Abstract
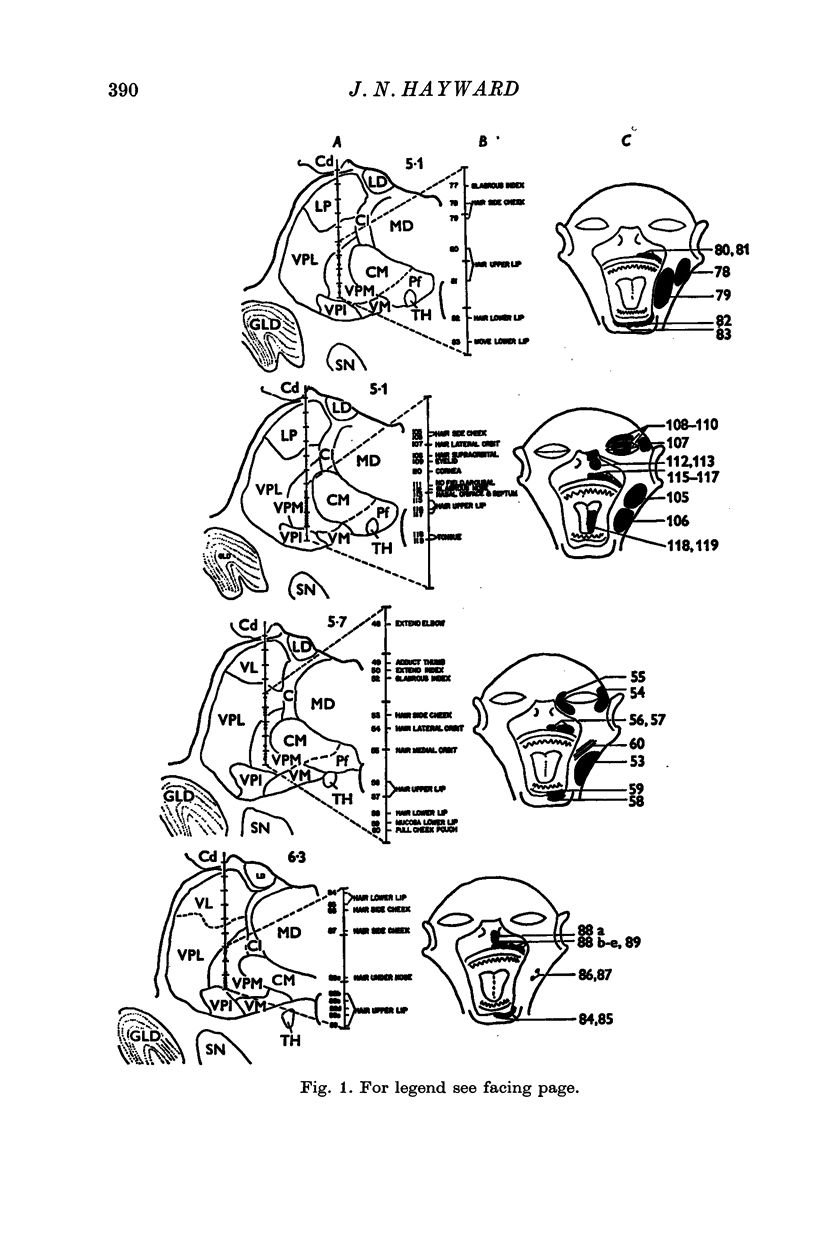
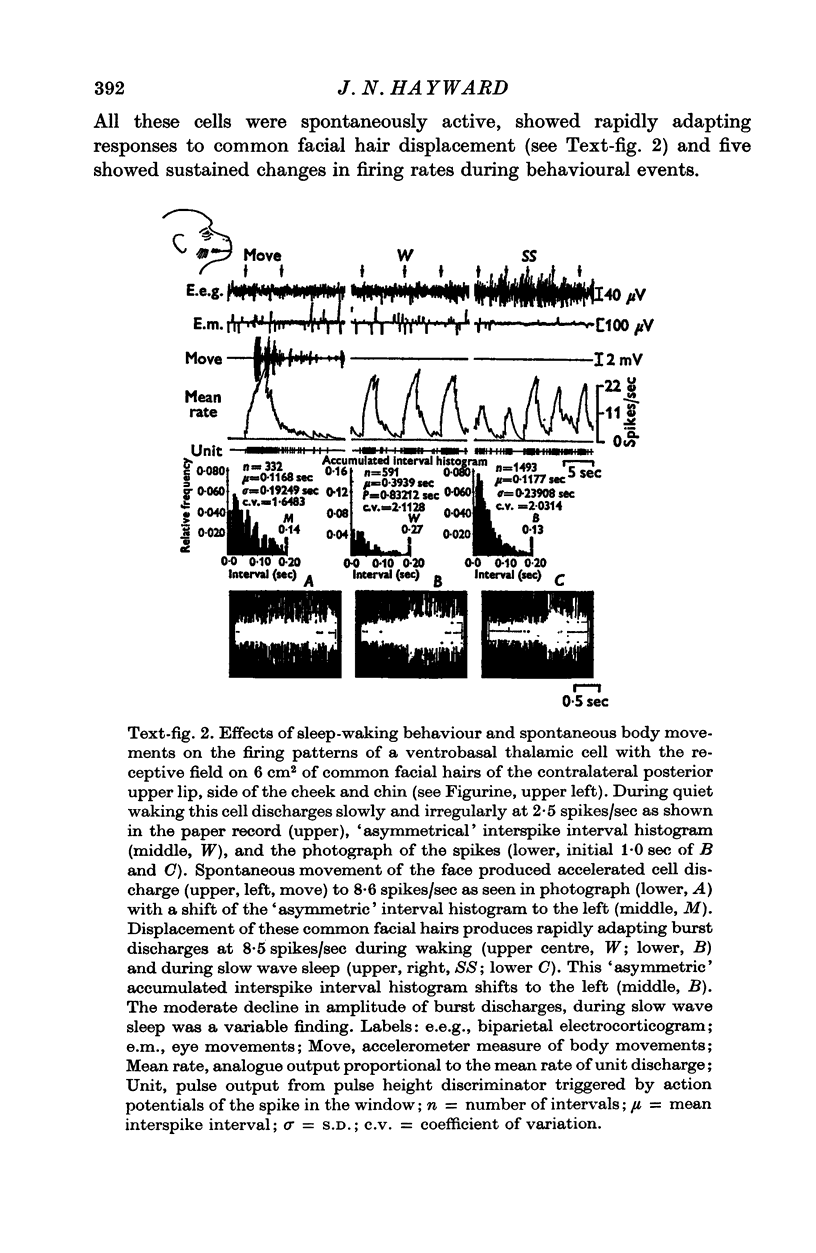
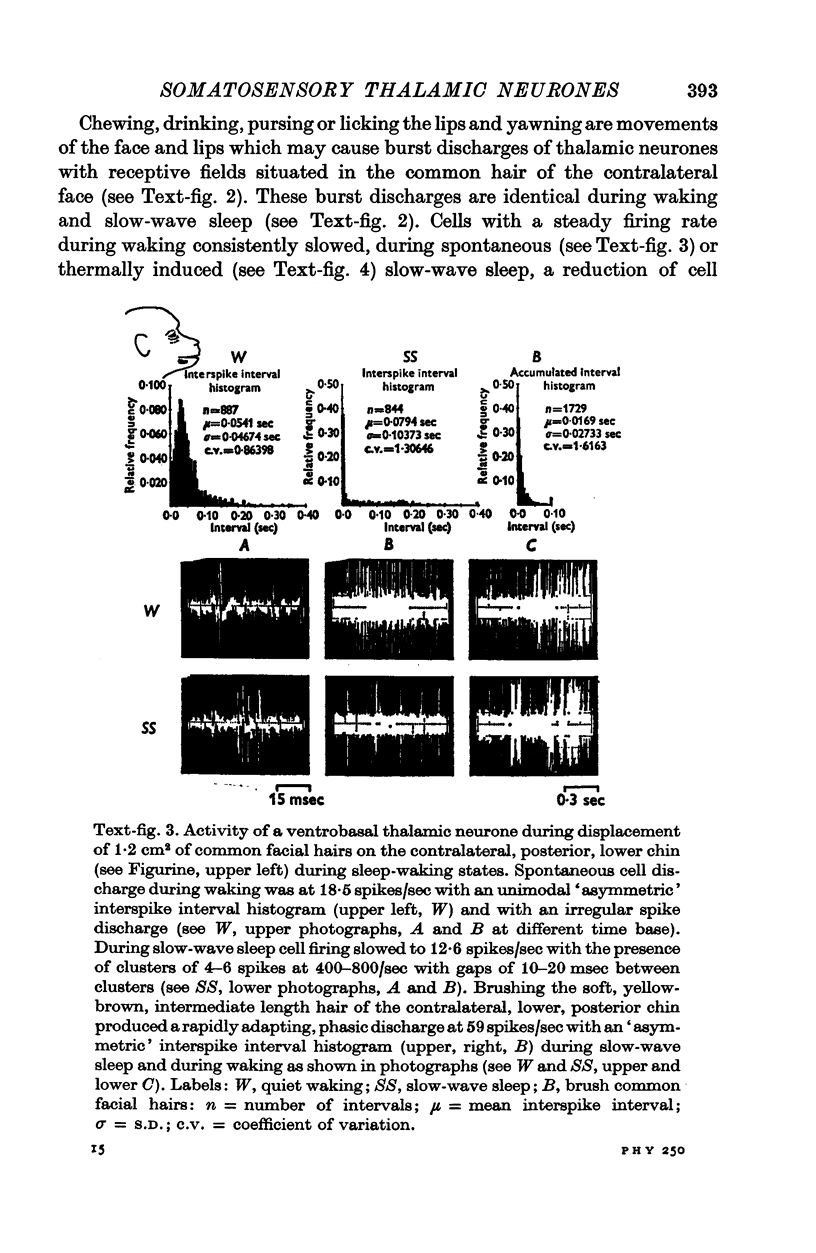
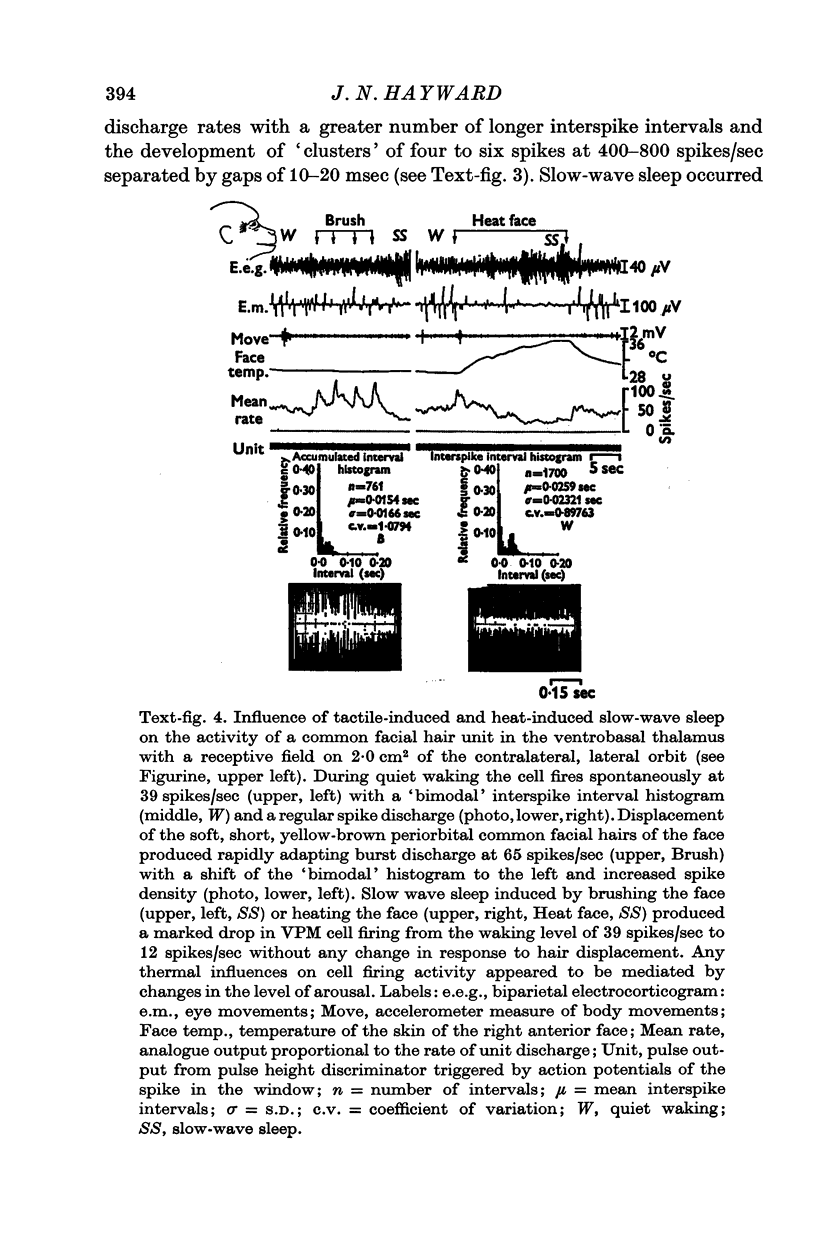
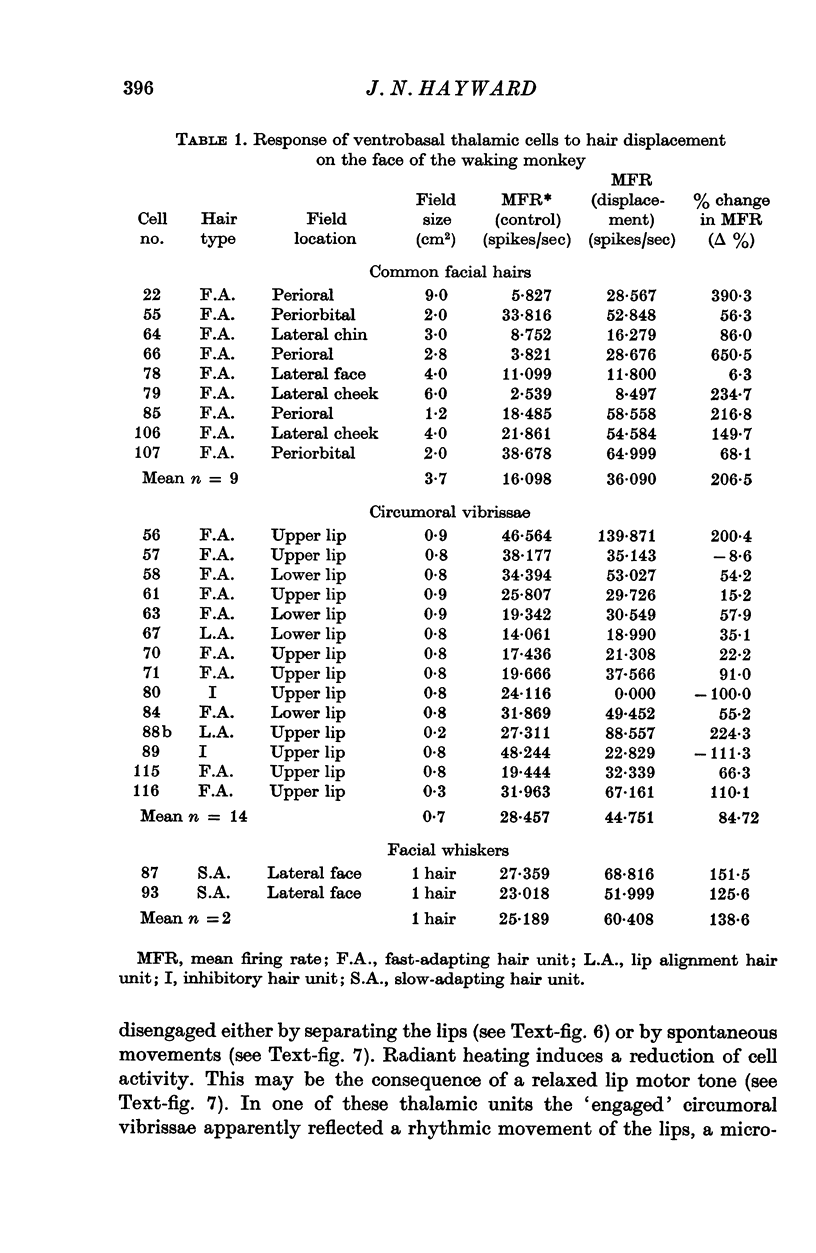
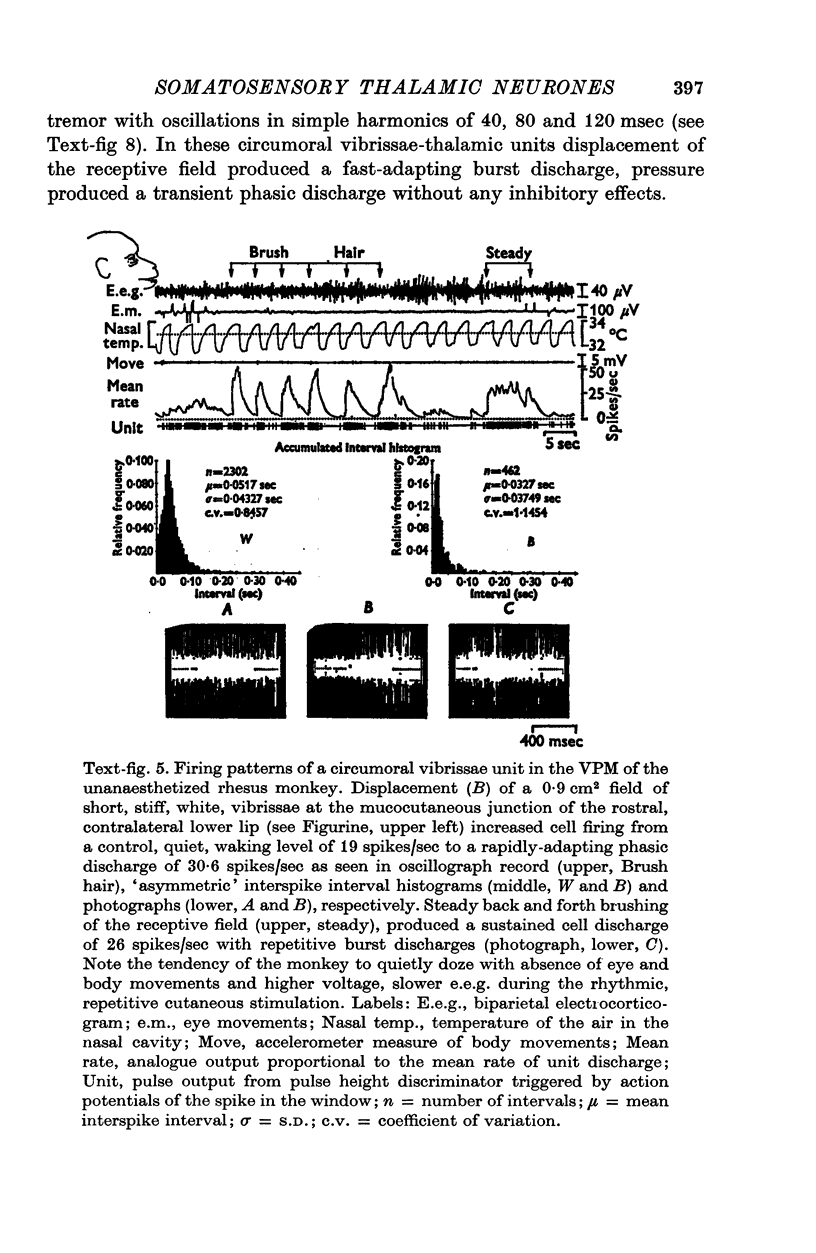
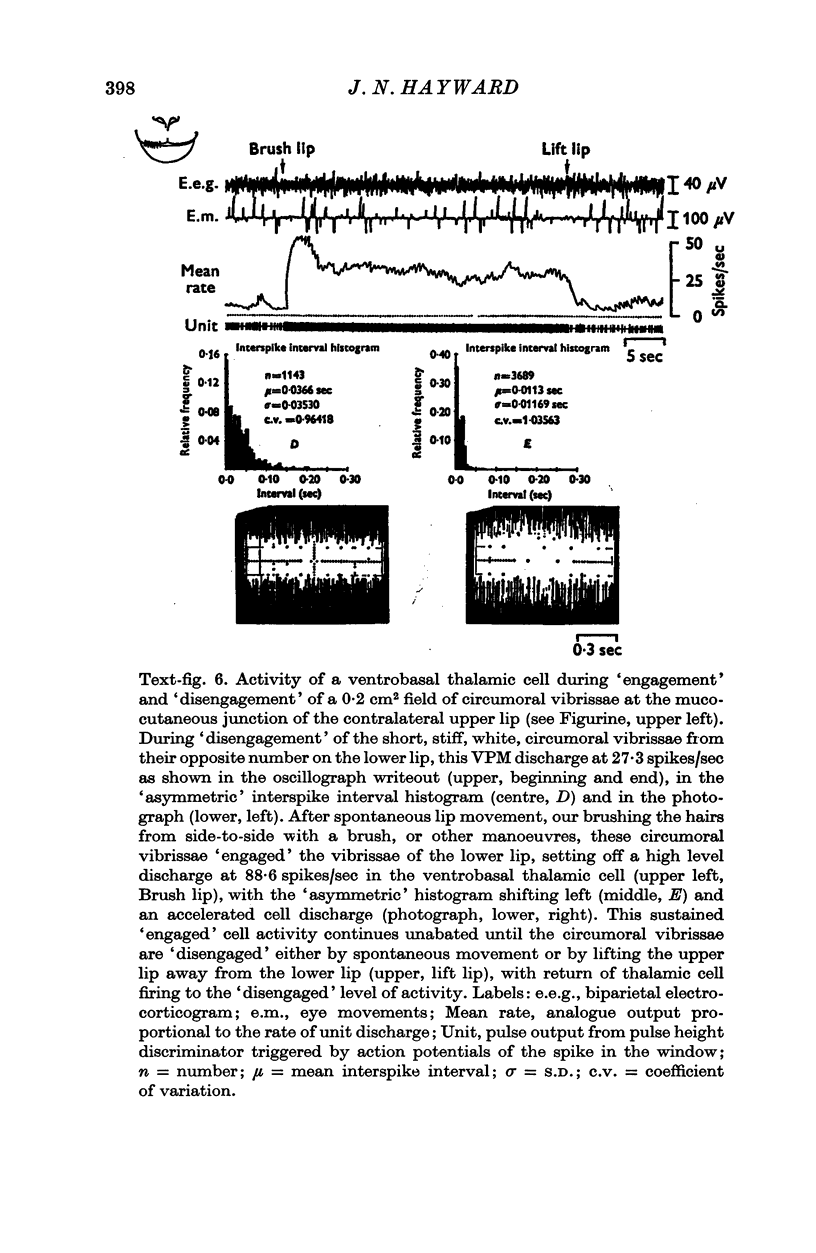
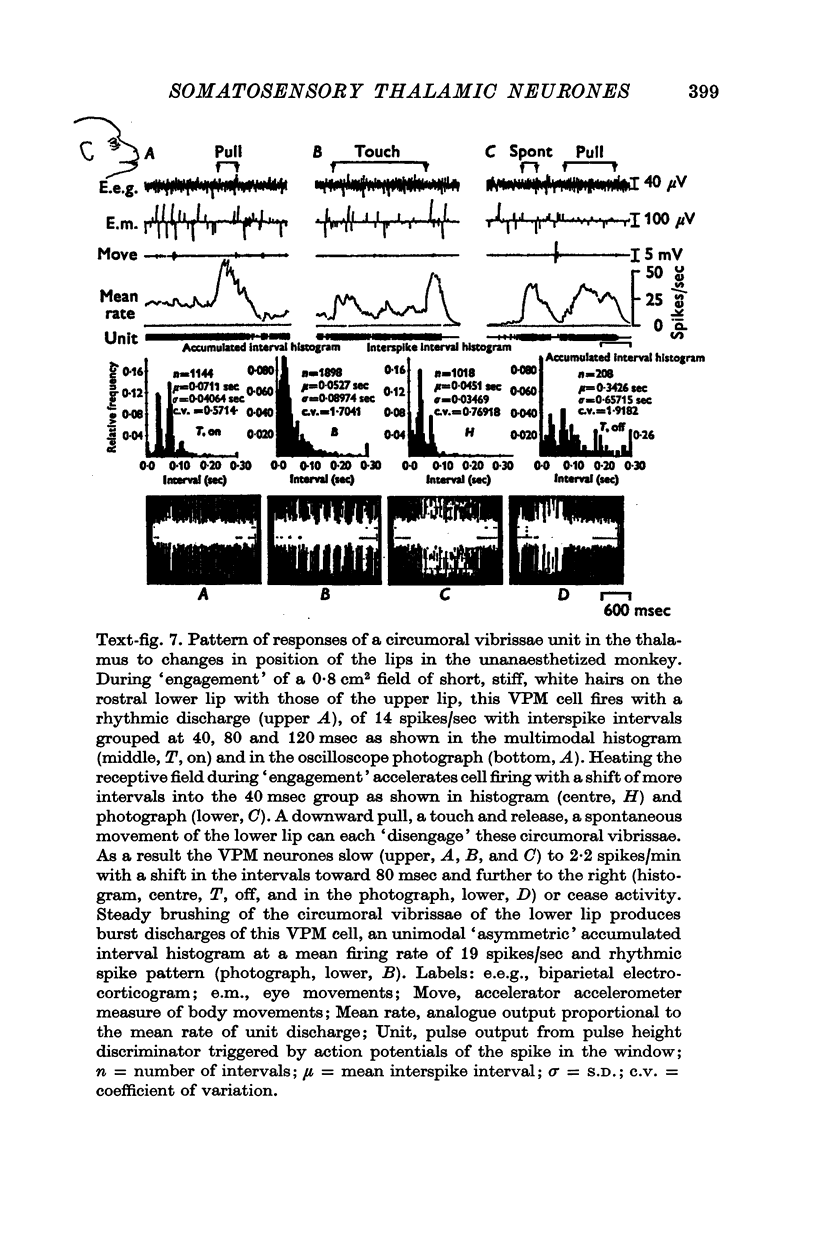
1. In the unanaesthetized, moving monkey, single cell firing patterns in the region of the ventrobasal complex (VB) of the thalamus that respond to facial hair displacement were the basis for a statistical analysis of the effects of tactile, thermal and behavioural stimuli. 2. There were facial hair responses throughout the dorsoventral extent of the ventralis posterior medialis (VPM) nucleus of the contralateral thalamus over a rostro-caudal distance of about 2 mm (Fr. 5.1 to Fr. 7.1). 3. The three different anatomical types of facial hairs that activated thalamic neurones were common facial hairs, circumoral vibrissae and facial whiskers. 4. Displacement of the intermediate length, soft, yellow-brown common facial hairs on the central and lateral face from fields of 1-9 cm2 produced a fast-adapting burst discharge in single thalamic cells in the upper half of the contralateral VPM. 5. Tactile stimuli applied to the short, stiff, white circumoral vibrissae in fields of 0-2-0-9 cm2 along the margins of the upper and lower lips resulted in fast-adapting phasic firing of units in the lower half of the contralateral VPM. Engagement or disengagement of the interlocking hairs of upper and lower lips resulted in increased or decreased, respectively, firing of these thalamic units. 6. Bending a single, long, stiff, black facial whisker extending out from the side of the face resulted in a sustained increased firing of contralateral VPM cells with directional sensitivity. 7. Cells in the ventrobasal thalamus relay mechanoreceptor input from three specialized hair types on the face of the monkey. These somatotopically organized hairy receptive fields are unique, registering response patterns from tactile, thermal and behavioural stimuli. Facial hairs must play an important part in primate feeding, drinking, and oral-exploration.
Full text
PDF













Images in this article
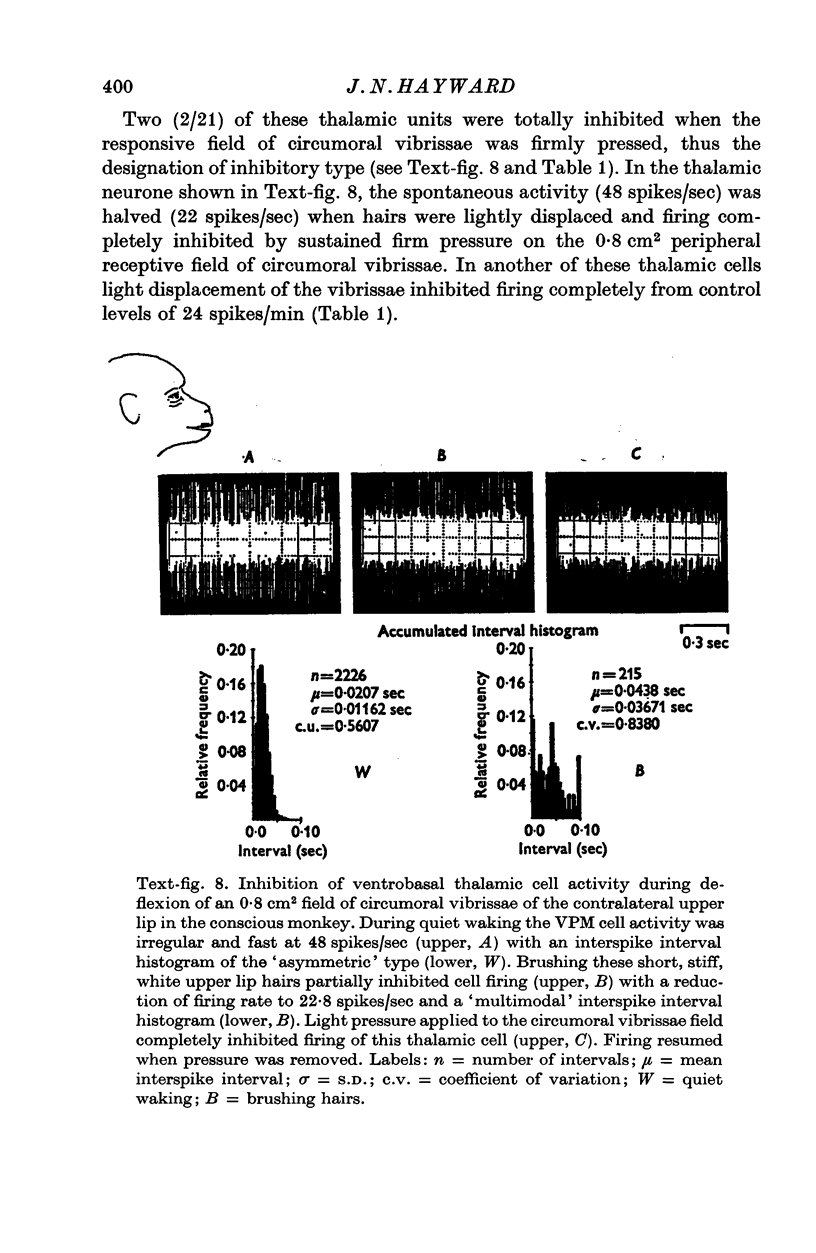
Selected References
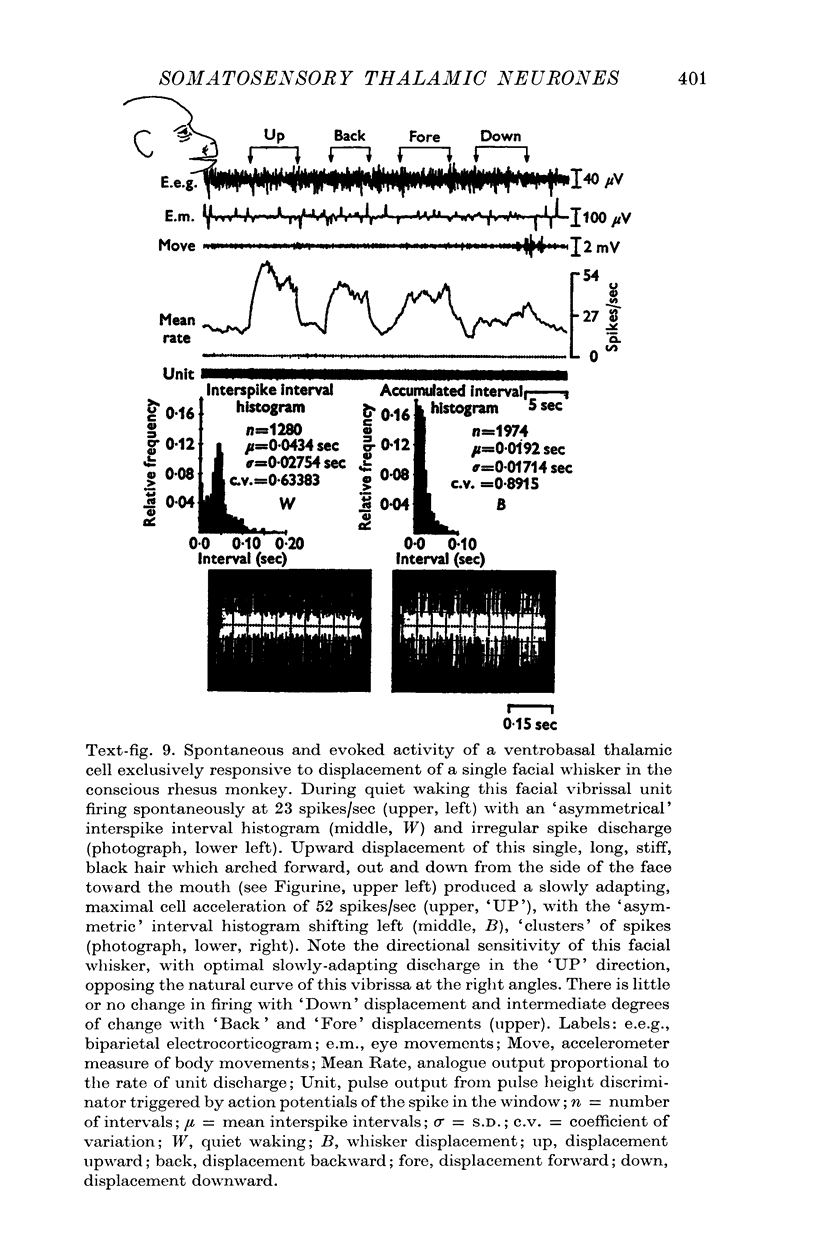
These references are in PubMed. This may not be the complete list of references from this article.
- Baker M. A., Burrell E., Penkhus J., Hayward J. N. Capping and stabilizing chronic intravascular cannulae. J Appl Physiol. 1968 Apr;24(4):577–579. doi: 10.1152/jappl.1968.24.4.577. [DOI] [PubMed] [Google Scholar]
- Baker M. A. Spontaneous and evoked activity of neurones in the somatosensory thalamus of the waking cat. J Physiol. 1971 Sep;217(2):359–379. doi: 10.1113/jphysiol.1971.sp009576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. W., Waite P. M. Responses in the rat thalamus to whisker movements produced by motor nerve stimulation. J Physiol. 1974 Apr;238(2):387–401. doi: 10.1113/jphysiol.1974.sp010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmers R. Separate relays of tactile, pressure, thermal, and gustatory modalities in the cat thalamus. Proc Soc Exp Biol Med. 1966 Feb;121(2):527–531. doi: 10.3181/00379727-121-30821. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. A technique for recording activity of subcortical neurons in moving animals. Electroencephalogr Clin Neurophysiol. 1968 Jan;24(1):83–86. doi: 10.1016/0013-4694(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Findlay A. L., Hayward J. N. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969 Mar;201(1):237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol. 1968 Aug;215(2):389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Jennings D. P. Activity of magnocellular neuroendocrine cells in the hypothalamus of unanaesthetized monkeys. I. Functional cell types and their anatomical distribution in the supraoptic nucleus and the internuclear zone. J Physiol. 1973 Aug;232(3):515–543. doi: 10.1113/jphysiol.1973.sp010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Jennings D. P. Activity of magnocellular neuroendocrine cells in the hypothalamus of unanaesthetized monkeys. II. Osmosensitivity of functional cell types in the supraoptic nucleus and the internuclear zone. J Physiol. 1973 Aug;232(3):545–572. doi: 10.1113/jphysiol.1973.sp010285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Vincent J. D. Osmosensitive single neurones in the hypothalamus of unanaesthetized monkeys. J Physiol. 1970 Nov;210(4):947–972. doi: 10.1113/jphysiol.1970.sp009251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967 Apr;47(2):117–177. doi: 10.1152/physrev.1967.47.2.117. [DOI] [PubMed] [Google Scholar]
- KERR F. W., LYSAK W. R. SOMATOTOPIC ORGANIZATION OF TRIGEMINAL-GANGLION NEURONES. Arch Neurol. 1964 Dec;11:593–602. doi: 10.1001/archneur.1964.00460240025003. [DOI] [PubMed] [Google Scholar]
- LANDGREN S. Thalamic neurones responding to cooling of the cat's tongue. Acta Physiol Scand. 1960 Mar 18;48:255–267. doi: 10.1111/j.1748-1716.1960.tb01860.x. [DOI] [PubMed] [Google Scholar]
- LANDGREN S. Thalamic neurones responding to tactile stimulation of the cat's tongue. Acta Physiol Scand. 1960 Mar 18;48:238–254. doi: 10.1111/j.1748-1716.1960.tb01859.x. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., HENNEMAN E. The representation of tactile sensibility in the thalamus of the monkey. J Comp Neurol. 1952 Dec;97(3):409–439. doi: 10.1002/cne.900970302. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POGGIO G. F., WERNER G. THE RELATION OF THALAMIC CELL RESPONSE TO PERIPHERAL STIMULI VARIED OVER AN INTENSIVE CONTINUUM. J Neurophysiol. 1963 Sep;26:807–834. doi: 10.1152/jn.1963.26.5.807. [DOI] [PubMed] [Google Scholar]
- Martin A. R. A peak amplitude selector for electrophysiological data analysis. IEEE Trans Biomed Eng. 1969 Apr;16(2):152–159. doi: 10.1109/tbme.1969.4502629. [DOI] [PubMed] [Google Scholar]
- POGGIO G. F., MOUNTCASTLE V. B. THE FUNCTIONAL PROPERTIES OF VENTROBASAL THALAMIC NEURONSSTUDIED IN UNANESTHETIZED MONKEYS. J Neurophysiol. 1963 Sep;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Munger B. L. The ultrastructure and innervation of rat vibrissae. J Comp Neurol. 1966 Mar;126(3):423–435. doi: 10.1002/cne.901260305. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos D. A., Benjamin R. M. Response of thalamic neurons to thermal stimulation of the tongue. J Neurophysiol. 1968 Jan;31(1):28–43. doi: 10.1152/jn.1968.31.1.28. [DOI] [PubMed] [Google Scholar]
- ROSE J. E., MOUNTCASTLE V. B. Activity of single neurons in the tactile thalamic region of the cat in response to a transient peripheral stimulus. Bull Johns Hopkins Hosp. 1954 May;94(5):238–282. [PubMed] [Google Scholar]
- ROSE J. E., MOUNTCASTLE V. B. The thalamic tactile region in rabbit and cat. J Comp Neurol. 1952 Dec;97(3):441–489. doi: 10.1002/cne.900970303. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol. 1973 Jan;228(2):541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]



