Abstract
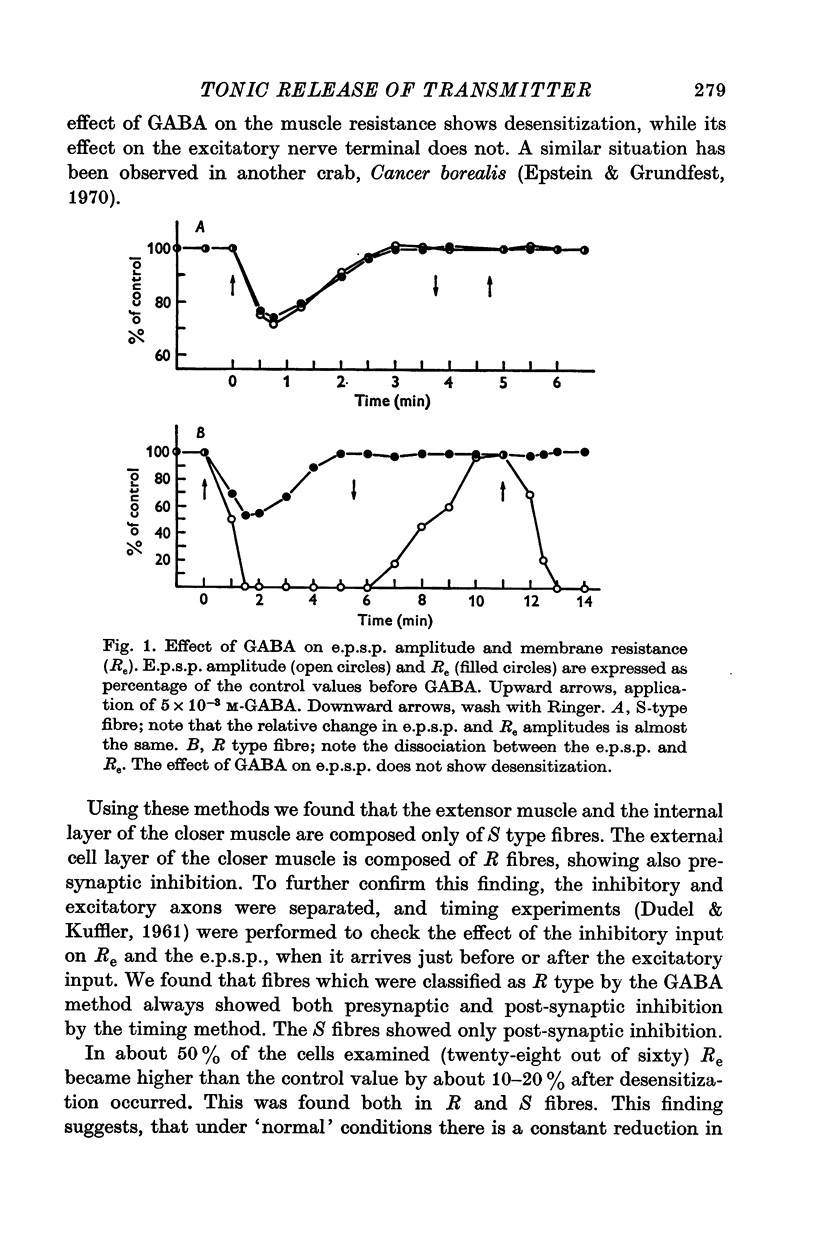
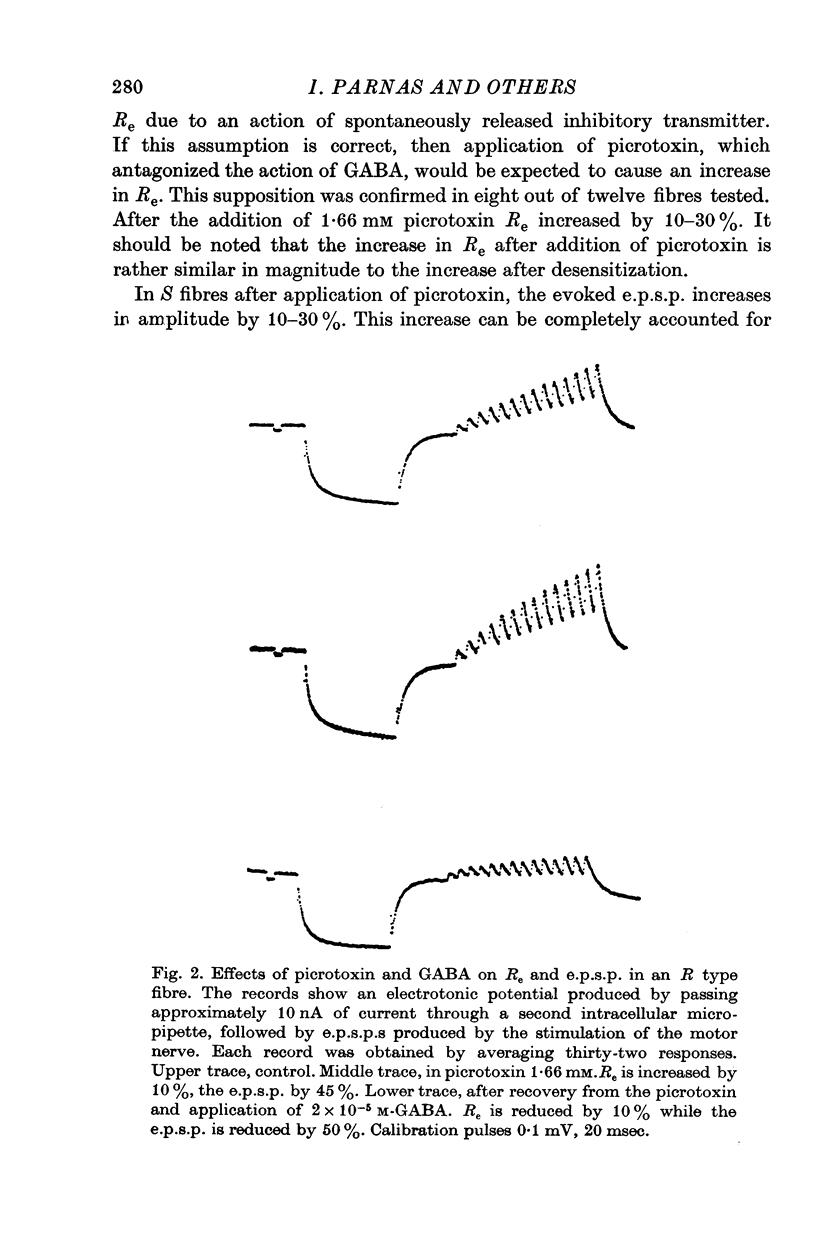
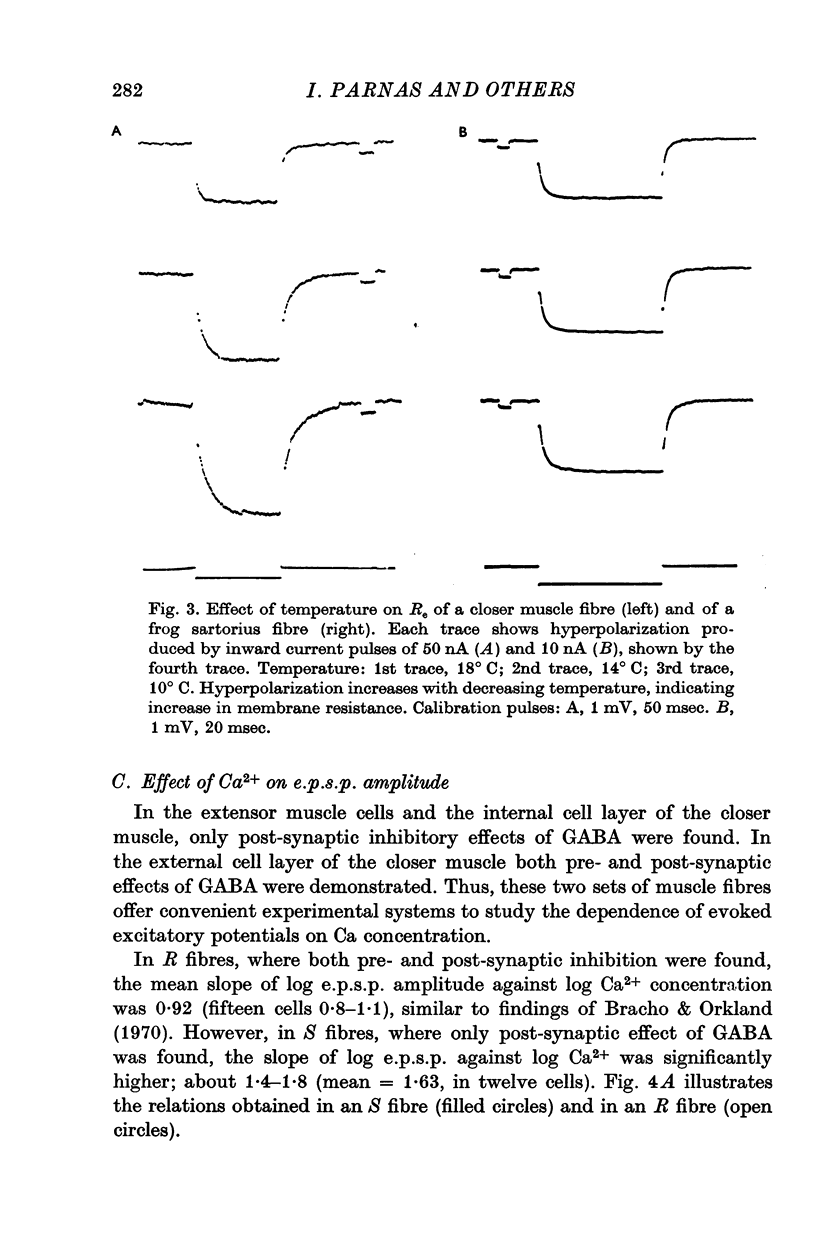
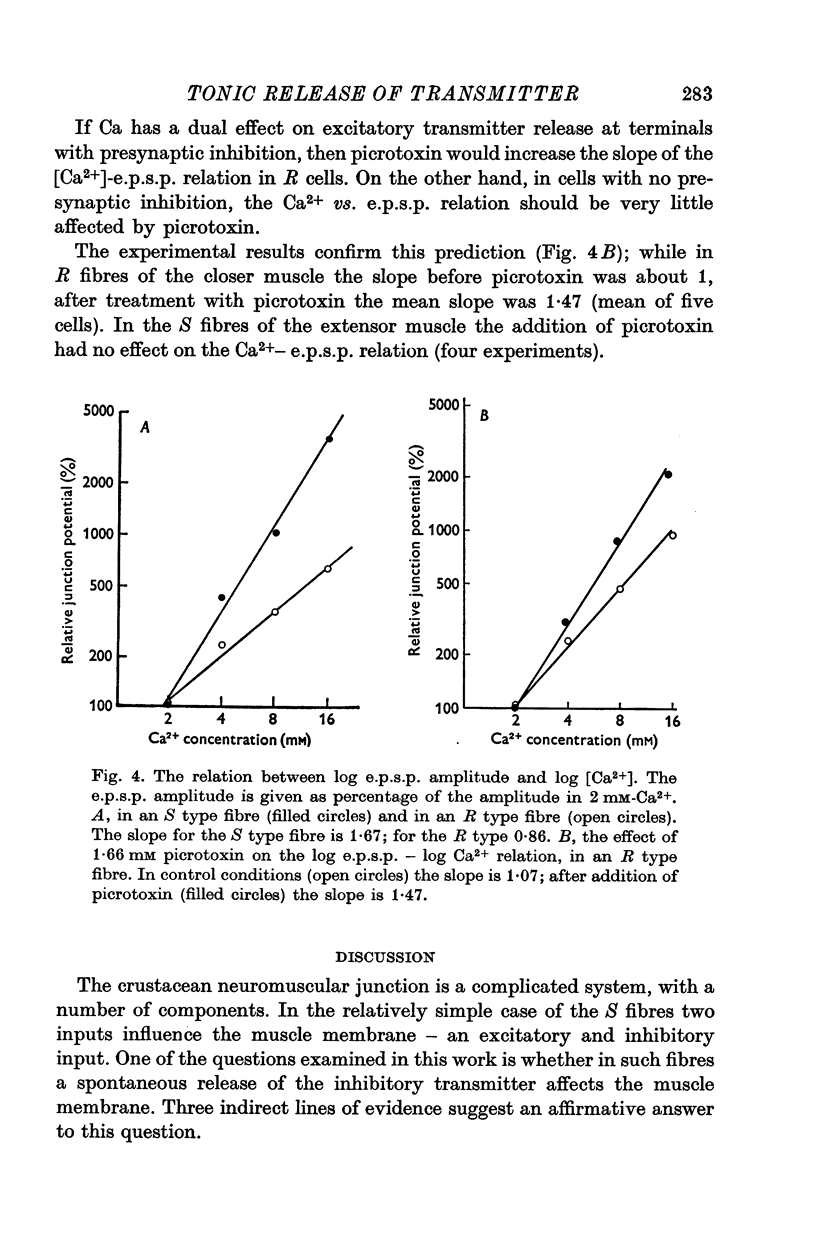
1. Synaptic transmission was studied at the neuromuscular junction of the crab Ocypoda cursor, using conventional electrophysiological technique. 2. It was found that fibres of the extensor muscle and those composing the internal layer of the closer muscle have only post-synaptic inhibition (S fibres) while the fibres at the external layer of the closer muscle have in addition presynaptic inhibition (R fibres). 3. In S fibres, addition of GABA reduces input membrane resistance (Rm) and e.p.s.p. amplitude approximately to the same degree. The effect shows desensitization. In R type fibres, GABA reduces the e.p.s.p. much more than expected from changes in Rm. The post-synaptic effect of GABA on Rm shows desensitization, while the presynaptic effect does not show desensitization. 4. In about 50 percent of the cases, after desensitization occurred, Rm increased by about 10-30 percent above the control. Similar increase in Rm occurred after application of picrotoxin. These results suggest that initially the membrane resistance was lower due to tonic release of inhibitory transmitter. 5. The Q10 of Rm was found to vary between 2 and 3. In Ca2+ free media, Cl- free media, or in picrotoxin the Q10 is about 1-3. 6. In R fibres, addition of picrotoxin increased the amplitude of the e.p.s.p. by 30-60 percent above the expected increase due to changes in Rm. 7. In S fibres the mean slope of log e.p.s.p. vs. log [Ca2+] was found to be 1-63, while in R fibres the slope was 0-93. These results suggest the presence of tonic release of the inhibitory transmitter which acts both post-synaptically and presynaptically.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho H., Orkand R. K. Effect of calcium on excitatory neuromuscular transmission in the crayfish. J Physiol. 1970 Jan;206(1):61–71. doi: 10.1113/jphysiol.1970.sp008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., MACHNE X. Effect of temperature on the passive electrical properties of the muscle fibre membrane. J Physiol. 1953 May 28;120(3):431–434. doi: 10.1113/jphysiol.1953.sp004906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J. PRESYNAPTIC AND POSTSYNAPTIC EFFECTS OF INHIBITORY DRUGS ON THE CRAYFISH NEUROMUSCULAR JUNCTION. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Mar 18;283:104–118. doi: 10.1007/BF00363182. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R., Grundfest H. Desensitization of gamma aminobutyric acid (GABA) receptors in muscle fibers of the crab Cancer borealis. J Gen Physiol. 1970 Jul;56(1):33–45. doi: 10.1085/jgp.56.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- GRUNDFEST H., REUBEN J. P., RICKLES W. H., Jr The electrophysiology and pharmacology of lobster neuromuscular synapses. J Gen Physiol. 1959 Jul 20;42(6):1301–1323. doi: 10.1085/jgp.42.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further study of the role of calcium in synaptic transmission. J Physiol. 1970 May;207(3):789–801. doi: 10.1113/jphysiol.1970.sp009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T. M. Calcium and facilitation at two classes of crustacean neuromuscular synapses. J Gen Physiol. 1973 Jan;61(1):56–73. doi: 10.1085/jgp.61.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz C. L., Bracho H. Effect of reduced calcium on excitatory transmitter release at the crayfish neuromuscular junction. Comp Biochem Physiol A Comp Physiol. 1972 Apr 1;41(4):805–812. doi: 10.1016/0300-9629(72)90343-x. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Iversen L. L., Hall Z. W., Kravitz E. A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Alnaes E. Inhibitory action of Ruthenium red on neuromuscular transmission. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3613–3616. doi: 10.1073/pnas.70.12.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. A study of the inhibitory action of gamma-amino-butyric acid on neuromuscular transmission in the crayfish. J Physiol. 1966 Mar;183(2):418–432. doi: 10.1113/jphysiol.1966.sp007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Takeuchi N. On the permeability of the presynaptic terminal of the crayfish neuromuscular junction during synaptic inhibition and the action of gamma-aminobutyric acid. J Physiol. 1966 Mar;183(2):433–449. doi: 10.1113/jphysiol.1966.sp007875. [DOI] [PMC free article] [PubMed] [Google Scholar]


