Abstract
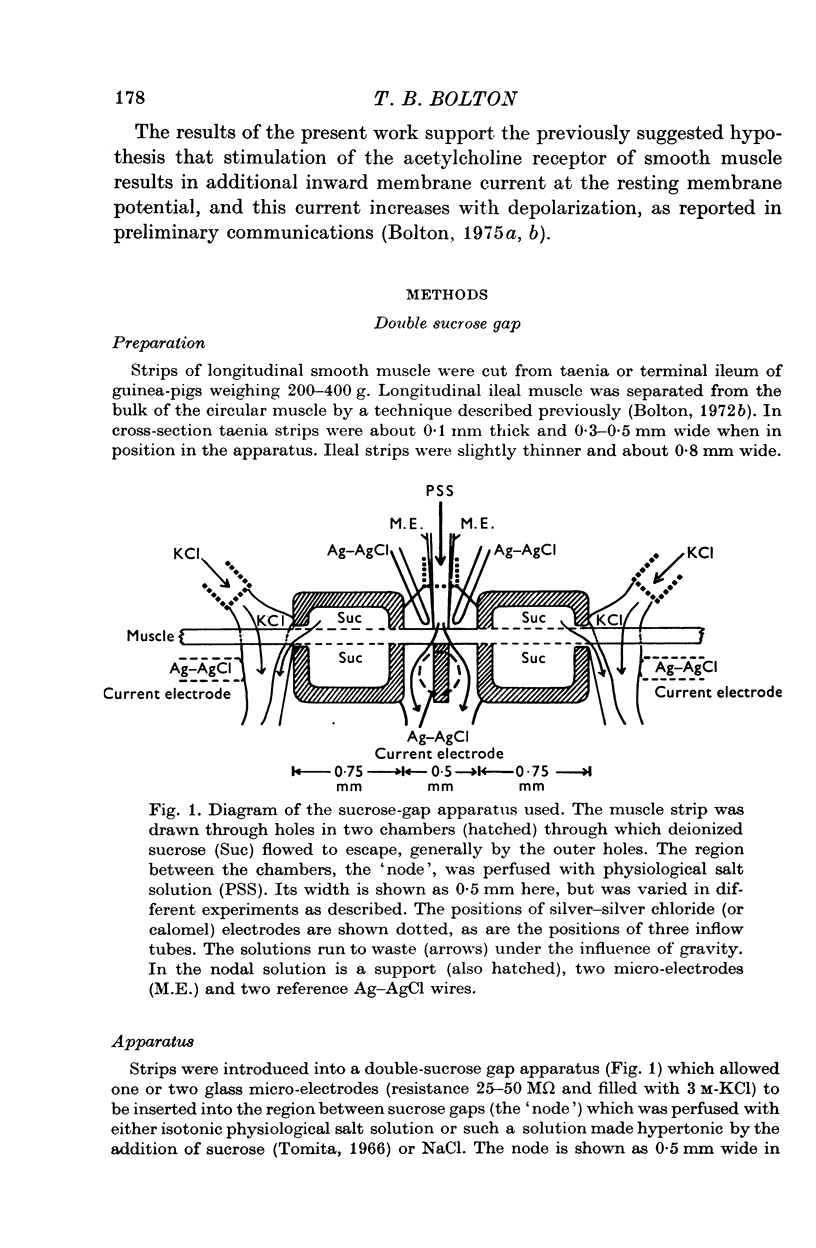
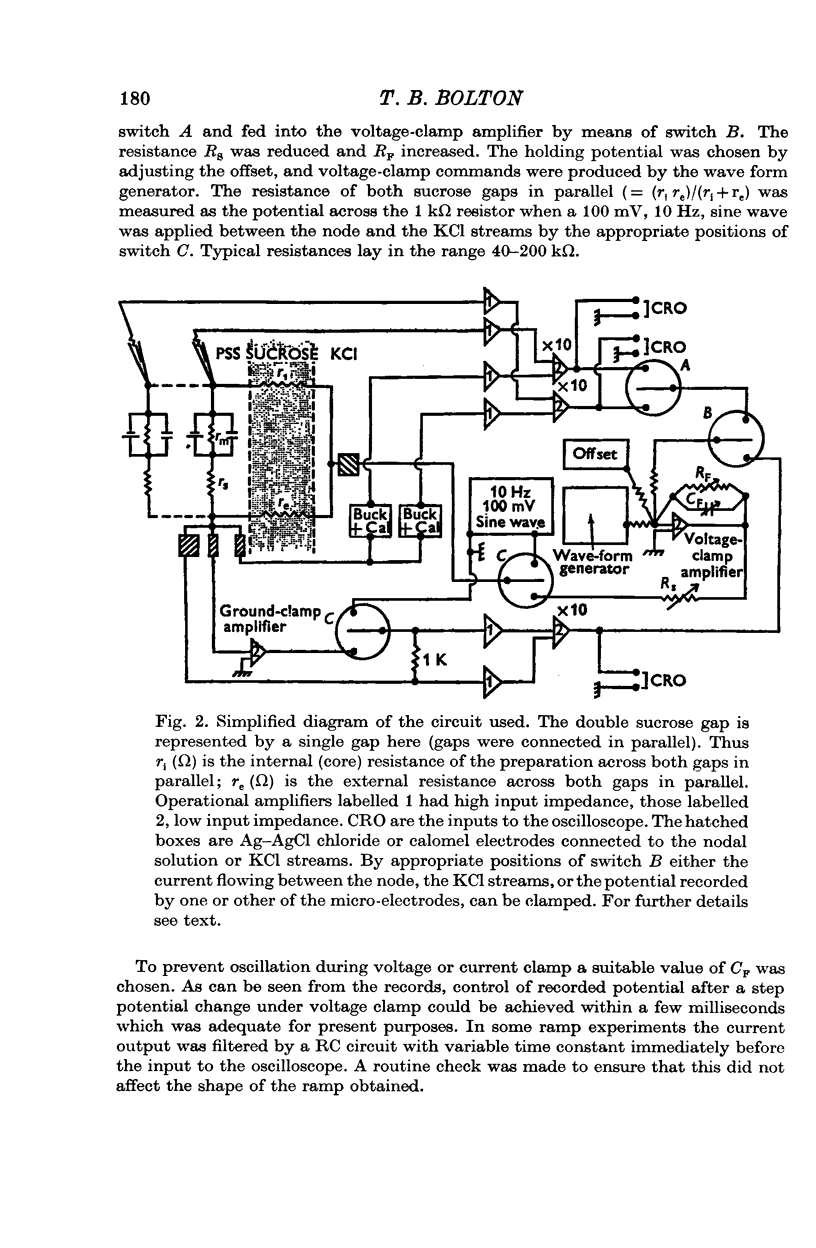
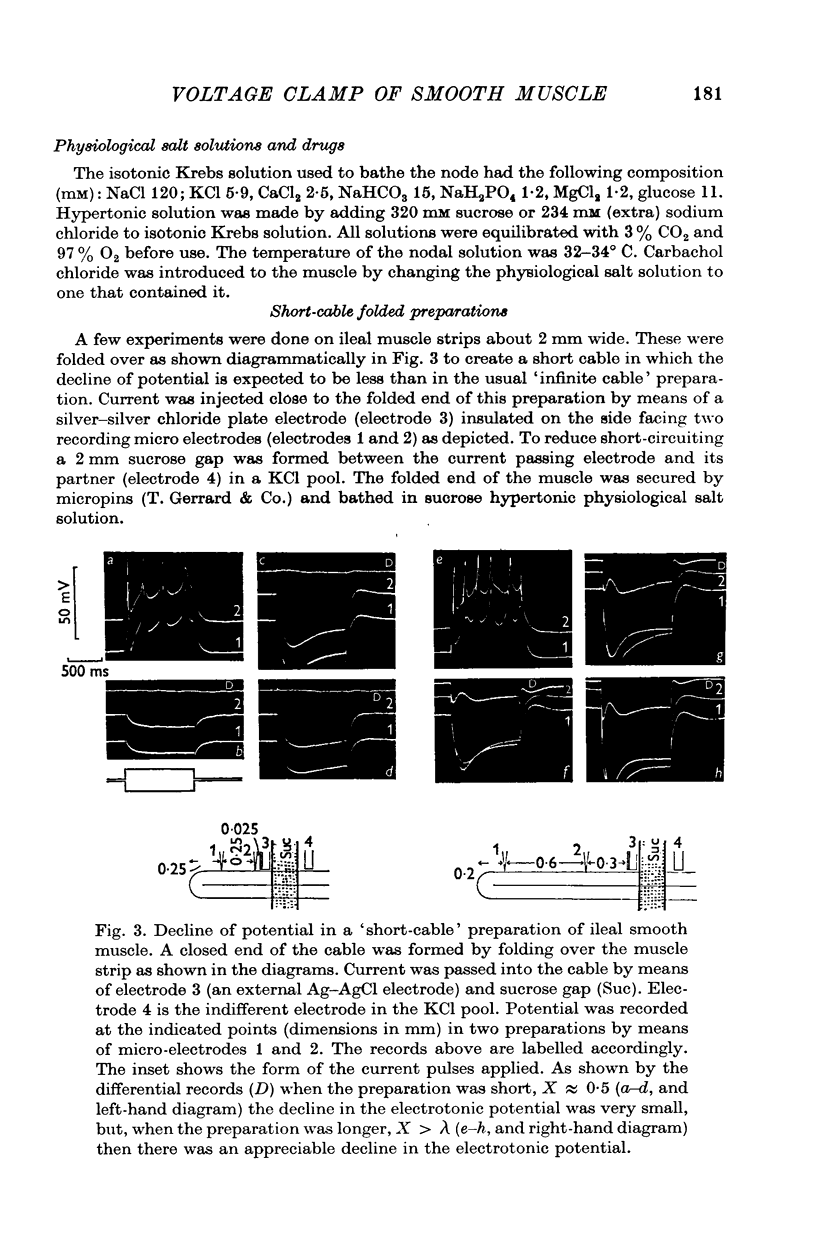
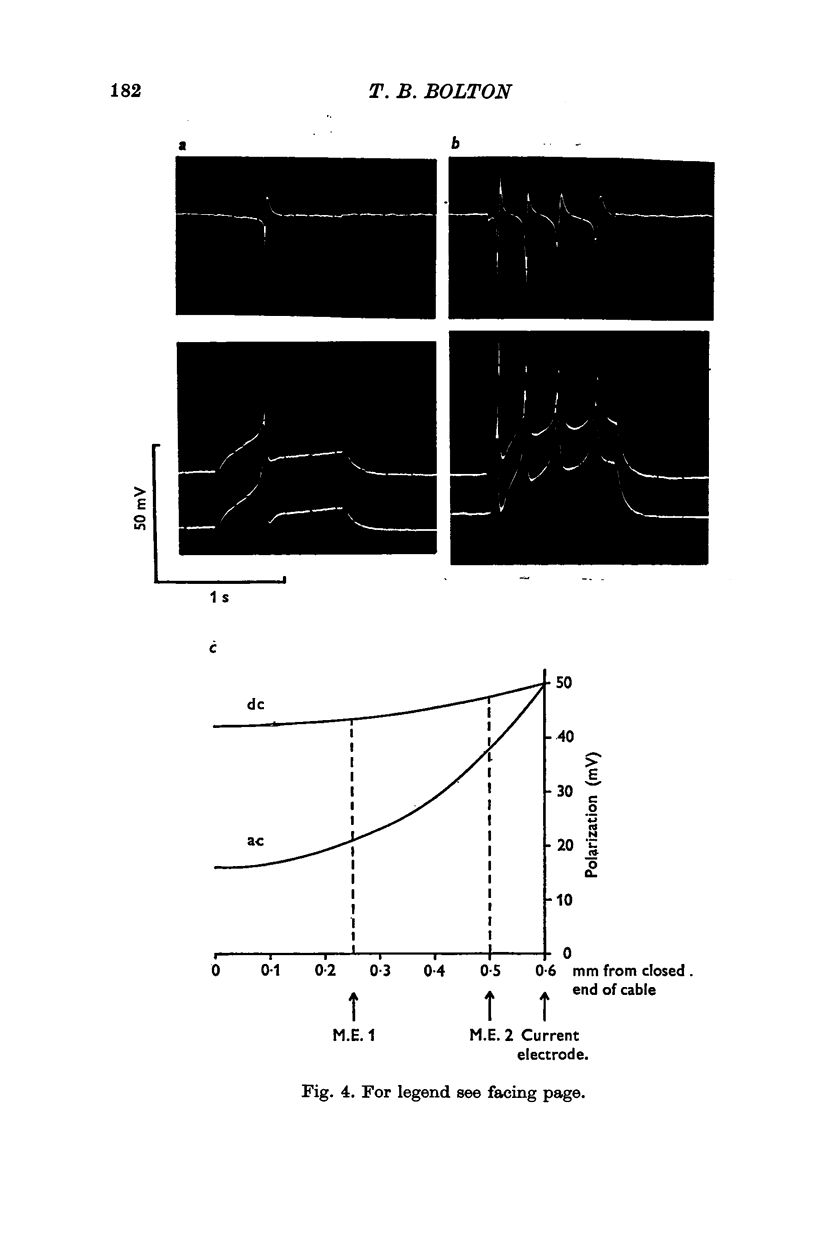
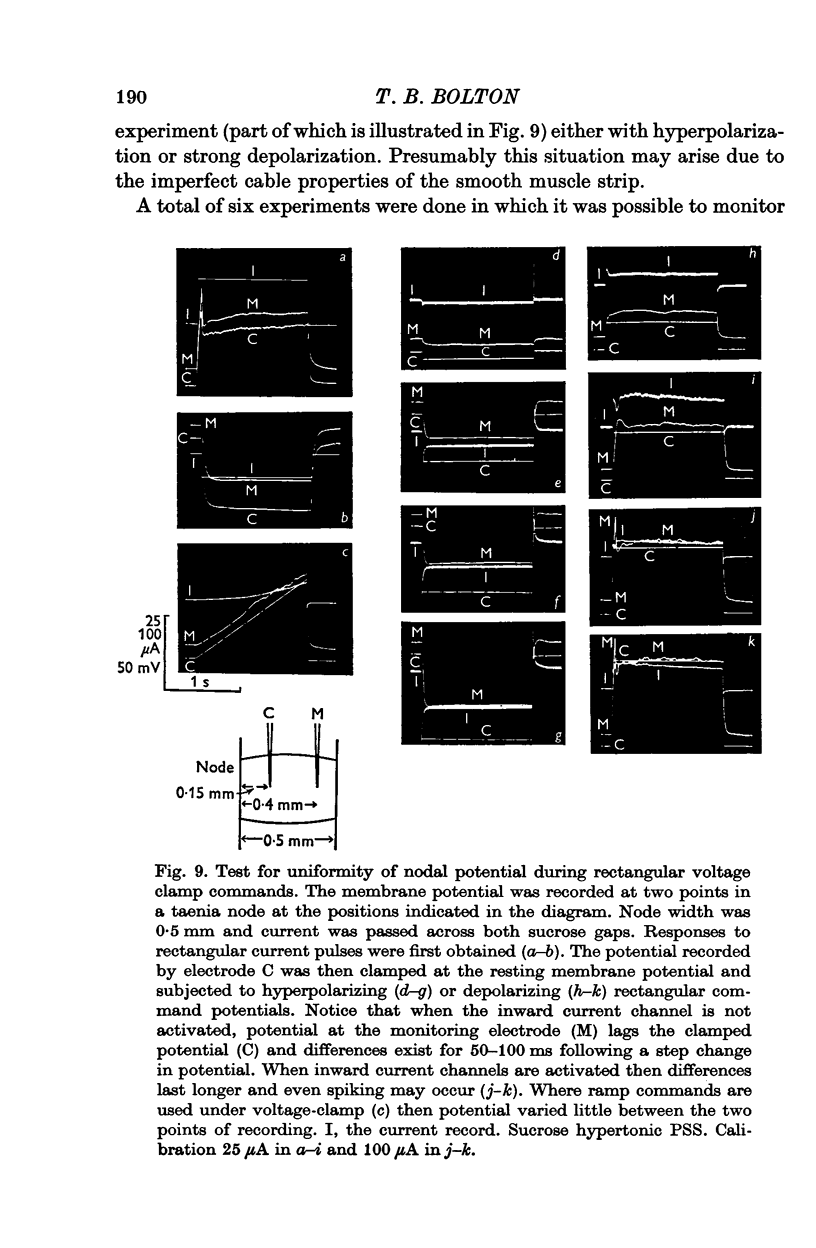
1. A double sucrose-gap voltage-clamp technique is described for use on smooth muscle strips longer than about 2 mm. It involves intracellular recording by microelectrode of the membrane potential of a narrow region of the strip ("node") sandwiched between two streams of deionized sucrose solution. Current was passed into the node across one or both sucrose streams. 2. Preliminary experiments in which potential was recorded intracellularly at two points during polarization of a "short cable" preparation, formed by folding over a strip of smooth muscle, suggested that a node width of less than 0-15 mm was needed to achieve uniform potential during inward current flow. However, when node width between sucrose-gaps was reduced to 0-5 mm, spontaneous electrical activity was lost, and below 0-5 mm spike threshold was raised and the regenerative spike became graded. The currents flowing during the application of rectangular voltage-clamp command potentials were described. 3. Using taenia smooth muscle it was shown by recording with a second, independent micro-electrode that potential was not uniform for up to 200 ms or more following a step change in potential under voltage-clamp in nodes 0-4-0-5 mm wide where current was passed across both sucrose gaps. However, reasonably uniform nodal potentials were obtained using ramps with relatively slow rates of rise (25 mV/s). 4. Using such slow ramp commands under voltage clamp, the effects of carbachol on the current-voltage relationship of longitudinal muscle of ileum and taenia were studied in hypertonic solution. 5. In the presence of carbachol (10(-6) to 10(-5) g/ml.) additional inward current flowed across the membrane (in some experiments an equilibrium potential was observed at which this current reversed direction). The magnitude of this additional current was linearly related to potential at potentials negative to the resting potential. At potentials positive to the resting membrane potential, this additional current increased with depolarization over the range -40 to -10 mV; in ileum the effect of this additional inward current on the current-voltage relationship was to produce a region of net inward current where before, in the absence of carbachol, a net outward current existed. In taenia the additional inward current flowing in the presence of carbachol was too small to produce a region of net inward current; thus carbachol produced regenerative slow oscillations of potential (slow waves) in ileum but not in taenia. 6. These results support a previous suggestion that activation of the acetylcholine receptor of ileal smooth muscle produces an additional inward current in the membrane which increases with depolarization and is responsible for the regenerative slow waves seen when muscarinic stimulants are applied. A similar effect apparently operates in taenia but the additional inward current is too small to produce regenerative slow waves.
Full text
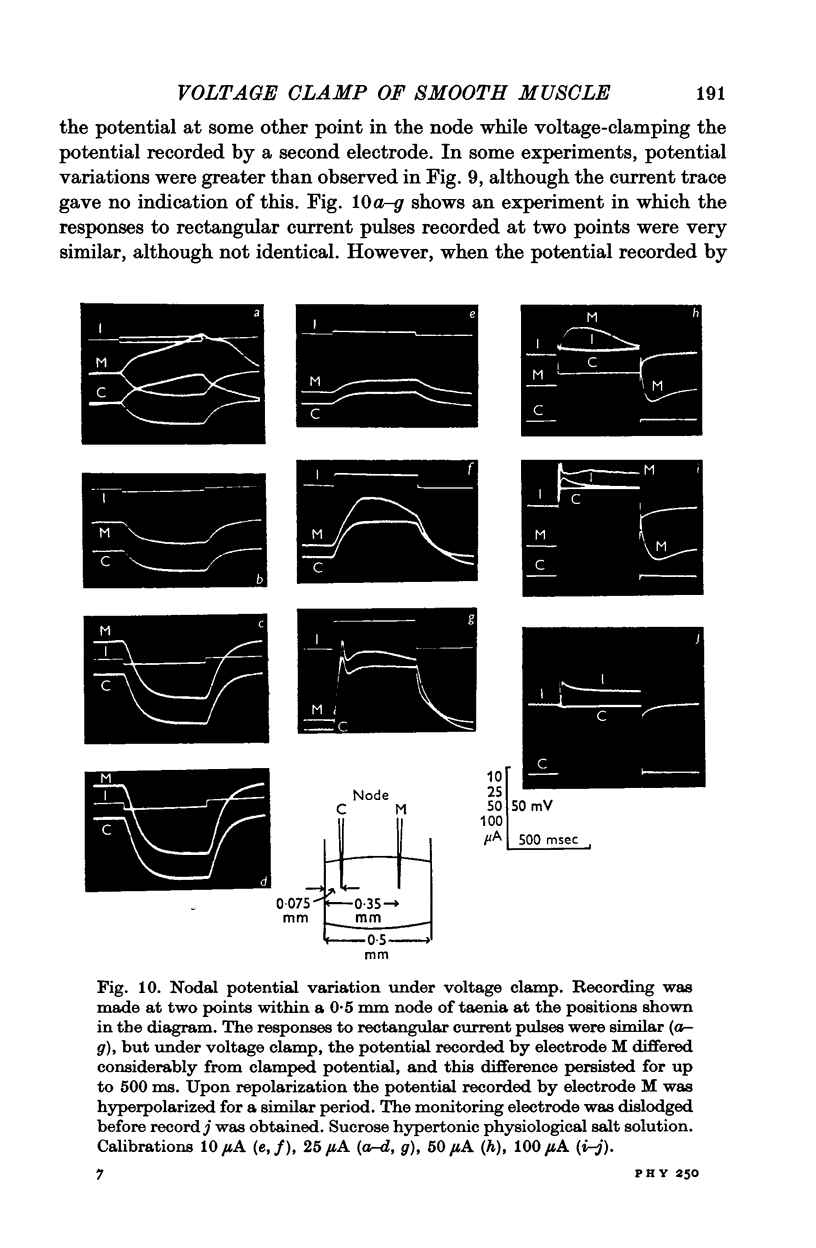
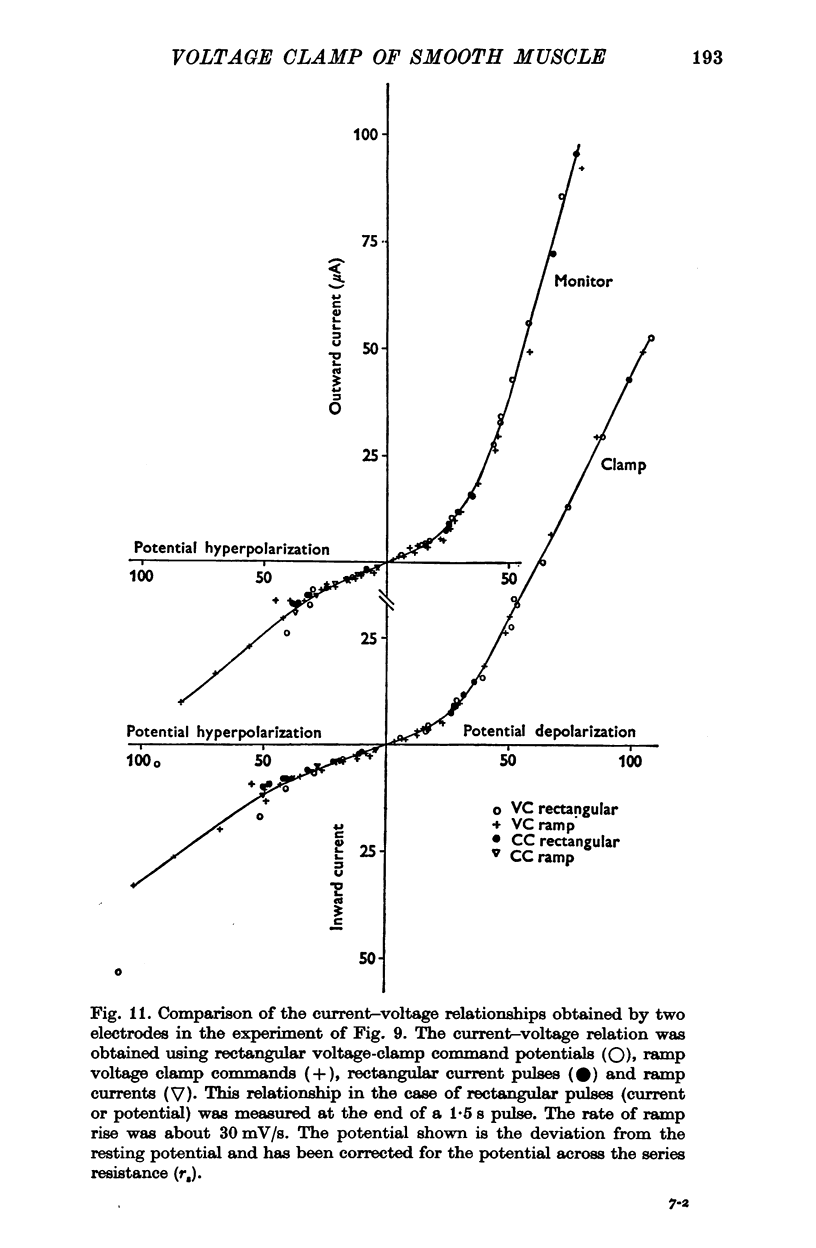
PDF




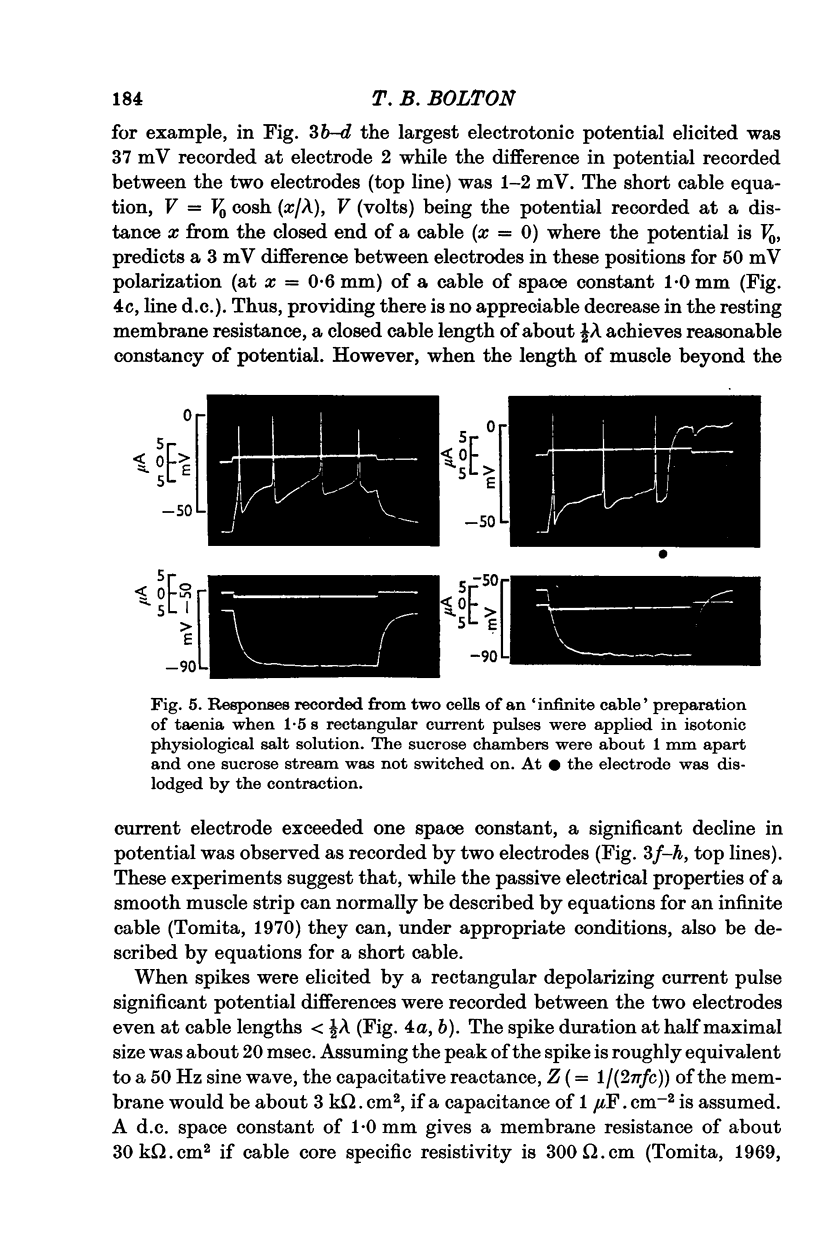

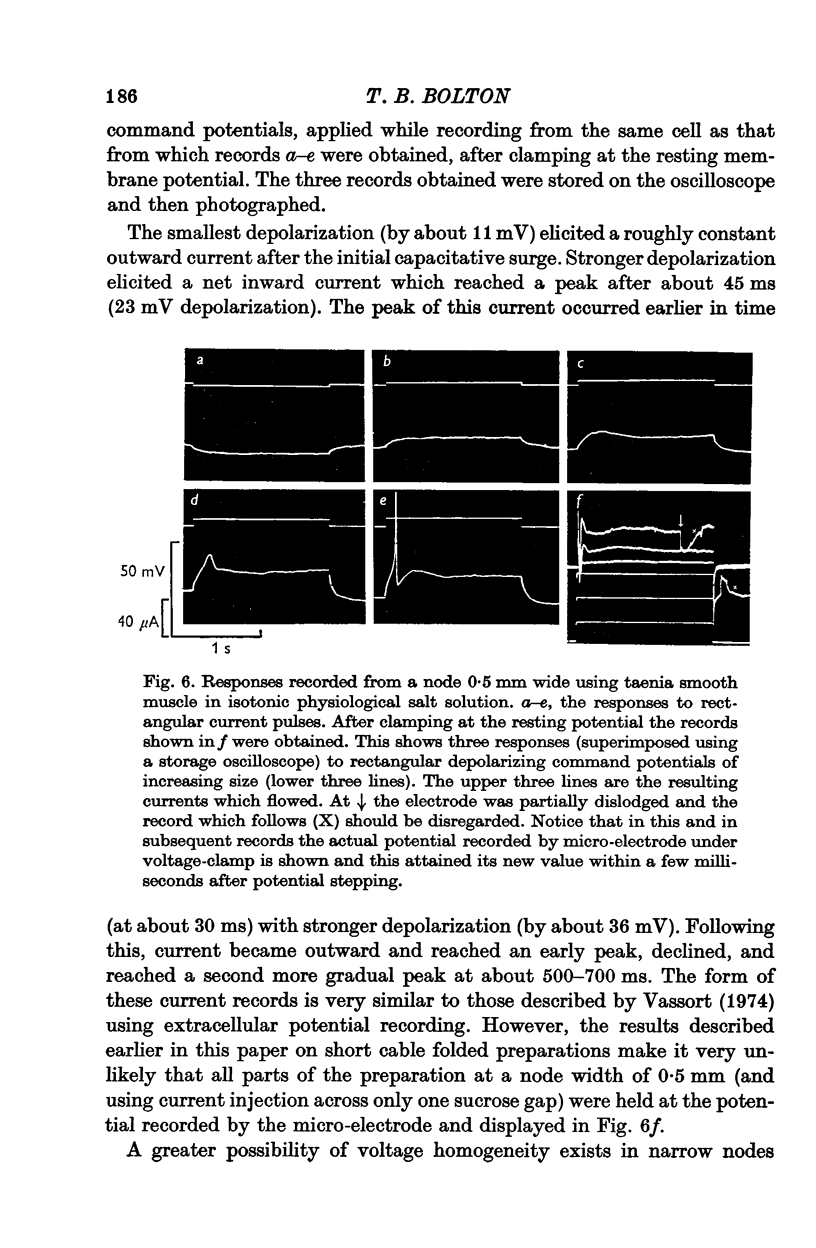
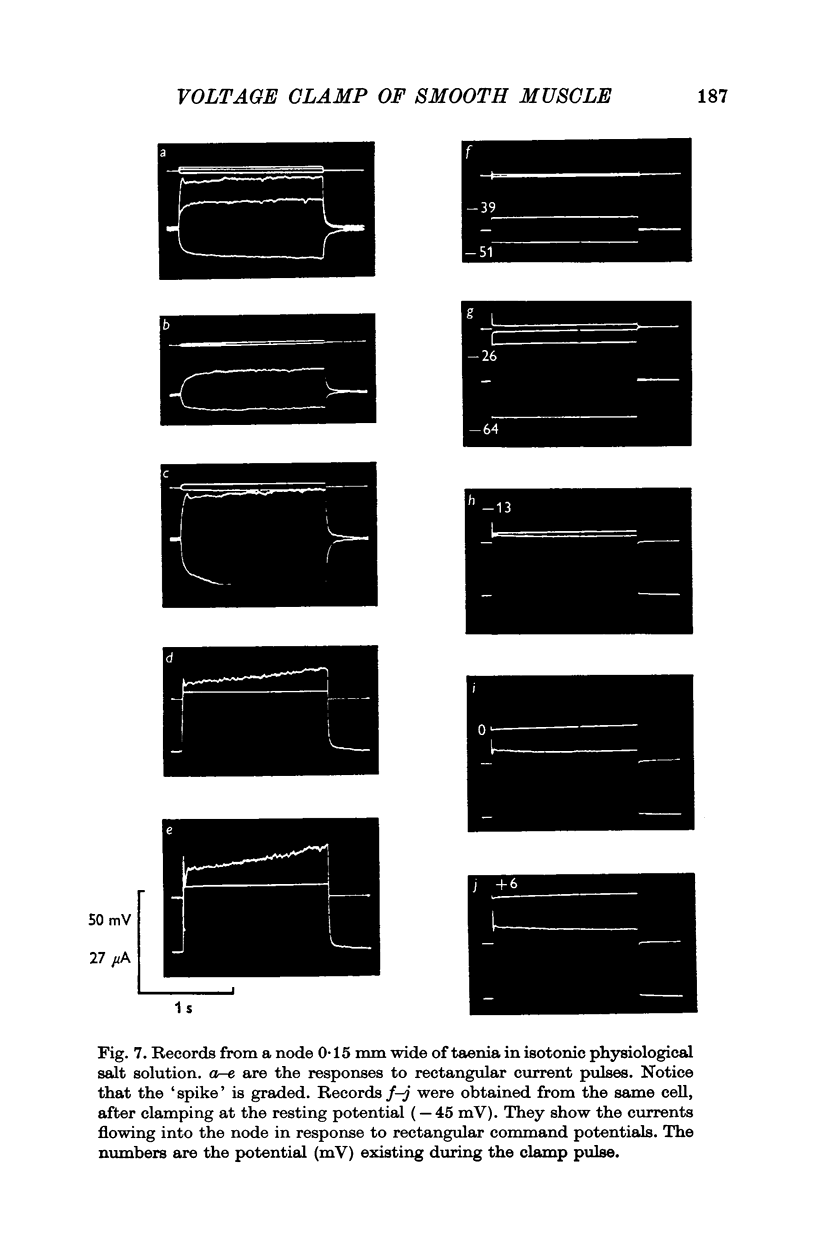
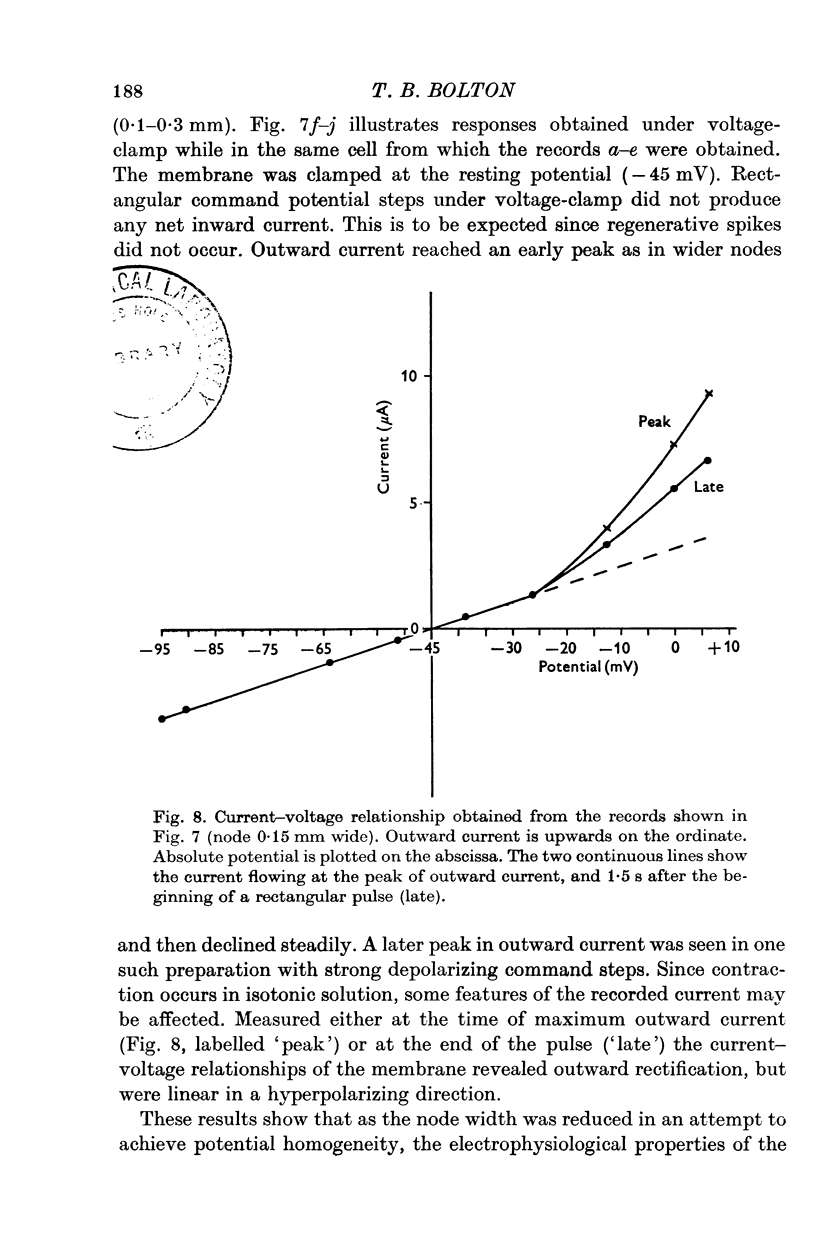


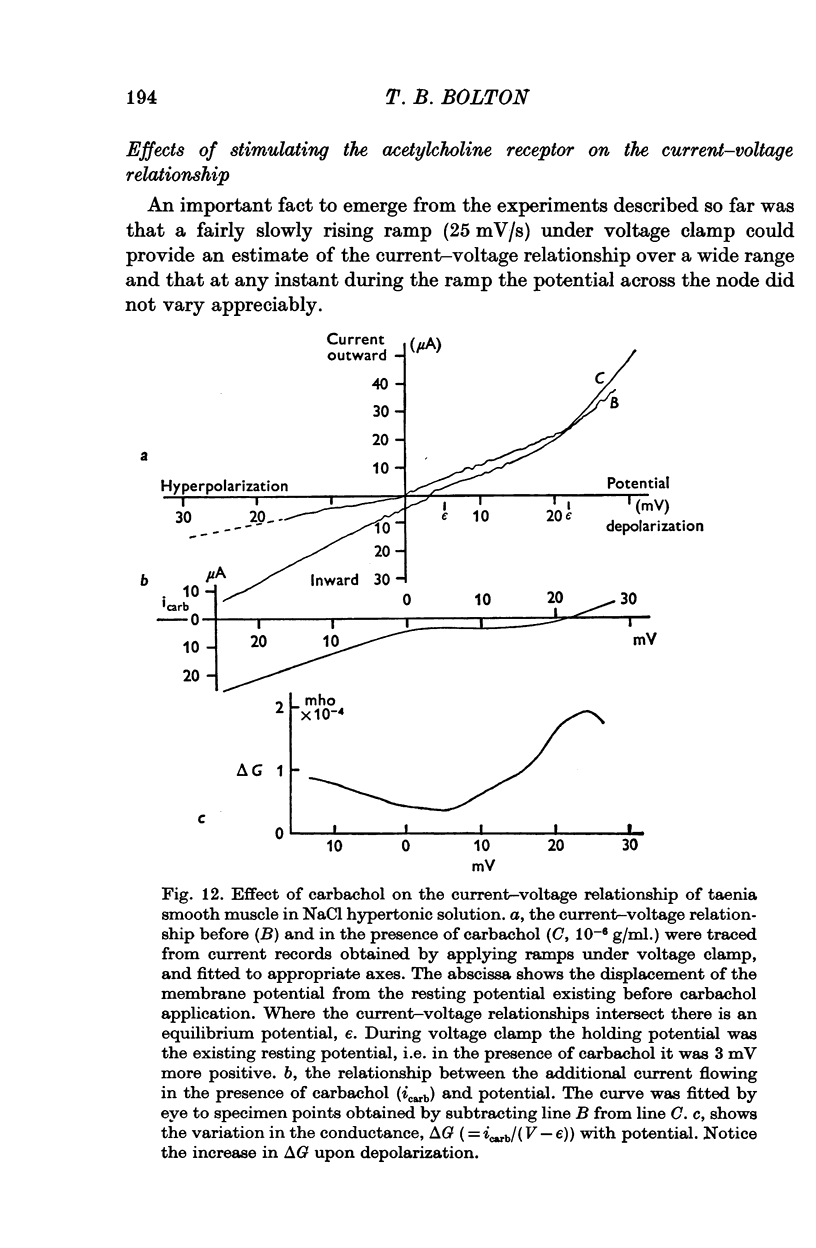







Selected References
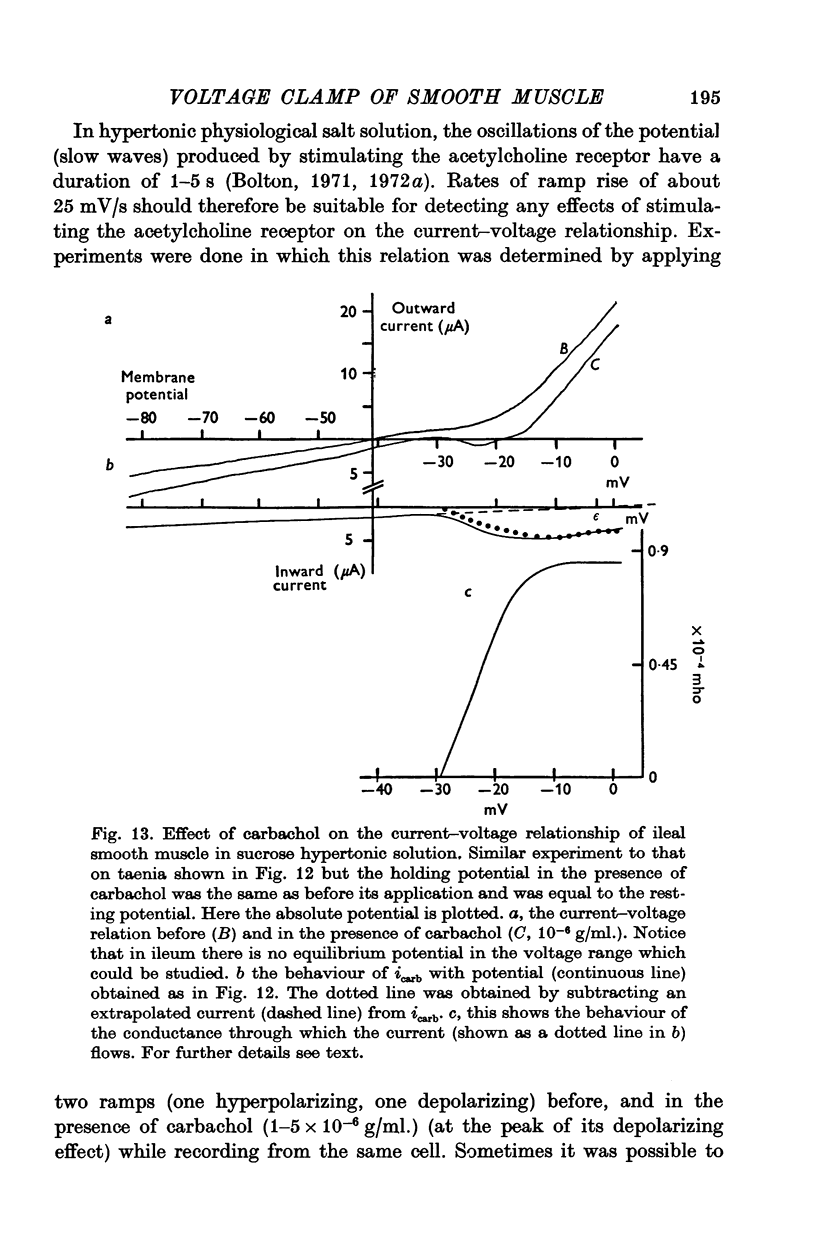
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Ramon F., Snyder A. Studies on calcium and sodium in uterine smooth muscle excitation under current-clamp and voltage-clamp conditions. J Gen Physiol. 1971 Sep;58(3):322–339. doi: 10.1085/jgp.58.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Evidence of a non-linear voltage dependence of the additional current flowing in the membrane of smooth muscle when the acetylcholine receptor is timulated. J Physiol. 1975 Mar;246(2):63P–64P. [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Proceedings: Voltage-clamp experiments on the potential-dependent behaviour of membrane ion channels operated by the muscarinic receptor of smooth muscle. Br J Pharmacol. 1974 May;51(1):129P–130P. [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The effects of varying the concentrations of ions in the external solution on the oscillations of the membrane potential (slow waves) produced by carbachol in longitudinal ileal miscle. Pflugers Arch. 1972;335(2):85–96. doi: 10.1007/BF00592036. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The role of electrogenic sodium pumping in the response of smooth muscle to acetylcholine. J Physiol. 1973 Feb;228(3):713–731. doi: 10.1113/jphysiol.1973.sp010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Voltage-clamp of potential recorded intracellularly with microelectrodes in smooth muscle. J Physiol. 1975 Jan;244(1):25P–26P. [PubMed] [Google Scholar]
- Burgen A. S., Hiley C. R. Proceedings: Two populations of acetylcholine receptors in guinea-pig ileum. Br J Pharmacol. 1974 May;51(1):127P–127P. [PMC free article] [PubMed] [Google Scholar]
- Burgen A. S., Spero L. The action of acetylcholine and other drugs on the efflux of potassium and rubidium from smooth muscle of the guinea-pig intestine. Br J Pharmacol. 1968 Sep;34(1):99–115. doi: 10.1111/j.1476-5381.1968.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgen A. S., Spero L. The effects of calcium and magnesium on the response of intestine smooth muscle to drugs. Br J Pharmacol. 1970 Nov;40(3):492–500. doi: 10.1111/j.1476-5381.1970.tb10630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Szurszewski J. H. The stimulant action of noradrenaline (alpha-action) on guinea-pig myometrium compared with that of acetylcholine. Proc R Soc Lond B Biol Sci. 1974 Jan 29;185(1079):225–262. doi: 10.1098/rspb.1974.0018. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Creed K. E., Muir T. C. The mechanisms of action of neurotransmitters. Electrical changes underlying excitation and inhibition in intestinal and related smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):95–106. doi: 10.1098/rstb.1973.0012. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Ionic currents in the uterine smooth muscle. J Physiol. 1975 Mar;246(1):1–36. doi: 10.1113/jphysiol.1975.sp010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M., Horn L. Voltage clamping of smooth muscle from Taenia coli. Microvasc Res. 1970 Apr;2(2):188–201. doi: 10.1016/0026-2862(70)90007-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaribuchi T., Ito Y., Kuriyama H. Desensitization of smooth muscle cells in the guinea pig taenia coli to prolonged application of carbachol. Jpn J Physiol. 1973 Oct;23(5):447–464. doi: 10.2170/jjphysiol.23.447. [DOI] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Lenfant J. Activité électrique du faisceau musculaire lisse de l'utérua. Mise en évidence et analyse d'un courant ionique sortant. J Physiol (Paris) 1972;64(2):97–105. [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Osa T., Taga F. Electrophysiological comparison of the action of oxytocin and carbachol on pregnant mouse myometrium. Jpn J Physiol. 1973 Feb;23(1):81–96. doi: 10.2170/jjphysiol.23.81. [DOI] [PubMed] [Google Scholar]
- Purves R. D. Muscarinic excitation: a microelectrophoretic study on cultured smooth muscle cells. Br J Pharmacol. 1974 Sep;52(1):77–86. doi: 10.1111/j.1476-5381.1974.tb09689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- Tarr M., Trank J. W. An assessment of the double sucrose-gap voltage clamp technique as applied to frog atrial muscle. Biophys J. 1974 Sep;14(9):627–643. doi: 10.1016/S0006-3495(74)85940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. The longitudinal tissue impedance of the smooth muscle of guinea-pig taenia coli. J Physiol. 1969 Mar;201(1):145–159. doi: 10.1113/jphysiol.1969.sp008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. Initial ionic currents in guinea-pig myometrium. J Physiol. 1974 Mar;237(2):50P–51P. [PubMed] [Google Scholar]
- Zieglgänsberger W., Reiter C. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology. 1974 Jun;13(6):519–527. doi: 10.1016/0028-3908(74)90141-5. [DOI] [PubMed] [Google Scholar]


