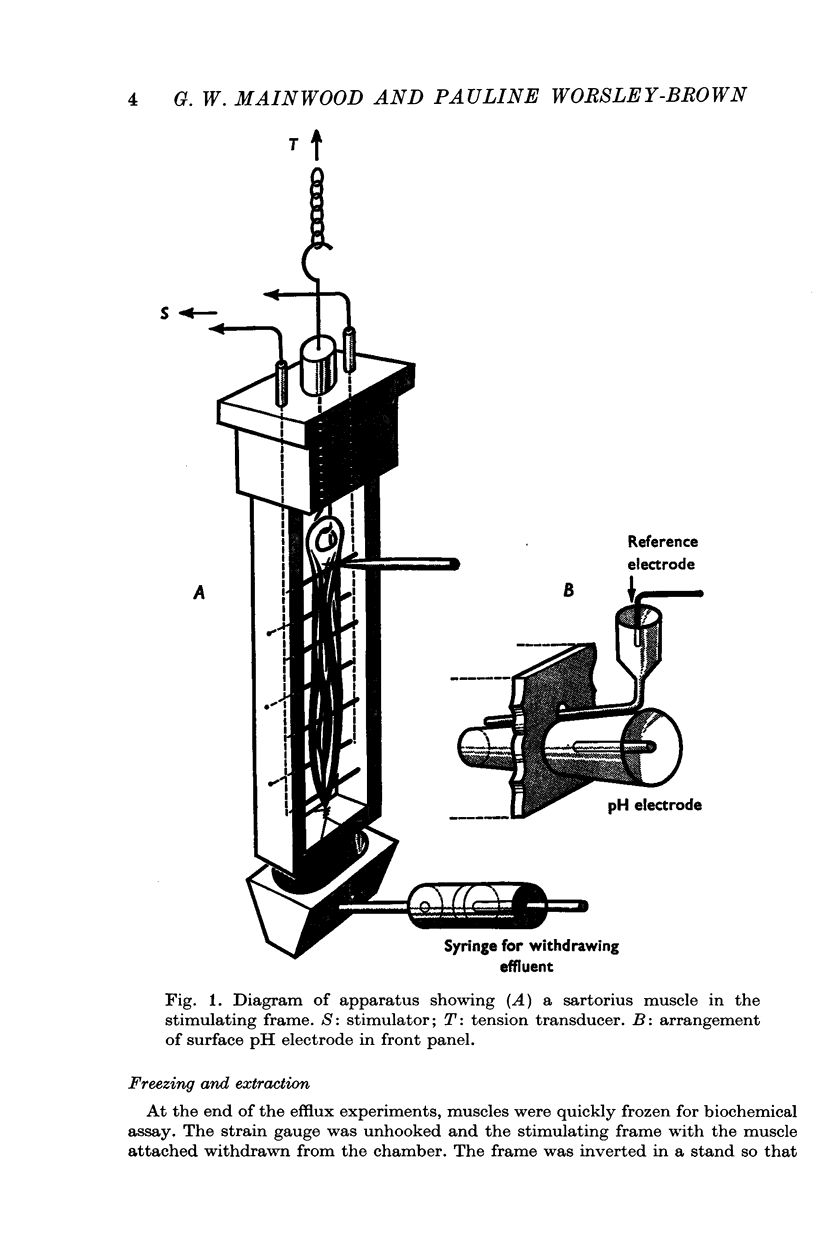


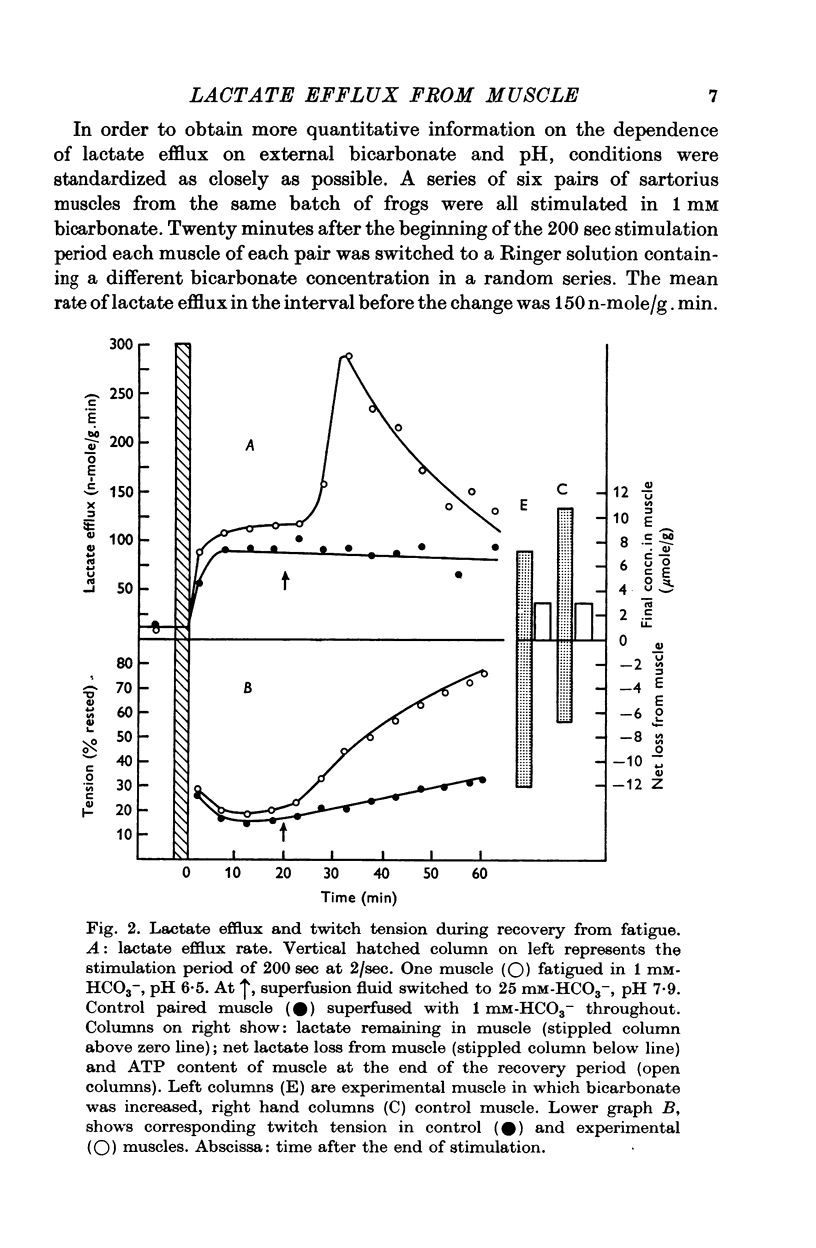
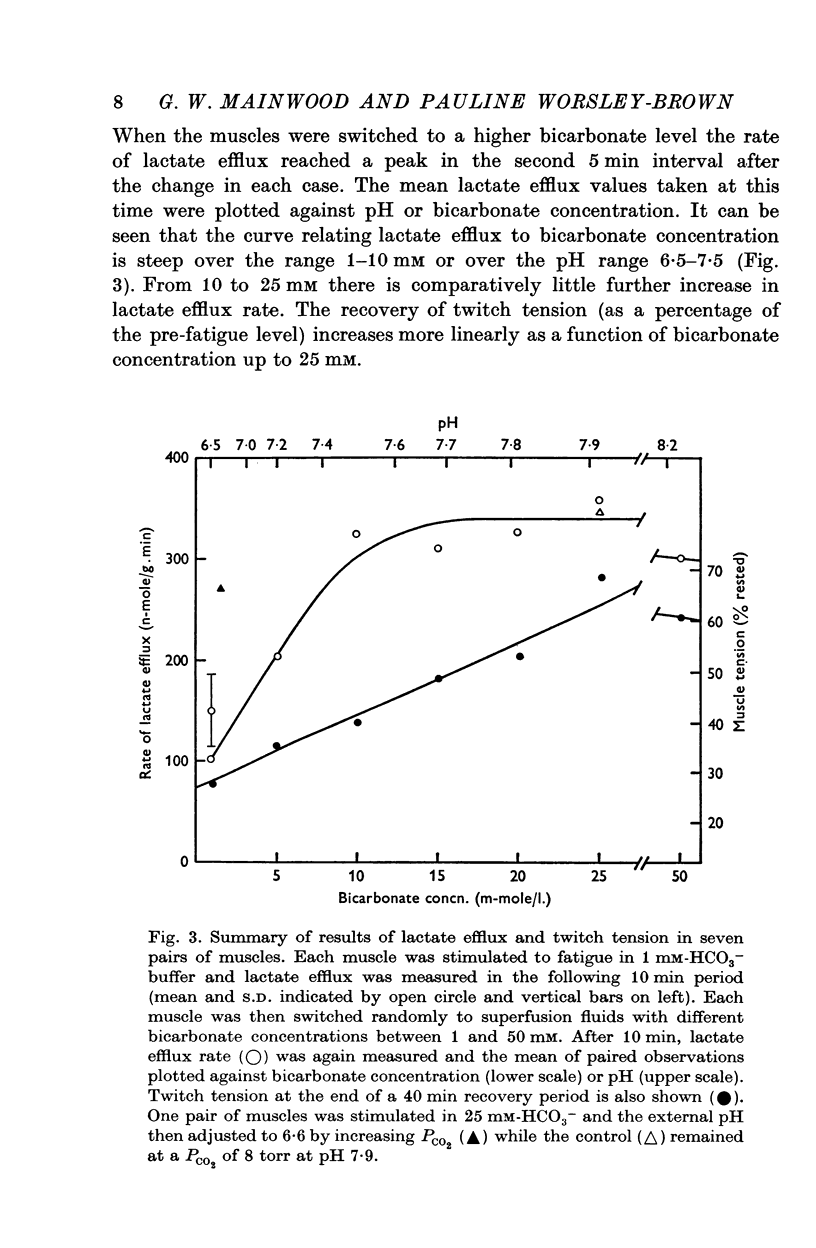






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Diamant B., Karlsson J., Saltin B. Muscle tissue lactate after maximal exercise in man. Acta Physiol Scand. 1968 Mar;72(3):383–384. doi: 10.1111/j.1748-1716.1968.tb03861.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler N., Piiper J. The buffer value of rat diaphragm muscle tissue determined by P CO2 equilibration of homogenates. Respir Physiol. 1971 Jun;12(2):169–178. doi: 10.1016/0034-5687(71)90050-8. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izutsu K. T. Intracellular pH, H ion flux and H ion permeability coefficient in bullfrog toe muscle. J Physiol. 1972 Feb;221(1):15–27. doi: 10.1113/jphysiol.1972.sp009735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARPATKIN S., HELMREICH E., CORI C. F. REGULATION OF GLYCOLYSIS IN MUSCLE. II. EFFECT OF STIMULATION AND EPINEPHRINE IN ISOLATED FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3139–3145. [PubMed] [Google Scholar]
- Lockwood L. B., Yoder D. E., Zienty M. Lactic acid. Ann N Y Acad Sci. 1965 Jul 31;119(3):854–867. doi: 10.1111/j.1749-6632.1965.tb47447.x. [DOI] [PubMed] [Google Scholar]
- Lucier G. E., Mainwood G. W. The measurement of potassium efflux in superfused frog sartorius muscles. Can J Physiol Pharmacol. 1972 Feb;50(2):123–131. doi: 10.1139/y72-019. [DOI] [PubMed] [Google Scholar]
- MARECHAL G., MOMMAERTS W. F. The metabolism of phosphocreatine during an isometric tetanus in the frog sartorius muscle. Biochim Biophys Acta. 1963 Feb 19;70:53–67. doi: 10.1016/0006-3002(63)90718-2. [DOI] [PubMed] [Google Scholar]
- MARGARIA R., CERRETELLI P., DIPRAMPERO P. E., MASSARI C., TORELLI G. Kinetics and mechanism of oxygen debt contraction in man. J Appl Physiol. 1963 Mar;18:371–377. doi: 10.1152/jappl.1963.18.2.371. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Lucier G. E. Fatigue and recovery in isolated frog sartorius muscles: the effects of bicarbonate concentration and associated potassium loss. Can J Physiol Pharmacol. 1972 Feb;50(2):132–142. doi: 10.1139/y72-020. [DOI] [PubMed] [Google Scholar]
- Mainwood G. W., Worsley-Brown P., Paterson R. A. The metabolic changes in frog sartorius muscles during recovery from fatigue at different external bicarbonate concentrations. Can J Physiol Pharmacol. 1972 Feb;50(2):143–155. doi: 10.1139/y72-021. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Koss P. G. Hydrogen ion buffers and enzymatic activity: myosin B adenosinetriphosphatase. Arch Biochem Biophys. 1968 Oct;128(1):236–242. doi: 10.1016/0003-9861(68)90027-1. [DOI] [PubMed] [Google Scholar]
- OLSON G. F. Optimal conditions for the enzymatic determination of L-lactic acid. Clin Chem. 1962 Feb;8:1–10. [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Woodbury J. W., Miles P. R. Anion conductance of frog muscle membranes: one channel, two kinds of pH dependence. J Gen Physiol. 1973 Sep;62(3):324–353. doi: 10.1085/jgp.62.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]