Abstract
Overexpression of NorA, an endogenous efflux transporter of Staphylococcus aureus, confers resistance to certain fluoroquinolone antimicrobials and diverse other substrates. The norA gene was amplified by PCR and cloned in the expression vector pTrcHis2. Histidine-tagged NorA (NorA-His) was overexpressed in Escherichia coli cells to prepare two experimental systems, everted membrane vesicles enriched with NorA-His and proteoliposomes reconstituted with purified NorA-His. In membrane vesicles, NorA-His actively transported Hoechst 33342, a dye that is strongly fluorescent in the membrane but has low fluorescence in an aqueous environment. Transport was activated by the addition of ATP or lactate and reversed by the addition of nigericin, with the addition of K+-valinomycin having little effect. Transport of Hoechst 33342 was inhibited competitively by verapamil, a known inhibitor of NorA, and by other NorA substrates, including tetraphenyl phosphonium and the fluoroquinolones norfloxacin and ciprofloxacin. In contrast, sparfloxacin, a fluoroquinolone whose antimicrobial activity is not affected by NorA expression, exhibited noncompetitive inhibition. NorA induction and overexpression yielded 0.5 to 1 mg of a largely homogeneous 40- to 43-kDa protein per liter of culture. NorA-His incorporated into proteoliposomes retained the ability to transport Hoechst 33342 in response to an artificial proton gradient, and transport was blocked by nigericin and verapamil. These data provide the first experimental evidence of NorA functioning as a self-sufficient multidrug transporter.
Fluoroquinolone antimicrobial agents have been used for treatment of a broad range of bacterial infections. The development of drug resistance in some species, particularly Pseudomonas aeruginosa and Staphylococcus aureus, however, has limited the utilities of these antimicrobials in some clinical settings. The genetics and mechanisms of bacterial fluoroquinolone resistance have been studied most extensively for Escherichia coli and P. aeruginosa. Fluoroquinolones act on DNA gyrase and topoisomerase IV to inhibit bacterial DNA replication (6). Single mutations in the gyrA and gyrB genes of E. coli, encoding the subunits of DNA gyrase (9, 11), and single mutations in the grlA and grlB genes of S. aureus, encoding the subunits of S. aureus DNA topoisomerase IV (7, 18), have been shown to cause resistance.
In addition, in E. coli, mutations in genes that affect the expression of porin outer membrane proteins and the AcrAB efflux pump have been shown to cause resistance and to be associated with reduced levels of drug accumulation in intact cells (3, 5, 10, 19). Furthermore, in these mutant gram-negative bacteria, reduced levels of drug accumulation appear to be dependent on energy, because drug accumulation returns to wild-type levels after treatment with protonophores, such as dinitrophenol or carbonyl cyanide m-chlorophenylhydrazone (CCCP) (4, 10).
For gram-positive bacteria, efflux pumps have also contributed to fluoroquinolone resistance. The norA gene, which is located on the SmaI D fragment of the S. aureus chromosome (17), was initially identified as a fluoroquinolone resistance gene (32) and was subsequently shown also to confer resistance to a broad range of compounds, similar in breadth to that encoded by the bmr gene, a homolog of norA in Bacillus subtilis (16). The NorA protein is predicted to have 388 amino acids and a molecular mass of 42,385 Da and is presumed to be localized in the cytoplasmic membrane. NorA belongs to the major facilitator superfamily of transporters with 12 transmembrane segments (20, 32). Overexpression of norA in E. coli as a cloned gene and in S. aureus produces resistance to a broad range of substrates, including fluoroquinolones, and reduced levels of drug accumulation in intact E. coli and S. aureus (12, 13, 16). Furthermore, it has been reported that everted membrane vesicles prepared from norA-expressing E. coli cells exhibited energy-dependent transport of the hydrophilic quinolone norfloxacin (17). This transport was not demonstrable in cells expressing NorA and was abolished by cyanide m-chlorophenylhydrazone and nigericin but not by valinomycin, indicating that NorA-mediated quinolone transport is coupled to the proton gradient across the cell membrane.
NorA itself has not been shown to transport substrates in the absence of other membrane proteins, leaving open the possibility that NorA overexpression acts in concert with other transporters to mediate multidrug resistance. In addition, the mechanisms of transport of multiple structurally distinct compounds by NorA have not been investigated systematically, and it is not known whether diverse substrates, particularly those with different hydrophobicities, are transported by distinct or common pathways.
To characterize the function of NorA further, the norA gene was cloned into an E. coli expression vector, and histidine-tagged NorA (NorA-His) was overexpressed in and purified from E. coli. Purified NorA was also reconstituted in proteoliposomes and shown for the first time to function alone as a drug transporter. A robust fluorescence assay for the NorA-mediated transport of hydrophilic and hydrophobic chemical entities was developed in everted membrane vesicles of E. coli. The use of the NorA-enriched vesicles described in this work allows for the determination of NorA activity and introduces the possibility of using these vesicles in high-throughput screens to identify NorA inhibitors.
MATERIALS AND METHODS
Antimicrobials and chemicals.
Norfloxacin, verapamil, valinomycin, nigericin, 2"-(4-ethoxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5"-bi-1H-benzimidazole (Hoechst 33342), and tetraphenyl phosphonium (TPP) were purchased from Sigma Chemicals (St. Louis, Mo.). Ethidium bromide, ampicillin, acridine orange, and isopropyl-β-d-thiogalactopyranoside (IPTG) were from Fisher Scientific (Pittsburgh, Pa.). Ciprofloxacin was a gift from Bayer Pharmaceuticals (West Haven, Conn.), and sparfloxacin was from Dainippon Pharmaceutical Co., Ltd. (Osaka, Japan). E. coli polar lipids and egg phosphatidylcholine were purchased from Avanti Polar Lipids (Alabaster, Ala.). All other chemicals were purchased from either Sigma Chemicals or Fisher Scientific and were analytic grade.
Bacterial strains and growth conditions.
E. coli strain DH10B [F− araD139Δ(ara-leu)7697 ΔlacX74 galU galK mcrA (mrr-hsdRMS-mcrBC) rpsL dor φ80ΔlacZM15 endA1 nupG recA1] was used for competent cells. E. coli strains containing cloned norA or control plasmids were grown aerobically at 37°C in Luria-Bertani broth containing ampicillin (100 μg/ml) and norfloxacin (0.128 μg/ml).
DNA manipulations and plasmid constructions.
General procedures for cloning and DNA manipulations were performed as described by Sambrook et al. (24). The norA gene was amplified from plasmid pMT102, which was derived from pUC19 and contained the norA gene amplified and cloned from S. aureus strain MT1222 (17), by using primers GAGGGGATCCTATGAATAAACAGA and GCCGAATTCCGCCATATTTTGTTC (underlined regions indicate the engineered BamHI and EcoRI sites). The PCR product was ligated in frame to the BamHI and EcoRI sites of the expression vector pTrcHis2C (Invitrogen Inc., Carlsbad, Calif.). The product of this vector contains six histidines and a myc epitope at the C terminus. The plasmids constructed were then transformed into E. coli DH10B. The gene and the junctions of vector DNA were confirmed to be correct by DNA sequencing.
Preparation of everted membrane vesicles.
An overnight culture of E. coli DH10B bearing pTrcHis2C-norA or control plasmids was diluted 1:50 in fresh Luria-Bertani broth with ampicillin (100 μg/ml) and norfloxacin (0.128 μg/ml) and grown at 37°C. IPTG was added to a final concentration of 0.5 mM when the A600 reached 0.8. Growth was continued for another 4 h, and cells were harvested and resuspended in 50 mM potassium phosphate buffer (pH 7.2). Everted membrane vesicles were prepared by cell lysis with a French press at 5,000 lb/in2. The cell lysate was centrifuged at 12,000 × g for 10 min to remove unlysed cells and cell debris, and the supernatant fluid was centrifuged at 100,000 × g for 1 h. The pelleted vesicles were then resuspended at a concentration of 8 mg of protein/ml in 50 mM potassium phosphate buffer (pH 7.2) supplemented with 10% glycerol. The vesicles were stored in aliquots at −70°C.
Preparation of liposomes.
E. coli polar lipids and egg phosphatidylcholine, both dissolved in chloroform, were mixed in a ratio of 3:1, dried under nitrogen, and kept under low pressure for at least 2 h. The lipids were then rehydrated with 20 mM potassium phosphate buffer-100 mM potassium acetate-2 mM magnesium sulfate (pH 7.0) at a final lipid concentration of 20 mg/ml. The liposomes were prepared by two cycles of freezing and thawing with brief bath-type sonication between each freezing and thawing cycle as described previously (23) and were kept at −70°C.
Solubilization and purification of NorA-His.
Everted membrane vesicles prepared from NorA-overexpressing cells were diluted in 20 mM potassium phosphate buffer-100 mM NaCl (pH 7.0) supplemented with 1% Triton X-100 at a protein concentration of 0.8 mg/ml and were incubated at 20°C for 30 min. Insoluble material was removed by centrifugation at 100,000 × g for 30 min at 4°C. After the addition of imidazole at a final concentration of 10 mM, the supernatant fluid was promptly loaded on a Ni column (Amersham Pharmacia Biotech, Uppsala, Sweden) equilibrated with start buffer that contained 20 mM potassium phosphate buffer-100 mM NaCl (pH 7.0) and had been supplemented with 10 mM imidazole. The column was washed with start buffer supplemented with concentrations of imidazole increasing from 10 to 60 mM. NorA-His was then eluted in buffer containing 300 mM imidazole. The yield of NorA-His was 0.5 to 1.0 mg of protein from 1 liter of culture.
Western blotting was performed with mouse monoclonal anti-c-myc antibody (1:5,000) and horseradish peroxidase-conjugated polyclonal rabbit anti-mouse immunoglobulin G (1:10,000).
Functional reconstitution of NorA-His.
The method of Putman et al. (23) was used with modifications. The liposomes in 20 mM potassium phosphate buffer-100 mM potassium acetate-2 mM magnesium sulfate (pH 7.0), prepared as described above, were thawed, diluted to a concentration of 5 mg of lipids/ml, and then extruded through a polycarbonate filter (pore size, 0.4 μm). Triton X-100 was added at a final concentration of 0.01% to destabilize the liposomes. NorA was mixed with detergent-treated liposomes at a protein-to-lipid ratio of 1:75 to 1:100. The mixture was incubated at 20°C for 30 min. Lipid concentrations in both protein-containing and protein-free liposomes were adjusted to the same level. To remove the detergent, polystyrene Bio-Beads SM-2 (Bio-Rad, Hercules, Calif.), which had been extensively washed in methanol and then in distilled water, were added at a concentration of 80 mg (wet weight)/ml and incubated at 4°C. The beads were replaced at 2 and 18 h. The proteoliposomes and protein-free liposomes in control preparations were then collected by centrifugation at 100,000 × g for 1 h at 4°C, resuspended in 20 mM potassium phosphate buffer-100 mM potassium acetate-2 mM magnesium sulfate (pH 7.0) at a protein concentration of 0.5 mg/ml, and kept at −70°C.
Measurement of the proton gradient across everted membrane vesicles.
Membrane vesicles were diluted in 50 mM potassium HEPES (pH 7.2)-8.5 mM sodium chloride-2 mM magnesium sulfate at a final protein concentration of 40 μg/ml. Acridine orange was added at a final concentration of 5 nM. Proton motive force was generated by the F0F1 H+-ATPase upon the addition of 0.1 mM Mg2+-ATP. The ionophores valinomycin (at a 1.8 μM final concentration) and nigericin (at a 2.7 μM final concentration) were added to dissipate the electrical gradient (Δψ) and proton gradient (ΔpH), respectively. Acridine orange fluorescence was monitored at excitation and emission wavelengths of 494 and 530 nm, respectively, in a FluoroMax SPEX Fluorolog series 2 spectrofluorometer (SPEX INdustries, Inc., Edison, N.J.) with SPEX dm3000 software.
Hoechst 33342 transport by NorA in everted membrane vesicles.
Everted membrane vesicles prepared from the NorA-overexpressing cells or from control cells were diluted into 2 ml of 50 mM potassium HEPES-8.5 mM sodium chloride-2 mM magnesium (pH 7.2) at a protein concentration of 40 μg/ml. NorA was activated following the generation of proton motive force upon the addition of lactate at 0.5 mM (final concentration) or Mg2+-ATP at 0.1 mM (final concentration). Fluorescence change was monitored at excitation and emission wavelengths of 355 and 457 nm, respectively, in a FluoroMax SPEX Fluorolog series 2 spectrofluorometer. It was not possible to measure Hoechst 33342 transport after the addition of NADH because of the overlap of the fluorescence parameters of NADH with those of Hoechst 33342.
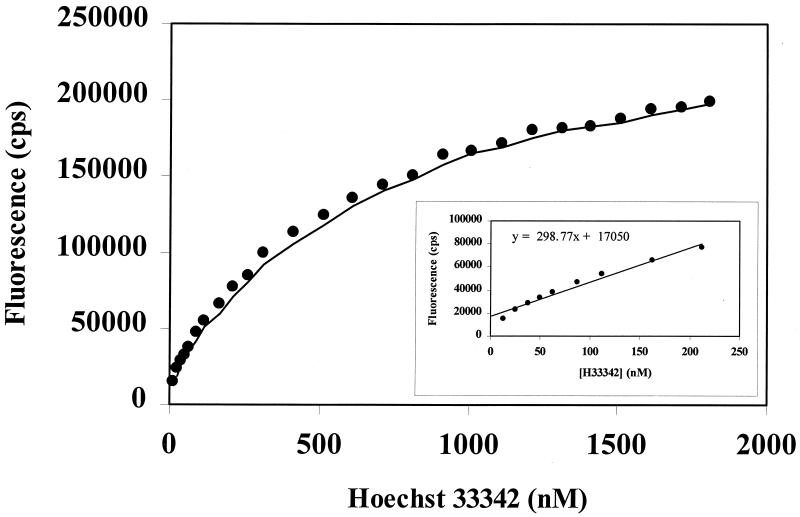
To determine the binding capacity of membrane vesicles for Hoechst 33342, Hoechst 33342 was added in a range of concentrations from 12.5 nM to 1.6 μM, and maximal fluorescence units were recorded. For everted membrane vesicles at 40 μg of protein/ml, increasing concentrations of Hoechst 33342 between 12.5 and 200 nM produced linear increases in fluorescence signal, reflecting linear membrane binding. Hoechst 33342 at higher concentrations, however, produced a plateau of fluorescence, suggesting saturation of membrane binding capacity. Therefore, the kinetic studies were performed in the concentration range of 12.5 to 200 nM Hoechst 33342.
In order to assess the relationship between fluorescence intensity and the intramembrane concentration of Hoechst 33342, we measured the fluorescence of Hoechst 33342 in the presence of increasing amounts of membrane vesicles. The maximal fluorescence value was taken as being equivalent to that of the added dye when it was fully dissolved in the membrane. Thus, for kinetic experiments, fluorescence values can be converted to concentrations of Hoechst 33342 by using this derived value for the coefficient of fluorescence (a) and the equation F = a · Hadded + b, where F is fluorescence, H is the concentration of Hoechst 33342, and b is the intrinsic fluorescence of the membrane vesicle preparation in the absence of Hoechst 33342. The concentration of Hoechst 33342 in the membrane can then be calculated as Hbound = (F − b)/a. The value of a was estimated as 300 cps per 1 nM Hoechst 33342 under the same conditions as those used in the kinetic experiments.
To study the kinetics of Hoechst 33342 transport by NorA-containing everted membrane vesicles, Hoechst 33342 was added in a range of concentrations from 12.5 to 200 nM and fluorescence change was recorded continuously within the first 20 s after the addition of lactate or ATP. Double reciprocal plots of Hoechst 33342 concentration and the rate of fluorescence change were then generated.
To study the effects and patterns of inhibition of Hoechst 33342 transport by quinolones, TPP, and verapamil, the inhibitors were added to the cuvettes in ranges of concentrations (2.5 to 10 μM for norfloxacin, 3 to 12 μM for ciprofloxacin, 10 to 20 μM for sparfloxacin, 50 to 100 μM for TPP, and 10 to 80 μM for verapamil) prior to the addition of Hoechst 33342. Inhibition patterns were determined by the concurrence of the y or x intercepts from plots of 1/V and 1/S, where V is initial velocity of transport and S is concentration of the substrate, Hoechst 33342. In the case of competitive inhibition, apparent Ki values were calculated by comparing Km values in the presence and absence of the inhibitor by using the equation Ki=I/(Kmi/Kmo−1) in which Kmo and Kmi are the apparent Km values in the absence and presence, respectively, of an inhibitor of concentration I (17, 27). In the case of noncompetitive inhibition, apparent Ki values were calculated by comparing maximum rates of transport (Vmax values) in the presence and absence of the inhibitor by using the equation Ki=(I)(Vmaxi)/(Vmaxo−Vmaxi), in which Vmaxo and Vmaxo are the apparent Vmax values in the absence and presence, respectively, of an inhibitor of concentration I (27).
Hoechst 33342 transport by proteoliposomes reconstituted with purified NorA-His.
The NorA proteoliposomes, prepared as described above, were thawed at 20°C and subjected to a brief bath-type sonication. The proteoliposomes were diluted into a cuvette containing 2 ml of 20 mM potassium phosphate buffer-50 mM potassium sulfate-2 mM magnesium sulfate (pH 7.0) at a protein concentration of 10 μg/ml, and then the fluorescence of Hoechst 33342 was measured as described previously (23). The assay was started with the addition of Hoechst 33342.
RESULTS
Heterologous expression of NorA and characterization of the protein.
NorA was heterologously expressed in E. coli with the expression vector pTrcHis2, in which transcription is from an inducible trc promoter. The expressed protein contains a six-His tag that allows purification by Ni affinity chromatography and a c-myc epitope that facilitates monitoring of protein purification. Without induction, there was expression of NorA in cells containing amounts of pTrcHis2-norA sufficient to produce increases in the minimal growth inhibitory concentrations of norfloxacin and ciprofloxacin, both of which are known NorA substrates, with little effect on the inhibitory concentration of sparfloxacin, a poor substrate (Table 1). Thus, NorA expressed with a histidine tag appeared to be functional.
TABLE.
1. Susceptibilities of E. coli strains containing cloned norA to quinolones and Hoechst 33342
| Plasmid carried by strain | MIC (μg/ml) of:
|
|||
|---|---|---|---|---|
| Norfloxacin | Ciprofloxacin | Sparfloxacin | Hoechst 33342 | |
| None | 0.05 | 0.0125 | 0.0015 | 64 |
| pMT102(norA) | 3.2 | 0.2 | 0.0015 | 512 |
| pTrcHis2C | 0.05 | 0.0125 | 0.0015 | 64 |
| pTrcHis2C-norA | 3.2 | 0.2 | 0.0015 | 512 |
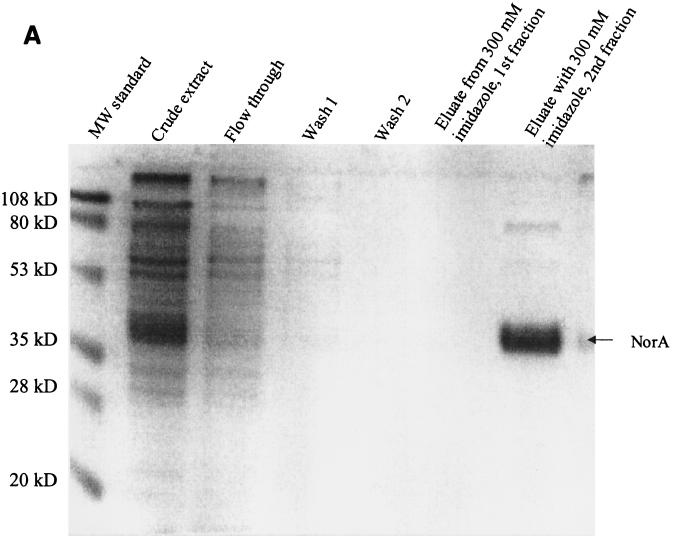
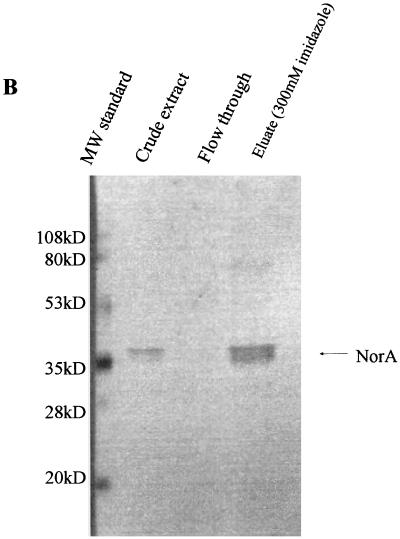
Induction with IPTG resulted in NorA overexpression to the level of approximately 5 to 10% of membrane proteins as determined by protein assay and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Cell growth ceased shortly after induction, making it impossible to measure growth-inhibitory concentrations of quinolone after induction. Purification yielded 0.5 to 1.0 mg of protein per liter of culture. By SDS-PAGE, NorA-His was largely homogeneous and had an apparent molecular mass of 40 to 43 kDa (Fig. 1). A minor additional band of approximately twice the molecular mass was present on both the SDS-polyacrylamide gel and the Western blot and likely represents a dimeric or aggregated form of NorA.
FIG. 1.
SDS-PAGE (A) and Western blot (B) analysis of NorA purification. Under the conditions described in Materials and Methods, NorA protein in the crude extract was adsorbed to the Ni column. The column was washed with buffers containing 10 and 60 mM imidazole, and the pure protein was eluted with buffer containing 300 mM imidazole. MW, molecular mass.
NorA-mediated extrusion of Hoechst 33342 from everted membrane vesicles.
NorA function has been studied by transport of the fluoroquinolone norfloxacin (17), but this method is cumbersome and requires radiolabeled norfloxacin of high specific activity in order to measure transport kinetics at physiologically relevant concentrations of the drug. Ethidium bromide is a known substrate of NorA and, as a fluorescent dye, could be a better tool for studying NorA transport. Our attempts to evaluate NorA function by changes in ethidium bromide fluorescence, however, were unsuccessful (data not shown), likely due to the absence of a major change in the fluorescence properties of ethidium bromide present outside and within the membrane vesicle. Thus, we sought another dye that might be suitable for studying NorA function by measurement of fluorescence. Hoechst 33342 is a hydrophobic dye that has been shown to be a substrate for a number of multidrug resistance efflux pumps, including P-glycoprotein (25) and LmrP (23). E. coli cells containing pTrcHis-norA but not those containing the vector alone exhibited an eightfold increase in the growth-inhibitory concentration of Hoechst 33342 (Table 1), suggesting that it was a substrate for NorA.
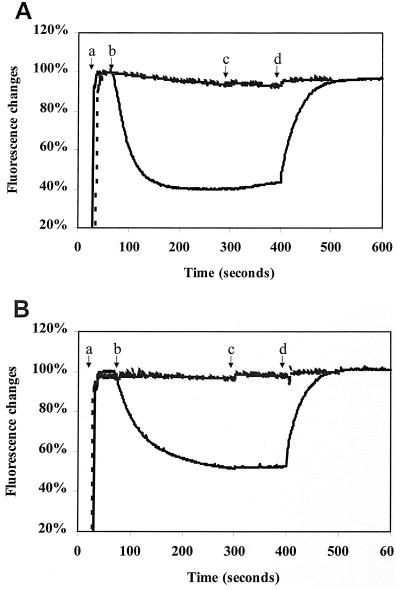
Hoechst 33342 is largely nonfluorescent in an aqueous environment but has strong fluorescence when bound to membranes. Thus, a decrease in fluorescence reflects a net movement of dye from membrane to aqueous environment. Everted membrane vesicles prepared from NorA-overexpressing cells exhibited rapid quenching of the fluorescence of Hoechst 33342 after energization by the addition of Mg+-ATP or lithium lactate (Fig. 2). In contrast, no quenching was observed with control vesicles prepared from cells containing the expression vector plasmid without norA. Thus, under the conditions of these experiments, NorA expression is required for measurable transport of Hoechst 33342 from everted membrane vesicles, as was the case with the transport of norfloxacin (17).
FIG. 2.
Hoechst 33342 transport in everted membrane vesicles energized by ATP (A) or lactate (B). Sequential additions of substances are indicated by arrows: a, 0.2 μM Hoechst 33342; b, 0.1 mM ATP (panel A) or 0.5 mM lactate (panel B); c, 1.8 μM valinomycin; and d, 2.7 μM nigericin. Dotted lines indicate control vesicles. Solid lines indicate NorA-His-containing vesicles. A value of 100% was defined as maximal Hoechst 33342 binding before ATP or lactate was added.
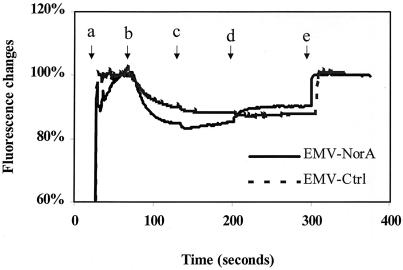
The NorA-enriched vesicles were capable of generating a pH gradient, as indicated by the change in fluorescence of acridine orange after the addition of ATP. It is noteworthy that the magnitude of this change did not differ between NorA-containing and control vesicles (Fig. 3). Thus, the ability of the NorA-containing vesicles to quench the fluorescence of Hoechst 33342 upon energization is most likely caused by the NorA-His present in their membranes.
FIG. 3.
Measurement of the proton gradient across everted membrane vesicles (EMV) by change in fluorescence of acridine orange. Sequential additions of substances are indicated by arrows: a, 5 nM acridine orange; b, 0.1 mM ATP; c, 1.8 μM valinomycin; d, 10 μM norfloxacin; and e, 2.7 μM nigericin. A value of 100% was defined as maximal Hoechst 33342 before ATP was added.
The transport of Hoechst 33342 in NorA-containing vesicles was reversed by the protonophore nigericin, but valinomycin had little or no effect (Fig. 2). Thus, the proton gradient across the vesicle membrane is necessary for the transport of Hoechst 33342, as it is for other NorA substrates such as norfloxacin (17). In accordance with such a mechanism, the addition of norfloxacin to energized NorA-containing vesicles in the presence of valinomycin enhanced the decay of the proton gradient as measured by acridine orange fluorescence (Fig. 3).
The binding capacity of membrane vesicles was determined by monitoring the fluorescence in the presence of vesicles and increasing concentrations of Hoechst 33342. For everted membrane vesicles at 40 μg of protein/ml, increasing concentrations of Hoechst 33342 between 12.5 and 200 nM produced linear increases in fluorescence signal, reflecting linear membrane binding (Fig. 4). Hoechst 33342 at higher concentrations, however, produced a plateau of fluorescence, suggesting saturation of membrane binding capacity. Therefore, kinetic studies were performed with Hoechst 33342 at a concentration range of 12.5 to 200 nM.
FIG. 4.
Saturation of the binding capacities of everted membrane vesicles with Hoechst 33342. Note that increasing concentrations of Hoechst 33342 between 12.5 and 200 nM produced linear increases in fluorescence signal, reflecting linear membrane binding capacity (inset).
Kinetics and inhibition of Hoechst 33342 transport.
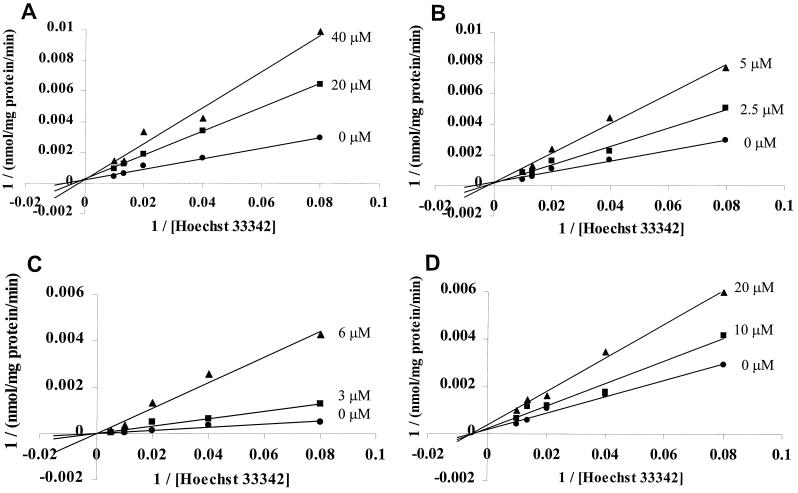
The kinetics of Hoechst 33342 transport by NorA in everted membrane vesicles were saturable, with an estimated Vmax of 89 nmol/s/mg of protein and an estimated Km of 0.17 μM (Fig. 5).
FIG. 5.
Double reciprocal plots of inhibition of Hoechst 33342 transport in NorA-containing everted membrane vesicles by verapamil (A), norfloxacin (B), ciprofloxacin (C), and sparfloxacin (D). Hoechst 33342 is measured in nanomolar units.
NorA-mediated transport of fluoroquinolones such as norfloxacin is known to be inhibited by reserpine and verapamil (17). Because of its fluorescence properties, reserpine could not be studied, but verapamil inhibited the transport of Hoechst 33342. Furthermore, inhibition was competitive with Hoechst 33342 (apparent Ki = 16 μM) (Fig. 5A), suggesting that the inhibitor is itself a transport substrate. NorA transport of fluoroquinolones is thought to result from the aqueous interface of NorA embedded in the membrane (17), since the antimicrobial activities of hydrophilic members of the fluoroquinolone class are more affected by NorA expression than are those of hydrophobic members of the class (32). To determine if Hoechst 33342 and fluoroquinolones differed in NorA transport pathways, we evaluated the effect of quinolones on Hoechst 33342 transport. Both norfloxacin, whose transport by NorA has been demonstrated directly, and ciprofloxacin, whose transport by NorA was demonstrated indirectly by competition with norfloxacin (17), competitively inhibited transport of Hoechst 33342, with apparent Ki values of 3.2 and 2.1 μM (Fig. 5B and C), respectively. The apparent Ki of norfloxacin (3.2 μM) was somewhat lower than its apparent Km (6 μM) when its transport by NorA was studied directly (17). The antimicrobial activities of both norfloxacin and ciprofloxacin are reduced by NorA overexpression (17), but that of sparfloxacin, a more hydrophobic fluoroquinolone, is not (32). Sparfloxacin, in contrast, required higher concentrations to inhibit the transport of Hoechst 33342 by NorA, and inhibition was noncompetitive (Fig. 5D), with an apparent Ki value of 20 μM. TPP, another known substrate of NorA (16) that is structurally unrelated to fluoroquinolones or Hoechst 33342, also inhibited Hoechst 33342 transport competitively, but only at substantially higher concentrations (apparent Ki = 130 μM).
Transport of Hoechst 33342 in NorA-containing proteoliposomes.
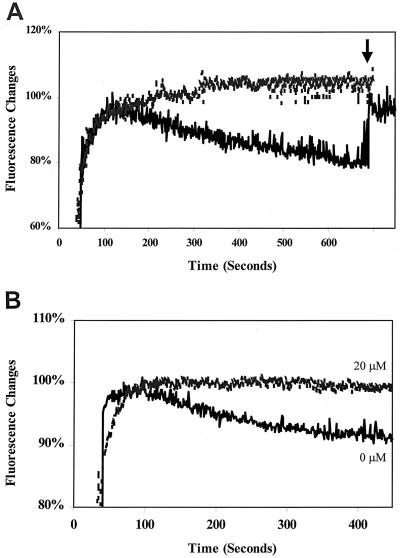
NorA was reconstituted in unilamellar proteoliposomes by mixing the purified protein with preformed Triton X-100-destabilized liposomes composed of E. coli phospholipids and egg phosphatidylcholine. A ratio of lipids to protein of 75:1 to 100:1 produced a maximum reconstitution rate. After the generation of an artificial proton gradient, the fluorescence of Hoechst 33342 in NorA-containing liposomes decreased with time. In contrast, fluorescence remained stable in control liposomes. The addition of nigericin abolished the decrease in fluorescence in the NorA liposomes, indicating that NorA-mediated transport of Hoechst 33342 was dependent on the artificial proton gradient (Fig. 6A). Verapamil also inhibited Hoechst 33342 transport by NorA reconstituted in proteoliposomes (Fig. 6B), further supporting the similarity of the properties of NorA in proteoliposomes to those of NorA in everted membrane vesicles and establishing the ability of NorA itself to transport Hoechst 33342 without interaction with other membrane proteins.
FIG. 6.
Hoechst 33342 transport in NorA proteoliposomes upon the generation of an artificial proton gradient. (A) Effect of nigericin. Nigericin at 3.4 μM was added as indicated by the arrow. (B) Effect of verapamil. A value of 100% was defined as maximal binding of Hoechst 33342 before it was dissociated from proteoliposomes. The upper curves represent control liposomes; the lower curves represent NorA proteoliposomes.
DISCUSSION
The norA gene encoding NorA-His was cloned and expressed in E. coli, conferring resistance to norfloxacin and certain other fluoroquinolones to the same level observed with native NorA (16, 17, 32).
The fluorescent dye Hoechst 33342 was used to determine NorA activity in membrane vesicles and proteoliposomes. This dye is a substrate for a number of membrane transporters (23). We observed that the antibacterial effect of Hoechst 33342 required an eightfold increase in concentration in NorA-expressing cells. This finding indicates that this dye is also a substrate for NorA in intact cells, thereby providing us with a tool to evaluate the interactions of diverse substrates with NorA.
In NorA-containing everted membrane vesicles, the manner of transport of Hoechst 33342 is like that of other NorA substrates, such as norfloxacin (17), in that the dye is able to saturate the membrane, dependent on the proton gradient across the membrane, and its transport inhibited by verapamil. The competitive nature of the inhibition of Hoechst 33342 transport suggested that verapamil too is a substrate for NorA.
The mechanisms by which multidrug efflux transporters handle structurally diverse substrates constitute an area of considerable interest (1, 29, 30). Structural data from regulatory proteins, such as BmrR, which controls expression of the inducible multidrug resistance transporter Bmr by interacting with a similar diversity of substrates in mediating induction of Bmr expression, have suggested models for how diverse structures can be accommodated by a single protein molecule (33, 34). In addition, for transporters that appear to interact with both aqueous and membrane-associated substrates, information about common or parallel sites of interaction may be helpful for model building.
The effect of hydrophilic fluoroquinolones on the transport of Hoechst 33342 allows direct study of whether these two different types of substrates interact with common or entirely distinct sites when they are transported by NorA. The competitive inhibition of Hoechst 33342 transport by hydrophilic fluoroquinolones, which are established NorA substrates, strongly suggests that there is an overlap in the transport pathways for both Hoechst 33342, an intramembrane substrate, and hydrophilic fluoroquinolones, which are likely extramembrane substrates. The fact that the Ki of norfloxacin inhibition of Hoechst 33342 transport was within twofold of the Km of norfloxacin transport by NorA that was measured directly further suggests that processes measured by the two methods are similar. Although we cannot rule out the possibility that fluoroquinolones dissolve in the inner leaflet of the cytoplasmic membrane, as appears to occur for certain hydrophobic cephalosporins affected by the AcrAB pump of E. coli (15), the positive correlation of hydrophilicity and the magnitude of the effect of NorA expression on drug resistance (17, 28) suggest that this explanation is unlikely. In addition, the lack of change in the fluorescence properties of norfloxacin in the presence and absence of control everted membrane vesicles suggests that there is no major interaction of this substrate with the membrane (data not shown).
It is interesting that the hydrophobic fluoroquinolone sparfloxacin, which appears to be a poor substrate for NorA in intact cells, behaves as a noncompetitive inhibitor of Hoechst 33342 transport by NorA. This finding suggests that sparfloxacin interacts with NorA in a manner that is different from that of other more hydrophilic fluoroquinolones and possibly accounts for the lack of an effect of NorA overexpression on its activity. Although we cannot exclude the possibility that rapid diffusional reuptake of sparfloxacin accounts in part for the lack of an effect of NorA overexpression on sparfloxacin activity, our data highlight a distinct mechanism of interaction of this fluoroquinolone with NorA. The transport of Hoechst 33342 by other transporters, such as LmrP, has also been shown to be inhibited competitively, noncompetitively, and uncompetitively by compounds of different classes (21), but this is the first report to our knowledge of a secondary transporter in which drugs of the same class exhibited different kinetic patterns of inhibition. Thus, NorA appears to have at least two functionally distinct drug binding sites for fluoroquinolones.
We were able to reconstitute functional NorA-His in proteoliposomes that lacked other protein species. The transport of Hoechst 33342 in proteoliposomes was dependent on a proton gradient, and it was inhibited by a NorA inhibitor, verapamil, thus establishing that NorA is a self-sufficient membrane transporter. Thus, NorA itself can contribute to drug resistance and in some conditions may be sufficient alone to cause drug resistance. Other multidrug transporters functionally reconstituted in proteoliposomes include Smr of S. aureus (8), LmrP and LmrA of Lactococcus lactis (14, 23), EmrE of E. coli (31), and mammalian P-glycoprotein (25), in addition to the unidrug transporter TetA of E. coli (26).
The physiologic role of NorA in the cell is uncertain, and fluoroquinolones as synthetic antimicrobials are likely only incidental substrates. That NorA can remove Hoechst 33342 from within the membrane suggests that at least one of its roles within the cell may be to remove toxic substances accumulating within the membrane, a suggestion consistent with the “hydrophobic membrane vacuum cleaner” hypothesis proposed for structurally and functionally related multidrug resistance pumps (1, 2). It is interesting that this same transporter is also capable of removing from the cell and causing resistance to antimicrobials with cytoplasmic targets, apparently through overlapping pathways, further highlighting the diversity of function of multidrug efflux pumps such as NorA (22).
Acknowledgments
We thank Irene Kochevar and Richard Bringhurst for use of the spectrofluorometers for these experiments and Hans-Christ Ludemann, Xin Chen, and Matthew Mahon for help with their use.
This work was supported by a grant from the U.S. Public Health Service, National Institutes of Health, (AI23988 to D.C.H.).
REFERENCES
- 1.Bolhuis, H., H. W. Van Veen, J. R. Brands, M. Putman, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1996. Energetics and mechanism of drug transport mediated by the lactococcal multidrug transporter LmrP. J. Biol. Chem. 271:24123-24128. [DOI] [PubMed] [Google Scholar]
- 2.Bolhuis, H., H. W. Van Veen, D. Molenaar, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 15:4239-4245. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen, S. P., H. Hächler, and S. B. Levy. 1993. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 175:1484-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, S. P., D. C. Hooper, J. S. Wolfson, K. S. Souza, L. M. McMurry, and S. B. Levy. 1988. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob. Agents Chemother. 32:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, S. P., L. M. McMurry, and S. B. Levy. 1988. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J. Bacteriol. 170:5416-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drlica, K., and X. Zhao. 1997. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 61:377-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero, L., B. Cameron, and J. Crouzet. 1995. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 39:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinius, L. L., and E. B. Goldberg. 1994. Bacterial multidrug resistance is due to a single membrane protein which functions as a drug pump. J. Biol. Chem. 269:29998-30004. [PubMed] [Google Scholar]
- 9.Hane, M. W., and T. H. Wood. 1969. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J. Bacteriol. 99:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper, D. C., J. S. Wolfson, K. S. Souza, E. Y. Ng, G. L. McHugh, and M. N. Swartz. 1989. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob. Agents Chemother. 33:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper, D. C., J. S. Wolfson, K. S. Souza, C. Tung, G. L. McHugh, and M. N. Swartz. 1986. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob. Agents Chemother. 29:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1991. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J. Infect. Dis. 163:1080-1086. [DOI] [PubMed] [Google Scholar]
- 13.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1993. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 37:1086-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolles, A., M. Putman, H. W. Van Veen, and W. N. Konings. 1999. The purified and functionally reconstituted multidrug transporter LmrA of Lactococcus lactis mediates the transbilayer movement of specific fluorescent phospholipids. Biochemistry 38:16298-16306. [DOI] [PubMed] [Google Scholar]
- 15.Mazzariol, A., G. Cornaglia, and H. Nikaido. 2000. Contributions of the AmpC β-lactamase and the AcrAB multidrug efflux system in intrinsic resistance of Escherichia coli K-12 to β-lactams. Antimicrob. Agents Chemother. 44:1387-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyfakh, A. A., C. M. Borsch, and G. W. Kaatz. 1993. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 37:128-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng, E. Y. W., M. Trucksis, and D. C. Hooper. 1994. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob. Agents Chemother. 38:1345-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng, E. Y., M. Trucksis, and D. C. Hooper. 1996. Quinolone resistance mutations in topoisomerase IV: relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putman, M., L. A. Koole, H. W. Van Veen, and W. N. Konings. 1999. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry 38:13900-13905. [DOI] [PubMed] [Google Scholar]
- 22.Putman, M., H. W. Van Veen, J. E. Degener, and W. N. Konings. 2000. Antibiotic resistance: era of the multidrug pump. Mol. Microbiol. 36:772-773. [DOI] [PubMed] [Google Scholar]
- 23.Putman, M., H. W. Van Veen, B. Poolman, and W. N. Konings. 1999. Restrictive use of detergents in the functional reconstitution of the secondary multidrug transporter LmrP. Biochemistry 38:1002-1008. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 1.1-18.88. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Shapiro, A. B., and V. Ling. 1998. The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiol. Scand. 163:227-234. [PubMed] [Google Scholar]
- 26.Someya, Y., Y. Moriyama, M. Futai, T. Sawai, and A. Yamaguchi. 1995. Reconstitution of the metal-tetracycline/H+ antiporter of Escherichia coli in proteoliposomes including F0F1-ATPase. FEBS Lett. 374:72-76. [DOI] [PubMed] [Google Scholar]
- 27.Stein, W. D. 1986. Transport and diffusion across cell membranes, p. 1-685. Academic Press, Inc., New York, N.Y.
- 28.Takenouchi, T., F. Tabata, Y. Iwata, H. Hanzawa, M. Sugawara, and S. Ohya. 1996. Hydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Veen, H. W., and W. N. Konings. 1998. Structure and function of multidrug transporters. Adv. Exp. Med. Biol. 456:145-158. [DOI] [PubMed] [Google Scholar]
- 30.Van Veen, H. W., A. Margolles, M. Müller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yerushalmi, H., M. Lebendiker, and S. Schuldiner. 1995. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J. Biol. Chem. 270:6856-6863. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida, H., M. Bogaki, S. Nakamura, K. Ubukata, and M. Konno. 1990. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 172:6942-6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheleznova, E. E., P. Markham, R. Edgar, E. Bibi, A. A. Neyfakh, and R. G. Brennan. 2000. A structure-based mechanism for drug binding by multidrug transporters. Trends Biochem. Sci. 25:39-43. [DOI] [PubMed] [Google Scholar]
- 34.Zheleznova, E. E., P. N. Markham, A. A. Neyfakh, and R. G. Brennan. 1999. Structural basis of multidrug recognition by BmrR, a transcription activator of a multidrug transporter. Cell 96:353-362. [DOI] [PubMed] [Google Scholar]