Abstract
We found that transcription of the pdxA and pdxB genes, which mediate steps in the biosynthesis of the essential coenzyme pyridoxal 5"-phosphate, and the ksgA gene, which encodes an rRNA modification enzyme and is partly cotranscribed with pdxA, is subject to positive growth rate regulation in Escherichia coli K-12. The amounts of the pdxA-ksgA cotranscript and pdxB- and ksgA-specific transcripts and expression from pdxA- and pdxB-lacZ fusions increased as the growth rate increased. The half-lives of ksgA- and pdxB-specific transcripts were not affected by the growth rate, whereas the half-life of the pdxA-ksgA cotranscript was too short to be measured accurately. A method of normalization was applied to determine the amount of mRNA synthesized per gene and the rate of protein accumulation per gene. Normalization removed an apparent anomaly at fast growth rates and demonstrated that positive regulation of pdxB occurs at the level of transcription initiation over the whole range of growth rates tested. RNA polymerase limitation and autoregulation could not account for the positive growth rate regulation of pdxA, pdxB, and ksgA transcription. On the other hand, growth rate regulation of the amount of the pdxA-ksgA cotranscript was abolished by a fis mutation, suggesting a role for the Fis protein. In contrast, the fis mutation had no effect on pdxB- or ksgA-specific transcript amounts. The amounts of the pdxA-ksgA cotranscript and ksgA-specific transcript were repressed in the presence of high intracellular concentrations of guanosine tetraphosphate; however, this effect was independent of relA function for the pdxA-ksgA cotranscript. Amounts of the pdxB-specific transcript remained unchanged during amino acid starvation in wild-type and relA mutant strains.
Pyridoxal 5"-phosphate (PLP) is a ubiquitous coenzyme that plays fundamental roles in the metabolism of all cells (9, 16, 21, 31). One property that makes PLP such a versatile coenzyme is its ability to form reactive Schiff bases with amino groups (9, 16, 21, 31). Consequently, many classes of enzymes involved in amino acid metabolism use PLP as a coenzyme, including racemases, transaminases, decarboxylases, oxidative deaminases, and enzymes that replace side chains in or eliminate side chains from carbon atoms (9, 16, 21, 31). In addition, PLP is the essential coenzyme of glycogen phosphorylases, where PLP is thought to act in acid-base catalysis, which is a completely different mechanism from its reactions with amino groups (29).
Intracellular PLP amounts appear to be regulated in E. coli, and deprivation or overproduction of PLP has deleterious effects on bacterial cell physiology and growth (9, 18, 19, 36, 39), although this regulation is incompletely understood. Moreover, recent findings suggest that de novo PLP biosynthesis occurs by two distinct pathways in different organisms (23, 30, 48, 51, 74). The well-characterized pathway found in E. coli is present in some bacterial species (48, 74), whereas other bacteria, fungi, and plants possess a recently discovered pathway that is largely uncharacterized (23, 48, 51). The same salvage pathway seems to be present in all organisms (16, 19, 21, 30, 74).
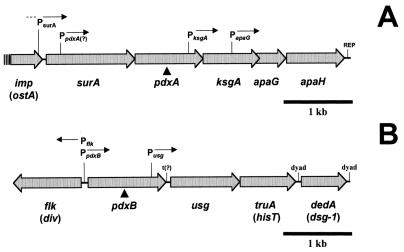
Most of the genes involved in the de novo biosynthesis of PLP in E. coli are located in multifunctional superoperons (74). The arrangements of the pdxA and pdxB genes that are the subject of this report are depicted in Fig. 1. pdxA encodes NAD+-dependent 4-hydroxythreonine 4-phosphate dehydrogenase, which synthesizes a substrate that is condensed with 1-deoxy-d-xylulose 5-phosphate by the PdxJ enzyme to form pyridoxine (PN) phosphate (PNP), which is the first B6 vitamer synthesized by the de novo pathway (11, 12, 26, 38, 77, 78). pdxA is located in a complex superoperon with surA (periplasmic peptidyl-prolyl isomerase; stationary-phase survival) and ksgA 16S rRNA methyltransferase (55, 66, 73; Fig. 1A). surA and pdxA are cotranscribed, and insertion mutations in surA cause PN auxotrophy (66). The transcription start and promoter upstream of surA-pdxA were preliminarily mapped at PsurA (Fig. 1A), and there is evidence for a minor promoter at PpdxA (Fig. 1A) (R. Kolter, personal communication). Work is under way to definitively map the promoters for the pdxA superoperon and determine which promoter(s) is regulated. About half of the amount of the ksgA transcript detected in exponentially growing cells is present in a surA-pdxA-ksgA cotranscript (55). The rest of the ksgA transcripts arise from an internal promoter within the pdxA reading frame (PksgA; Fig. 1A). It was reported that synthesis of ksgA depends on translation coupling to the upstream reading frame, which turned out to be pdxA (72, 73). It is unknown how such coupling, if necessary, could be accomplished for transcripts that initiate at PksgA.
FIG. 1.
Structures of the pdxA and pdxB superoperons. (A) pdxA superoperon. The site of insertion of the pdxA::mini-MudI lacZ element in strain NU1187 (determined by restriction analysis) (55) is indicated by the solid triangle. The product of the imp gene is involved in organic solvent tolerance (1), and surA encodes a periplasmic peptidyl-prolyl isomerase that assists in the proper folding of outer membrane proteins (42, 57, 66). The likely position of the PsurA promoter is indicated (66). The probe for detection of pdxA-ksgA cotranscripts and ksgA-specific transcripts covers the region between the BglI and PvuII sites (see Materials and Methods) (55). (B) pdxB superoperon. The site of insertion of the pdxB::mini-MudII lacZ translational fusion element in NU1702 (solid triangle) was determined to be in frame by DNA sequencing (59). The probes for detection of pdxB and Pflk cover the region between the HindIII and TaqI sites (see Materials and Methods; 59).
pdxB encodes a pivotal 4-phosphoerythronate dehydrogenase that is the first enzyme utilized solely by the de novo PLP biosynthetic pathway (40, 77). The pdxB gene is the first gene in a complex superoperon that encodes usg (paralog of asd, which encodes aspartate semialdehyde dehydrogenase), truA (hisT; encodes tRNA modification enzyme pseudouridine synthase I), and dsg (encodes a putative inner membrane protein of unknown function) (Fig. 1B) (2, 3). About half of the amount of usg-truA-dsg transcripts originates from an internal promoter located within the pdxB coding region (Pint; Fig. 1B) in exponentially growing E. coli (2, 3). pdxB is divergently transcribed from flk, which acts as a positive regulator that couples translation of the FlgM regulator to flagellar ring assembly (35, 59). The −10 regions of the PpdxB and Pflk promoters overlap; however, no link between pdxB and flk regulation and functions has been reported (35, 59).
To fully understand the control and integration of PLP biosynthesis, we have been determining whether the individual PLP biosynthetic genes are subject to forms of metabolic regulation. Previously, we reported that the serC (pdxF) gene is positively regulated by the Lrp protein and negatively regulated by the cyclic AMP receptor protein (43). Here we report that transcription of the pdxA and pdxB genes is positively growth rate regulated by two different mechanisms. The Fis regulatory protein mediates pdxA regulation and part of ksgA regulation, whereas pdxB is regulated by an unknown mechanism. We also report that pdxJ expression is constant with respect to growth rate.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains, plasmids, and phages used in this study are listed in Table 1. Markers were moved between strains by generalized transduction with P1 kc or P1 vir bacteriophage as previously described (46). Recombinant plasmids were constructed by using standard techniques (4). Plasmid clones of the pdxA upstream flanking region were derived from Kohara phage clone 8D2 (37), which was provided by K. Rudd. Insertional mutagenesis in plasmids with the mini-MudI (Kmr) and mini-MudII (Kmr) fusion elements was performed as previously described (14). Insertion sites in plasmids were determined by restriction analysis and by DNA sequencing for translational fusions obtained with mini-MudII (Kmr). Plasmids used for double stranded sequencing were prepared by a combined alkaline lysis and polyethylene glycol precipitation method (4). Kanamycin-resistant insertions were crossed from linearized plasmids into the chromosome of recBC sbc mutant JC7623 as described previously (3).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source or reference |
|---|---|---|
| Strains | ||
| AL0875 | thi-1 leu6 lacY1 lacIZ Δ(mlu) supE44 tonA21 rpsL rfbD1 lipB::mini-Tn10 | 54 |
| CAG12093 | MG1655 car-96::Tn10 | 62 |
| CF1693 | MG1655 ΔrelA251::kan ΔspoT207::cat; Cmr Kmr | M. Cashel collection |
| JC7623 | arg ara his leu pro recB21 recC22 sbcB15 thr | 55 |
| RJ1529 | MG1655 fis::767 Δ(lac-pro) ara str | 5 |
| NK7049 | ΔlacX74 galOP308 rpsL Smr | 61 |
| NU426 | W3110 prototroph | C. Yanofsky collection |
| NU816 | Single colony isolate of W3110 tnaA2 ΔlacU169 | C. Yanofsky collection |
| NU1187 | NU816 pdxA::mini-Mud-1-1 Kmr | 55 |
| NU1700 | JC7623 pdxB::mini-MudII Kmr | Transformation with linearized pNU250; this study |
| NU1702 | NU816 pdxB::mini-MudII Kmr | NU816 × P1 kc NU1700 |
| TX1892 | NU816 ΔrelA251::kan; Kmr | NU816 × P1 kc (CF1693) This study |
| TX2093 | NU1187(pNO2661, pNO2696) | This study |
| TX2094 | NU1187(pNO1301) | This study |
| TX2095 | NU1187(pNO1302) | This study |
| TX2096 | NU1187(pBR322) | This study |
| TX2169 | NU1187(pNU122) | This study |
| TX2171 | NU1187(pUC19) | This study |
| TX2243 | NU1187(pBR322, pACYC184) | This study |
| TX2254 | NU1187(pNU276) | This study |
| TX2269 | NU1187(pPR274) | This study |
| TX2283 | NU1702(pTX291) | This study |
| TX2357 | NU1187(pTX288) | This study |
| TX2737 | NU426(pALS13) | This study |
| TX2739 | NU426(pALS14) | This study |
| TX3333 | NU426 fis::767 | NU426 × P1 vir (RJ1529) |
| TX3338 | NU426 lipB::mini-Tn10; Tcr | NU426 × P1 vir (AL0875) |
| TX4080 | NK7049 with pdxJ-lacZ protein fusion λRS641; Smr | This study |
| TX4082 | NK7049 with pdxJ-lacZ protein fusion λRS643; Smr | This study |
| Plasmids | ||
| pACYC184 | Replicon P15A; Cmr Tcr | 46 |
| pALS13 | Ptac::relA" (445 N-terminal amino acids of relA) lacIq | 64 |
| pALS14 | Ptac::relA" (331 N-terminal amino acids of relA) lacIq, Apr | 64 |
| pBR322 | Replicon ColE1; Apr Tcr | 46 |
| pGEM3Z | pUC18 derivative with phage T7 and SP6 promoters | Promega |
| pNO1301 | pBR322 (rrnB+); Apr | 33 |
| pNO1302 | pNO1301 with 2.45-kb deletion in rrnB; Apr | 33 |
| pNO2661 | pACYC184 (RNA polymerase β and β") Cmr | 8 |
| pNO2696 | pBR322 (RNA polymerase α and σ70) Apr | 8 |
| pNU122 | pUC19 (pdxA+ksgA+apaG+apaH+); Apr | 55 |
| pNU180 | pGEM3Z (386-bp pdxA-ksgA region); Apr | 59 |
| pPR274 | mini-F" replicon; CmrtrpE+ | 47 |
| pNU276 | pBR322 (pdxA+ plus upstream region) 5.0-kb EcoRI fragment from Kohara phage clone 8D2; Apr | This study |
| pTX288 | pPR274 (pdxA+) same insert as plasmid pNU276; Cmr | This study |
| pTX291 | pPR274 (pdxB+) 4.4-kb insert containing entire pdxB operon; Cmr | This study |
| Phage λ-borne lacZ fusions | ||
| λRS641 | Δrnc-36 era+recO+pdxJ"::lacZ" | 45 |
| λRS643 | Δrnc-76 Δrnc-36 era+recO+pdxJ"::"lacZ | 45 |
Apr, Cmr, Kmr, and Tcr indicate resistance to ampicillin, chloramphenicol, kanamycin, and tetracycline, respectively.
Media and culture conditions.
E. coli strains were grown in Luria-Bertani (LB) medium or minimal 1×E media prepared as previously described (2). Minimal salts-glucose medium contained 1×E salts, 0.01 mM FeSO4, and 0.4% (wt/vol) glucose. α-Methylglucoside was added at a 5:1 ratio to glucose. Minimal salts-glycerol medium (MMGly) contained 0.6% (wt/vol) glycerol, and minimal salts-acetate medium contained 0.6% (wt/vol) potassium acetate. Enriched minimal salts-glucose medium (EMMG), enriched minimal salts-glycerol medium, enriched minimal salts-acetate medium, and enriched minimal salts medium all contained amino acids supplied in the form of 0.5% (wt/vol) vitamin assay Casamino Acids (Difco Laboratories, Detroit, Mich.) plus 0.1 mM l-tryptophan. When indicated, PN was supplemented at 5 × 10−7 M (17). When required, ampicillin and kanamycin were added to LB or minimal media at 50 and 25 μg/ml, respectively, and chloramphenicol was added at 25 and 12.5 μg/ml, respectively.
β-Galactosidase enzyme assays.
Strains were grown in different culture media to vary growth rates to cell densities of about 70 Klett units (≈4 × 108 cells/ml) on a Klett-Summerson colorimeter with a number 66 red filter, and cell extracts were prepared for determination of β-galactosidase activity as described previously (46). β-Galactosidase specific activities were expressed as Miller units (46).
RNA isolation and transcript analysis.
Bacteria were grown at 37°C with shaking in LB or minimal medium to cell densities of about 70 Klett units, except under the conditions of relA overproduction or amino acid starvation (see below). Total cellular RNA isolation and RNase T2 protection assays for transcript analysis were done as described previously (70). Plasmid template pNU180 was used for synthesis of an RNA probe that detects pdxA-ksgA cotranscripts and ksgA-specific transcripts (55). This probe covers the region between BglI and PvuII shown in Fig. 1A. pNU200 was used for the pdxB and Pflk probes (59). These probes cover the region between HindIII and TaqI shown in Fig. 1B. The pdxB probe was synthesized from linearized plasmid by using SP6 RNA polymerase, and the Pflk probe was synthesized from linearized plasmid by using T7 RNA polymerase. All RNA probes were synthesized by using the Riboprobe Gemini system in accordance with the manufacturer's instructions. For RNA preparations used in transcript half-life (t1/2) determinations, cells were grown in the indicated medium and rifampin was added to a final concentration of 0.5 mg/ml. Samples were taken immediately prior to rifampin addition (t = 0) and from 2 to 10 min after addition of the drug. Transcript analysis was performed on each sample, and t1/2s of mRNAs were calculated from the slope of a least-squares regression line of the amount of RNA remaining plotted semilogarithmically against time after rifampin addition.
Amino acid starvation.
E. coli prototroph strain NU426 or NU816 and strain TX1892 (NU426 ΔrelA) (Table 1) were grown in LB medium supplemented with 0.4% (wt/vol) glucose (LB-Glc) at 37°C to an optical density at 420 nm of 0.65 (15). Amino acid starvation was induced by the addition of 1 mg of serine hydroxymate per ml, which competitively inhibits seryl-tRNA synthetase and provokes the stringent response in wild-type E. coli (15). Samples for transcript analysis were taken 20 min after serine hydroxymate addition (15).
RelA overexpression.
TX2737 carrying pALS13 was used to overexpress the RelA protein, and TX2739 (Table 1) was used as a control. pALS13 encodes a truncated form of relA (the first 455 of the 743 amino acids of RelA) under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Ptac promoter. The truncated form of RelA is functional for guanosine tetraphosphate (ppGpp) synthesis independent of ribosomes (60, 64). pALS14, which encodes a form of RelA that is more truncated (331 amino acids) and is not functional for ppGpp synthesis, served as a control. Cells were grown to an optical density at 600 nm of 0.4 in glucose minimal medium supplemented with 0.5% amino acids, and RelA expression was induced with 100 μM IPTG. Samples for transcript analysis were taken immediately prior to IPTG addition and 20 min following IPTG addition.
Normalization of growth rate-regulated parameters.
Specific activities were converted to rate of enzyme accumulation per genome in accordance with the equation of Tribe et al. (68), q = μln2/60 × S × P/G, where P/G is the amount of protein per genome, extrapolated from the data of Bremer and Dennis (10), and S is specific activity. The term μln2/60 × S is equivalent to dP/dt (10), so q is equivalent to the instantaneous rate of accumulation of a protein normalized to the total DNA of the E. coli genome. To relate q to the relative gene frequency with respect to μ, Fg was first calculated as follows: Fg = cln2/60 × μ × 2(c)(μ/60)(1−x) × 1/2(c)(μ/60) − 1, where c is 45 min, which is the transit time for the chromosome replication fork (10), and x is the relative distance of the particular gene from the replication origin. qg was then calculated as follows: qg = q/Fg × 4.22 × 10−2 mg. The amount of a given mRNA is related to gene frequency as follows: r = amount of specific mRNA/R × R/G, where R is the total amount of RNA and R/G is the total amount of RNA per genome, extrapolated from the data of Bremer and Dennis (10). Then, rg = r/Fg × 4.22 × 10−2 mg.
RESULTS
Growth rate-regulated expression of lacZ fusions to pdxA and pdxB.
To analyze the regulation of pdxA and pdxB, we constructed lacZ fusions in pdxA and pdxB at their native chromosomal positions in a prototrophic Δlac strain of E. coli K-12. Strains NU1187 (55) and NU1702 contain a pdxA::mini-MudI lacZ transcriptional fusion and a pdxB::mini-MudII lacZ translational fusion, respectively, at the positions indicated in Fig. 1. We grew strains NU1187 and NU1702 in different media to analyze the expression of pdxA or pdxB in relation to the cellular growth rate. The corresponding β-galactosidase specific activities under these growth conditions were determined (Fig. 2). The expression of the pdxA and pdxB genes, as measured by lacZ fusions, increased in direct proportion to the growth rate up to 1.3 doublings per hour. The specific activities from the pdxB-lacZ translational fusion and, to a lesser extent, the pdxA-lacZ transcriptional fusion exhibited an apparent repression when cells were grown in rich LB medium, which supported a growth rate of approximately two doublings per hour (Fig. 2). This apparent reduction disappeared upon normalization (see below).
FIG. 2.
Growth rate regulation of pdxA- and pdxB-lacZ fusions in strains NU1187 (open symbols) and NU1702 (closed symbols), respectively. Cells were grown exponentially in minimal media containing different carbon sources (circles) or in LB (triangles) (see Materials and Methods). Specific activities of β-galactosidase were determined (see Materials and Methods) and plotted as a function of the growth rate expressed as doublings per hour (μ). The results shown are from at least three independent cultures at each growth rate. The error bars represent the standard error of the mean. Lack of error bars indicates that the standard error of the mean was negligible.
To find out how common positive growth rate regulation is among genes involved in PLP biosynthesis, we determined the response of pdxJ expression to growth rate changes. pdxJ is in a complex superoperon that includes the following (in order): rnc, era, recO, pdxJ, and acpS (41, 65). The major promoter for the operon is before the rnc gene (Prnc) with a minor promoter (PpdxJ) for pdxJ and acpS located at the distal end of recO (45). Matsunaga et al. (45) constructed pdxJ-lacZ protein fusions expressed from both Prnc and PpdxJ (strain TX4080) or from PpdxJ alone (strain TX4082), integrated into the E. coli chromosome at attλ. β-Galactosidase specific activities from strain TX4080 or TX4082 were constant within experimental error (scatter between 72 and 103 or 51 and 79 Miller units, respectively) over the eightfold range of growth rates between enriched minimal salts-acetate medium and LB (data not shown). These results were from at least three independent cultures grown in each of the media tested. Thus, we could not detect positive regulation of pdxJ expression with increasing growth rate.
Growth rate regulation of pdxA, ksgA, and pdxB transcript amounts.
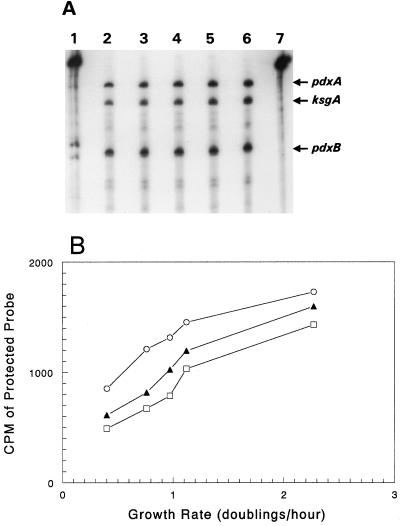
We performed RNase T2 transcript protection assays (70) to determine whether the observed changes in fusion-specific activities were matched by corresponding changes in pdxA and pdxB transcript amounts in cells grown at different rates. Equal amounts of total RNA from cells grown at different rates were probed for pdxA and pdxB transcripts, and the steady-state amounts of pdxA and pdxB mRNAs were compared. The probe used to detect pdxA-ksgA cotranscripts spans the 3" portion of pdxA gene and the 5" portion of the downstream ksgA gene and results in a 386-nucleotide (nt) full-length protected band (Fig. 1A and 3). The probe also detects a shorter protected band of 339 nt that corresponds to ksgA-specific transcripts that arise from the ksgA internal promoter (PksgA; Fig. 1A) (55). The pdxB probe covers the promoter and transcription start of pdxB and results in a single 252-nt protected band (Fig. 1B and 3) (59). A representative experiment showing quantitation of steady-state transcript amounts is shown in Fig. 3B. The amounts of the pdxA-ksgA cotranscript and ksgA- and pdxB-specific transcripts increased relative to the RNA amount in direct proportion to the growth rate, except at the highest growth rate in rich LB medium (Fig. 3B).
FIG. 3.
Amounts of pdxA-ksgA cotranscript and ksgA- and pdxB-specific transcripts in exponentially growing E. coli K-12. (A) Autoradiograph of RNase T2 protection assays. Equal amounts (50 μg) of RNA prepared from cultures of prototrophic strain NU426 grown in MMGly (lane 2), minimal salts-glucose medium (lane 3), enriched minimal salts-glycerol medium (lane 4), EMMG (lane 5), and LB (lane 6) were probed as described in Materials and Methods. Positions of bands corresponding to the pdxA-ksgA cotranscript (labeled pdxA) and the ksgA- and pdxB-specific transcripts are indicated. Lanes 1 and 7 show the undigested pdxA-ksgA and pdxB probes, respectively. The results were from at least three independent cultures at each growth rate. (B) Counts per minute corresponding to the pdxA-ksgA cotranscript (filled triangles), the ksgA-specific transcript (open rectangles), and the pdxB-specific transcript (open circles) were determined from gels such as the one in panel A by direct counting and plotted as a function of the growth rate.
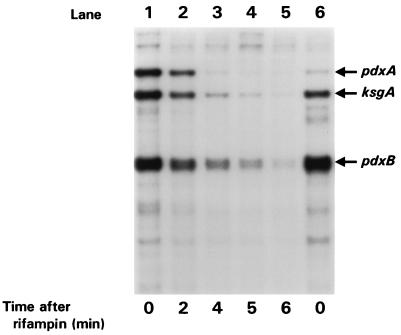
The changes in the amounts of steady-state pdxA-ksgA cotranscript and ksgA-specific and pdxB-specific transcripts at different growth rates could reflect changes in transcript stability, as has been demonstrated for the ompA and cat transcripts (50). Therefore, we analyzed the t1/2s of the pdxA-ksgA cotranscript and ksgA-specific and pdxB-specific transcripts with RNase T2 protection assays by using RNA prepared after rifampin addition to cultures grown at four different growth rates. Figure 4 shows a representative experiment with LB-Glc medium. The pdxB-specific and ksgA-specific transcripts exhibited a typical pattern for E. coli mRNAs of rapid exponential decay (Fig. 4). The stability of the pdxB-specific (t1/2 ≈ 1.1 min) and ksgA-specific (t1/2 ≈ 0.6 min) transcripts did not vary with the growth rate (data not shown). Thus, growth rate-dependent regulation of pdxB and ksgA expression is likely to occur at the level of transcription initiation. We could not reach a conclusion for the pdxA-ksgA cotranscript because it was extremely unstable. Between 2 and 4 min after rifampin addition, the amount of the pdxA-ksgA transcript became undetectable and growth rate-dependent variations in the stability of the short-lived pdxA message may not have been detected.
FIG. 4.
Determination of the t1/2s of the pdxA-ksgA cotranscript and ksgA- and pdxB-specific transcripts of E. coli K-12 growing exponentially in LB-Glc. Equal amounts of RNA isolated from NU426 growing in LB-Glc before (lane 1) and at various times after (lanes 2 to 5) rifampin addition were probed as described in Materials and Methods. Positions of bands corresponding to the pdxA-ksgA cotranscript and the ksgA- and pdxB-specific transcripts are indicated. Analogous experiments were performed for NU426 grown at additional growth rates (data not shown; Results). Lane 6, amounts of the same transcripts from NU426 subjected to starvation for amino acids (Results). The results shown are from at least three independent cultures at each growth rate.
Normalization of gene expression.
We wanted to compare and reconcile the determinations obtained for gene fusions and direct transcript amounts. Since a given amount of mRNA can be repeatedly translated, it is not meaningful simply to compare the amounts of transcripts and proteins present in exponentially growing cells (10, 68). Therefore, we applied a method of normalization (see Materials and Methods) to determine the amount of mRNA synthesized per gene and the rate of protein synthesis per gene in bacteria growing at different rates.
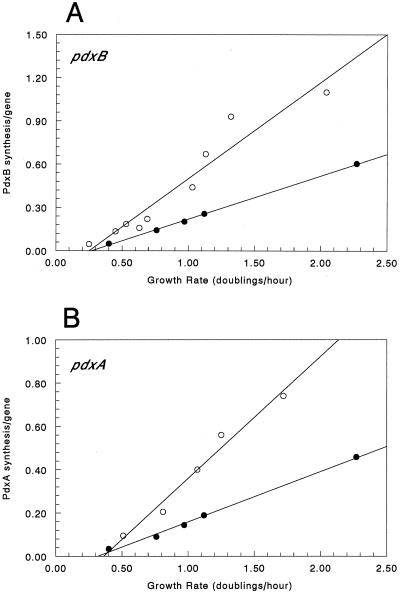
There is no simple assay for the activity the PdxB enzyme, so we relied on the β-galactosidase specific activities of a pdxB-lacZ translation fusion to reflect the PdxB level. We first assumed that transcription of the pdxB-lacZ fusion is the primary target for growth rate regulation. We normalized the specific activities from the pdxB-lacZ fusion and the amount of the pdxB-specific transcript on a per-gene basis in accordance with the equations of Tribe et al. (68) in order to compare the data in Fig. 2 and 3 directly. As shown in Fig. 5A, the curve for the rate of enzyme accumulation per gene for the pdxB-lacZ fusion is about twofold steeper than that of the corresponding curve for the amount of pdxB mRNA synthesized per gene. This difference corresponds to and likely reflects the increase in the overall translational efficiency with increasing growth rate over the growth rates used in these experiments (10).
FIG. 5.
pdxA and pdxB gene expression normalized per gene. The data from Fig. 2 was expressed as rates of protein accumulation per gene (open circles) (see Materials and Methods). The data from Fig. 3B was expressed as the amount of mRNA per gene (closed circles) (see Materials and Methods). Panels: A, pdxB gene expression normalized per gene; B, pdxA gene expression normalized per gene.
A similar analysis was performed for pdxA. In this case, a pdxA-lacZ transcription fusion was assayed, so this analysis tested whether expression of the pdxA-lacZ fusion was truly correlated with pdxA transcript accumulation. Similar to the case for pdxB, the curve for the rate of amount of enzyme accumulation per gene for the pdxA-lacZ fusion is about twofold steeper than that of the corresponding curve for the amount of pdxA mRNA synthesized per gene (Fig. 5B).
Figure 2 shows that the β-galactosidase specific activities encoded by the pdxB-lacZ and pdxA-lacZ fusions apparently failed to keep up with the growth rate change at the highest growth rate. When normalized on a per-gene basis, expression of both the pdxB and pdxA genes is proportional to the growth rate at all of the growth rates tested (Fig. 5). Thus, the apparent repression at the highest growth rate (Fig. 2) disappeared following normalization (Fig. 5).
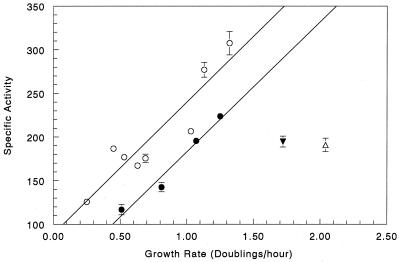
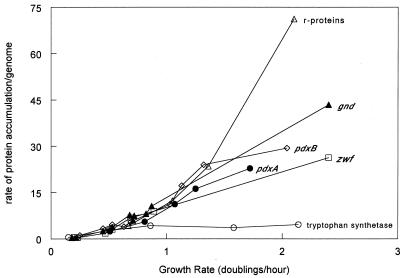
We compared the normalized growth rate regulation of pdxA and pdxB with that of other genes that have been reported to be growth rate regulated. For the following proteins, we used the data in the references cited to calculate the accumulation rate per genome (see Materials and Methods), which was plotted against the growth rate (μ) (Fig. 6) on the basis of the references cited: ribosomal protein (r-protein) gene expression (20), PdxB (from β-galactosidase activity of a translational fusion [Fig. 5A]), PdxA (from β-galactosidase activity of a transcriptional fusion [Fig. 5B]), 6-phosphogluconate (6PGD; encoded by gnd) (75), glucose 6-phosphate dehydrogenase (G6PD, encoded by zwf) (75), and tryptophan synthetase (56). The r-protein genes (20) and metabolic enzymes are found at several locations in the E. coli chromosome. Therefore, all expression was normalized on a per-genome basis, which gave only slightly different results from normalization on a per-gene basis. For example, the term Fg varies from 1.09 to 1.3 for μ = 0.5 to 1.7 for pdxA expression, whereas Fg is virtually invariant for pdxB over an even wider range of growth rates.
FIG. 6.
Rate of accumulation of several proteins normalized per genome (see Materials and Methods) plotted as a function of the growth rate (μ) in doublings per hour. Symbols: PdxB, open triangles; r-proteins, open triangles; tryptophan synthetase (TrpAB), open circles; PdxA, filled circles; Gnd, filled triangles; Zwf, open rectangles.
Normalization shows that growth rate regulation of pdxA, pdxB, gnd, and zwf (Fig. 6) extends over a much larger range of growth rates than is apparent from a simple comparison of enzyme specific activities (Fig. 2) (75). In cells grown in LB medium, the specific activities of pdxA, pdxB, gnd, and zwf apparently decrease compared to the specific activities from cells grown in glucose minimal medium (Fig. 2) (75). When normalized on a per-genome basis, pdxA, pdxB, gnd, and zwf gene expression is proportional to the growth rate at all of the growth rates tested (Fig. 6). Thus, the apparent repression at the highest growth rates (Fig. 2) (75) disappears following normalization (Fig. 6). Growth rate regulation of the r-protein genes is even greater than that of pdxA, pdxB, gnd, and zwf (Fig. 6). Tryptophan synthetase is far less affected by the growth rate than are pdxA, pdxB, gnd, and zwf (Fig. 6).
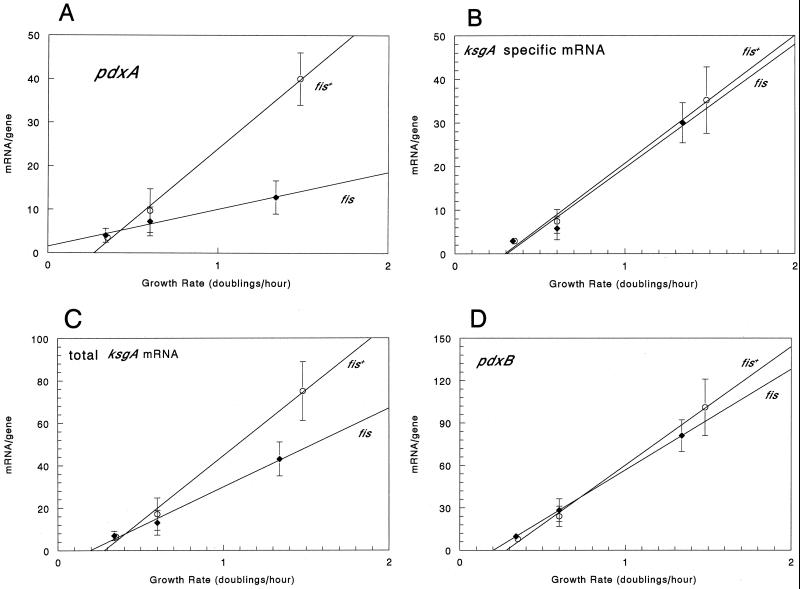
A fis mutation abolished the growth rate regulation of pdxA-ksgA but not that of pdxB.
We tested the effect of a null mutation in the fis gene on the growth rate-dependent regulation of pdxA and pdxB. The Δfis-767::kan allele (34) was transduced into NU426 by P1 transduction. The resulting strain, TX3333, had similar growth rates in three different minimal media, including acetate, glycerol, and glucose plus amino acids compared to that of NU426 (Fig. 7). The increase in the amount of pdxA-ksgA cotranscript as a fraction of the total RNA from TX3333 cells was only 25% of that seen in the fis+ parent strain as a function of the growth rate (Fig. 7A). In contrast, the increase, along with that of the growth rate, of the amounts of ksgA- and pdxB-specific transcripts was the same in the fis+ parent and fis mutant cells (Fig. 7B and D). Thus, only about half of the total amount of the ksgA transcript was subject to control by fis (Fig. 7C).
FIG. 7.
Effect of a fis mutation on the growth rate regulation of the amount of the pdxA-ksgA cotranscript, the amount of the ksgA- and pdxB-specific transcripts, and the total amount of the ksgA transcript. Total RNA isolated from NU426 (open symbols) or TX3333 (NU426 fis) (closed symbols) growing exponentially in minimal salts-acetate medium, MMGly, or EMMG was probed as described in Materials and Methods. Counts per minute corresponding to individual bands were expressed as the amount of mRNA per gene (see Materials and Methods), and the average amount of mRNA per gene for each transcript was calculated from three and four independent experiments for NU426 and TX3333, respectively, and plotted against the growth rate. Standard deviations of the mean are shown. Panels: A, pdxA-ksgA cotranscript; B, ksgA-specific transcript; C, total ksgA transcript (sum of panels A and B); D, pdxB-specific transcript.
ppGpp concentrations influence pdxA and ksgA, but not pdxB, transcript amounts.
We tested the effect of high levels of ppGpp on pdxA, ksgA, and pdxB expression by inducing the stringent response. We determined the amounts of the pdxA-ksgA cotranscript and ksgA- and pdxB-specific transcripts in relA+ (NU426) and ΔrelA251 (TX1892) strains grown in LB-Glc medium before and after amino acid starvation induced by the addition of 1 mg of serine hydroxymate per ml (15). The relA gene is necessary for the stringent control of rRNA (reviewed in reference 13).
A representative experiment comparing transcript levels is shown in Fig. 4 (lanes 1 and 6), and results from several experiments are averaged in Table 2. The amount of the pdxB-specific transcript was unaffected by amino acid starvation (Table 2). In contrast, the pdxA-ksgA cotranscript or ksgA-specific transcript amount was strongly (6-fold) or moderately (≈1.5-fold) decreased, respectively, during amino acid starvation in the relA+ parent strain. The decrease in the pdxA-ksgA cotranscript or ksgA-specific transcript amount was independent of or dependent on relA function, respectively (Table 2). The relA-independent decrease in the amount of the pdxA-ksgA cotranscript may simply reflect the decrease in the growth rate caused by amino acid limitation (Fig. 2 to 5) and was not examined further. The net effect of this regulatory pattern on the total ksgA transcript amount is a moderate stringent response of about 2.3-fold (Table 2).
TABLE.
2. Relative changes in transcript amounts of the pdxB-, flk-, and ksgA-specific transcripts and the pdxA-ksgA cotranscript in exponentially growing and amino acid-starved E. coli K-12
| Transcripta | Ratio of transcript amounts (unstarved/amino acid starved)b
|
|
|---|---|---|
| relA+ parent strainc | ΔrelA mutantd | |
| pdxB specific | 0.8 ± 0.1 | 0.8 ± 0.2 |
| flk specific | 1.7 ± 0.4 | 1.0 ± 0.5 |
| pdxA-ksgA cotranscript | 5.9 ± 0.8 | 6.6 ± 1.7 |
| ksgA specific | 1.6 ± 0.2 | 0.6 ± 0.1 |
| Total ksgA | 2.3 ± 0.3 | 0.8 ± 0.1 |
See Fig. 1 and the text for the locations and functions of genes.
Cells were grown in LB medium supplemented with 0.4% glucose at 37°C to an OD420 of 0.65 with shaking. Amino acid starvation was caused by addition of 1 mg of serine hydroxymate per ml (see Materials and Methods) (67). Samples for RNase T2 protection assays were taken immediately prior to serine hydroxymate addition (unstarved) and 20 min after serine hydroxymate addition (amino acid starved) (see Materials and Methods). Means with standard errors were obtained from several independent experiments
The relA+ strain was either NU426 or NU816 (Table 1), which gave similar results.
The ΔrelA strain was NU1892 (NU816 ΔrelA251) (Table 1).
We attempted to determine the effect of amino acid starvation on transcript t1/2s. However, we found that there was a marked lag after rifampin addition before decay of the transcripts became apparent (data not shown). This lag varied in length between experiments; consequently, transcript t1/2s could not be determined reliably following amino acid starvation. The permeability of E. coli cells to many molecules is altered during amino acid starvation (13), and it seems possible that amino acid starvation induced by serine hydroxymate decreases the permeability of E. coli for rifampin.
We also mimicked induction of the stringent response by overproducing the RelA protein under the control of the Ptac promoter in strain NU426 (pALS13 and pALS14, Table 1; data not shown; 60, 64). Addition of 100 μM IPTG has been shown to cause rapid accumulation of ppGpp in analogous strains approximately to the level detected during the stringent response (60, 64). Induction of RelA overexpression quickly inhibits the growth rate (60), which we observed for strain NU426 (data not shown). When RelA was overexpressed, the amounts of the pdxA-ksgA cotranscript and ksgA- and pdxB-specific transcripts changed in ways similar to those seen during amino-acid starvation (data not shown; Table 2).
RelA overexpression extended the t1/2s of the ksgA-specific transcript (t1/2 ≈ 1.2 min instead of 0.6 min) and the pdxA-ksgA cotranscript so that it could be determined (t1/2 ≈ 1.4 min). In contrast, the t1/2 of the pdxB-specific transcript remained about 1.1 min following RelA overexpression. In this experiment, transcript decay curves appeared normal and did not contain lags (data not shown). Thus, the relA-independent decrease in the pdxA-ksgA transcript amount may involve decreased transcription initiation that is partially offset by increased transcript stability. On the other hand, we cannot rule out complex differential effects of rifampin uptake that would affect the quantitation of the pdxA-ksgA cotranscript differently from that of the pdxB-specific transcript.
Other putative effectors did not influence pdxA and pdxB growth rate regulation.
We tested several other putative effectors of growth rate regulation to learn whether they influence pdxA and pdxB expression. lipB was proposed as a negative effector of the growth rate regulation of the dam gene (71). lipB encodes a lipoyltransferase that transfers endogenously synthesized lipoate to apoproteins (49). However, a lipB null mutation did not influence the amounts or growth rate regulation of the pdxA-ksgA cotranscript or the ksgA- and pdxB-specific transcripts (Table 1; data not shown). We tested whether RNA polymerase is limiting within the cell, resulting in some form of promoter competition (32). However, the growth rate regulation of the pdxA-lacZ fusion (Fig. 2) was not affected by plasmids (pNO2661 and pNO2096) expressing extra copies of the α, β, β", and σ70 subunits or RNA polymerase (Table 1; data not shown; 8). Similarly, growth rate regulation of the pdxA-lacZ fusion was not affected by the presence of a plasmid (pNO1301) containing the highly expressed rrnB operon (Table 1; data not shown).
Finally, we were unable to detect autoregulation of pdxA or regulation of pdxA or pdxB in response to added B6 vitamers such as PN. In these experiments, the pdxA+ gene, including the upstream promoter region, was cloned into a single-copy mini-F" vector (pTX288) or multicopy vectors (pNU122 and pNU276; Table 1). Transformation of these plasmids into the corresponding pdxA-lacZ (NU1187) chromosomal fusion strain resulted in prototrophy for PN (data not shown). Expression of pdxA+ from single-copy or multicopy plasmids did not significantly affect the expression level or the growth rate regulation of the pdxA-lacZ fusion (data not shown). In an analogous experiment, pdxB+ cloned into a single-copy mini F" vector (pTX291, Table 1) did not affect the expression or growth rate regulation of the pdxB-lacZ fusion NU1702 (data not shown). Addition of exogenous PN to 5 × 10−5 M, which was about 100-fold more than that required to support the full growth of auxotrophs, did not affect the expression of the pdxA- or pdxB-lacZ fusion (data not shown).
DISCUSSION
We report here that expression of the pdxA, ksgA, and pdxB genes is strongly regulated by cellular growth rate changes (Fig. 2 and 3). Expression of PdxA and PdxB normalized as the rate of accumulation per gene increased 6-fold and 20-fold over 2.5- and 5-fold ranges of increasing growth rates, respectively (Fig. 5). The levels of all E. coli proteins are coordinated in some way with the cellular growth rate. With two-dimensional gel electrophoresis, it was demonstrated that three basic patterns describe the relationship between the cellular growth rate and the relative amount of individual proteins (52). One pattern includes proteins that increase with growth rate increases over a wide range of growth rates, and PdxA and PdxB are within this class. As a fraction of the total protein, these proteins increase proportionately as the growth rate increases. The total amount of protein per cell itself increases as the growth rate increases (10). Thus, if the level of a protein increases as a fraction of the total protein with an increase in the growth rate, the absolute rate of accumulation of that protein is equal to at least the square of the increase in the growth rate. As a fraction of the total protein, both PdxA and PdxB increased when the growth rate increased (Fig. 2) and the absolute rate of accumulation per gene of both PdxA and PdxB increased well beyond the increase in the growth rate (Fig. 5). Other proteins in this class comprise a large fraction of the cellular bulk protein and include proteins involved in translation, such as r-proteins, tRNA synthetases, and elongation factors. The fraction of the total protein of those proteins varied from 20 to 55% over a fivefold range of growth rates. When we normalized these r-proteins as the absolute rate of accumulation per genome, we noticed that the rate of accumulation of these proteins was actually more than the square of the increase in the growth rate (Fig. 6). The amounts of rRNA and most tRNAs are also positively regulated over a wide range of growth rates (10).
The change in the rate of accumulation of the PdxA- and PdxB-LacZ fusion proteins per gene was greater than the change in pdxA and pdxB transcript amounts with changes in the growth rate (Fig. 5). This result is consistent with an increase in the overall cellular translational efficiency with an increase in the growth rate (10). Thus, the pdxA and pdxB transcripts seem to take advantage of the increased ribosome capacity that accompanies a growth rate increase.
We observed that the specific activity of β-galactosidase from the pdxA- and pdxB-lacZ fusions decreased at the highest growth rate in rich LB medium (Fig. 2). A similar pattern of apparent repression had been reported for 6PGD (Zwf), G6PD (Gnd) (75), and tryptophan synthetase (TrpAB) and was referred to as metabolic regulation (56). However, when normalized, the rates of accumulation of 6PGD, G6PD, PdxA, and PdxB per genome all still increase proportionally with the growth rate (μ) in rich media, whereas that of TrpAB levels off. Thus, normalization analysis revealed differences in gene expression with growth rate between the trp operon genes and the other genes described above that were not apparent by simply comparing the specific activities at different growth rates.
We attempted to find mechanisms that underlie the growth rate regulation of pdxA, ksgA, and pdxB. We found that growth rate regulation of the amount of pdxA-ksgA cotranscript, but not that of the pdxB- and ksgA-specific transcripts, was mediated by the Fis protein (Fig. 7). Fis is a small, basic, nucleoid-associated protein that functions as a dimer and binds to and bends DNA (25). Fis mediates a wide range of cellular processes, including site-specific recombination, positive and negative regulation of the transcription of many protein-encoding genes, and growth rate regulation of stable RNA genes (25, 76). Fis-dependent activation of rrn operons is not growth rate dependent and appears to be dispensable (6). However, under special circumstances, Fis can become the growth rate-dependent regulator of rrn operons (7). Fis mediates the positive growth rate regulation of 4.5S RNA but not that of M1 RNA (22). Fis positively and negatively regulates different tRNA operons with growth rate (24, 58).
The positive regulation of the pdxA-ksgA cotranscript amount by Fis with growth rate (Fig. 7) resembles that of tRNA operons (24, 58). We do know whether this regulation is direct or indirect. The promoter(s) for pdxA is located somewhere upstream of pdxA (Fig. 1A) (55). surA is located immediately upstream and overlaps pdxA (Fig. 1A) (66). The PsurA promoter, which must drive the formation of a surA-pdxA-ksgA cotranscript, has been preliminarily mapped to the end of imp (Fig. 1A), which immediately precedes surA and is involved in tolerance to organic solvents (1). Preliminary experiments indicate that transcription of the pdxA-ksgA cotranscript may also originate at a second promoter located in the 5" end of surA upstream from the insertion mutations in surA that are polar on pdxA expression and cause PN auxotrophy 66; Kolter, personal communication). The DNA sequence in the vicinity of the putative PsurA promoter contains several potential Fis binding sites (data not shown) (25). Whether these sites play a role in Fis-dependent growth rate regulation of the pdxA-ksgA cotranscript will be determined in future experiments.
We tested the effects of several other potential regulators of growth rate regulation on pdxA and pdxB expression. The amounts of the pdxA-ksgA- and pdxB-specific transcripts were not regulated by a ppGpp-mediated stringent response, although the amount of the pdxA-ksgA cotranscript was significantly decreased by conditions that limited bacterial growth (Table 2; Results). We did detect a modest level of stringent regulation of ksgA-specific and total ksgA transcript amounts (Table 2). Autoregulation of pdxA or pdxB expression was not detected, and pdxA and pdxB expression did not respond to the amount of B6 vitamer added to growth media or to starvation for B6 vitamers (Results; data not shown). We did not detect effects of a lipB mutation, overexpression of RNA polymerase subunits, or the presence of strong rrnB promoters on pdxA expression (Results). Thus, the growth rate regulation of the pdxA-ksgA cotranscript amount was directly or indirectly mediated by Fis whereas the mechanism of growth rate regulation of pdxB remains unknown.
It was recently reported that the concentration of the initiating nucleotide triphosphate may determine the growth rate regulation of rrn promoters (27), although this regulation may be more complicated than it first appeared (53). pdxA and pdxB expression is unlikely to be affected by the concentration of initiating nucleoside triphosphates because only strong promoters with short-lived complexes with RNA polymerase are affected by the initiating nucleoside triphosphate concentration (27). Moreover, the pdxB promoter does not contain any of the elements common to rRNA promoters and tRNA promoters, such as the GC-rich discriminator region that is necessary for the regulation of those promoters (27). Consequently, we did not test the effect of the cellular nucleotide concentration on pdxA and pdxB growth rate regulation.
Our present level of knowledge is not sufficient to predict what role, if any, the strong growth rate regulation of pdxA and pdxB (Fig. 7) has in the flux of intermediates through the de novo pathway of PLP biosynthesis. Moreover, other PLP biosynthetic genes do not follow the pattern of positive growth rate regulation reported here for pdxA and pdxB. For example, expression of the serC (pdxF)-encoded transaminase, which acts between PdxB and PdxA in the de novo PLP pathway (40, 43), is minimal in rich LB medium and maximal in minimal salts-glucose medium due to opposing positive and negative controls exerted by the Lrp and cyclic AMP receptor proteins, respectively (T.-K. Man and M. E. Winkler, unpublished results) (43). However, the regulation of the SerC (PdxF) transaminase may have been strongly set by its central role in serine biosynthesis (63).
Another PLP biosynthetic gene is not subject to growth rate regulation at all. For example, PNP synthase, encoded by pdxJ, catalyzes ring closure of the product of the PdxA reaction to form PNP as the last step in the de novo pathway of B6 vitamer biosynthesis (11, 26, 28, 38, 41, 44, 78). pdxJ is located in the complex rnc-era-recO-pdxJ-acpS superoperon (41, 45, 65). Extensive analyses of single-copy pdxJ-lacZ transcription and translation fusions indicated that pdxJ is not subject to growth rate regulation or to regulation by changes in fatty acid metabolism that might be expected to influence expression of the cotranscribed acpS gene (encodes holo-acyl-carrier protein synthase) (data not shown) (45). Understanding these diverse regulation patterns requires additional information about the cellular amounts of the PLP biosynthetic enzymes and pathway intermediates in E. coli growing at different rates. Another consideration is that the positive growth rate regulation in the complex superoperons that contain pdxA and pdxB (Fig. 1) may act primarily on expression of the downstream ksgA rRNA and truA (hisT) tRNA modification genes, respectively, which encode members of the translation apparatus. Thus, it may be that the genes encoding members of the translation apparatus, not the genes encoding PLP biosynthetic genes, are the targets of growth rate regulation in these superoperons. Consistent with this notion, truA (hisT) transcription is positively growth rate regulated in exponentially growing bacteria (69).
Acknowledgments
We thank the colleagues listed in Table 1 for bacterial strains and plasmids. We thank Karen Betchel for excellent technical assistance, Genshi Zhao and Tze-Kwong Man for helpful discussions, and Gregory Robertson for help preparing the figures.
This work was supported by Public Health Service grant GM37561 from the National Institute of General Medical Sciences and resources of the Lilly Research Laboratories.
REFERENCES
- 1.Aono, R., T. Negishi, and H. Nakajima. 1994. Cloning of organic solvent tolerance gene ostA that determines n-hexane tolerance in Escherichia coli. Appl. Environ. Microbiol. 60:4624-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arps, P. J., and M. E. Winkler. 1987. Structural analysis of the Escherichia coli K-12 hisT operon by using a kanamycin resistance cassette. J. Bacteriol. 169:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arps, P. J., and M. E. Winkler. 1987. An unusual genetic link between vitamin B6 biosynthesis and tRNA pseudouridine modification in Escherichia coli K-12. J. Bacteriol. 169:1071-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smaith, and K. Struhl. 1993. Current protocols in molecular biology. Wiley-Interscience, New York, N.Y.
- 5.Ball, C. A., R. Osuna, K. C. Ferguson, and R. C. Johnson. 1992. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J. Bacteriol. 174:8043-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartlett, M. S., and R. L. Gourse. 1994. Growth rate-dependent control of the rrnB P1 core promoter in Escherichia coli. J. Bacteriol. 176:5560-5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett, M. S., T. Gaal, W. Ross, and R. L. Gourse. 2000. Regulation of rRNA transcription is remarkably robust: Fis compensates for altered nucleoside triphosphate sensing by mutant RNA polymerases at Escherichia coli rrn P1 promoters. J. Bacteriol. 182:1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedwell, D. M., and M. Nomura. 1986. Feedback regulation of RNA polymerase subunit synthesis after the conditional overproduction of RNA polymerase in Escherichia coli. Mol. Gen. Genet. 204:17-23. [DOI] [PubMed] [Google Scholar]
- 9.Bender, D. A. 1985. Amino acid metabolism. John Wiley & Sons, Inc., New York, N.Y.
- 10.Bremer, H., and P. P. Dennis. 1996. Modulation of chemical composition and other parameters of the cell by growth rate, p. 1553-1569. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 2. ASM Press, Washington, D.C.
- 11.Cane, D. E., S. Du, J. K. Robinson, Y. Hsiung, and I. D. Spenser. 1999. Biosynthesis of vitamin B6: enzymatic conversion of 1-deoxy-d-xylulose-5-phosphate to pyridoxol phosphate. J. Am. Chem. Soc. 121:7722-7723.
- 12.Cane, D. E., Y. Hsiung, J. A. Cornish, J. K. Robinson, and I. D. Spenser. 1998. Biosynthesis of vitamin B6: the oxidation of 4-(phosphohydroxy)-l-threonine by PdxA. J. Am. Chem. Soc. 120:1936-1937. [Google Scholar]
- 13.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 14.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1985. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condon, C., J. Philips, Z.-Y. Fu, C. Squires, and C. L. Squires. 1992. Comparison of the expression of the seven ribosomal operons in Escherichia coli. EMBO J. 11:4175-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dakshinamurti, K. E. 1990. Vitamin B6. Ann. N.Y. Acad. Sci., vol. 585. [DOI] [PubMed]
- 17.Dempsey, W. B. 1965. Control of pyridoxine biosynthesis in Escherichia coli. J. Bacteriol. 90:431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempsey, W. B. 1971. Control of vitamin B6 biosynthesis in Escherichia coli. J. Bacteriol. 108:415-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dempsey, W. B. 1987. Synthesis of pyridoxal phosphate, p. 539-543. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 20.Dennis, P. P., and M. Nomura. 1975. Regulation of the expression of ribosomal protein genes in Escherichia coli. J. Mol. Biol. 97:61-76. [DOI] [PubMed] [Google Scholar]
- 21.Dolphin, D., R. Poulson, and O. Avramovic (ed.). 1986. Vitamin B6 pyridoxal phosphate: chemical, biochemical, and medical aspects. Wiley-Interscience, New York, N.Y.
- 22.Dong, H., L. A. Kirsebom, and L. Nilsson. 1996. Growth rate regulation of 4.5 S RNA and M1 RNA the catalytic subunit of Escherichia coli RNase P. J. Mol. Biol. 261:303-308. [DOI] [PubMed] [Google Scholar]
- 23.Ehrenshaft, M., P. Bilski, M. Y. Li, C. F. Chignell, and M. E. Daub. 1999. A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. USA 96:9374-9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emilsson, V., and L. Nilsson. 1995. Factor for inversion stimulation-dependent growth rate regulation of serine and threonine tRNA species. J. Biol. Chem. 270:16610-16614. [DOI] [PubMed] [Google Scholar]
- 25.Finkel, S. E., and R. C. Johnson. 1992. The Fis protein: it's not just for DNA inversion anymore. Mol. Microbiol. 6:3257-3265. [DOI] [PubMed] [Google Scholar]
- 26.Franco, M. G., B. Laber, R. Huber, and T. Clausen. 2001. Structural basis for the function of pyridoxine 5"-phosphate synthase. Structure 9:245-253. [DOI] [PubMed] [Google Scholar]
- 27.Gaal, T., M. S. Bartlett, W. Ross, C. L. Turnbough, Jr., and R. L. Gourse. 1997. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science 278:2092-2097. [DOI] [PubMed] [Google Scholar]
- 28.Garrido-Franco, M., R. Huber, F. S. Schmidt, B. Laber, and T. Clausen. 2000. Crystallization and preliminary X-ray crystallographic analysis of PdxJ, the pyridoxine 5"-phosphate synthesizing enzyme. Acta Crystallogr. D Biol. Crystallogr. 56:1045-1048. [DOI] [PubMed] [Google Scholar]
- 29.Helmreich, E. J. 1992. How pyridoxal 5"-phosphate could function in glycogen phosphorylase catalysis. Biofactors 3:159-172. [PubMed] [Google Scholar]
- 30.Hill, R. E., and I. D. Spenser. 1996. Biosynthesis of vitamin B6, p. 695-703. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 31.Iriarte, A., H. M. Kagen, and M. Martinez-Carrion (ed.). 2000. Biochemistry of vitamin B6 and PQQ-dependent proteins. Birkhäuser Verlag, Basel, Switzerland.
- 32.Jensen, K. F., and S. Pedersen. 1990. Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of the macromolecular biosynthetic apparatus with substrates and catalytic components. Microbiol. Rev. 54:89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jinks-Robertson, S., R. L. Gourse, and M. Nomura. 1983. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell 33:865-876. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, R. C., C. A. Ball, D. Pfeffer, and M. I. Simon. 1988. Isolation of the gene encoding the Hin recombinational enhancer. Proc. Natl. Acad. Sci. USA 85:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlinsey, J. E., A. J. Pease, M. E. Winkler, J. L. Bailey, and K. T. Hughes. 1997. The flk gene of Salmonella typhimurium couples flagellar P- and L-ring assembly to flagellar morphogenesis. J. Bacteriol. 179:2389-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karpeisky, M. Y., and H. B. F. Dixon. 1986. The use of pyridoxal phosphate for modification of proteins, p. 71-116. In D. Dolphin, R. Poulson, and O. Avramovic (ed.), Vitamin B6 pyridoxal phosphate: chemical, biochemical, and medical aspects, vol. B. Wiley-Interscience, New York, N.Y. [Google Scholar]
- 37.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 38.Laber, B., W. Maurer, S. Scharf, K. Stepusin, and F. S. Schmidt. 1999. Vitamin B6 biosynthesis: formation of pyridoxine 5"-phosphate from 4-(phosphohydroxyl)-l-threonine and 1-deoxy-d-xylulose-5-phosphate by PdxA and PdxJ protein. FEBS Lett. 449:45-48. [DOI] [PubMed] [Google Scholar]
- 39.Lam, H.-L., and M. E. Winkler. 1992. Characterization of the complex pdxH-tyrS operon of Escherichia coli K-12 and pleiotropic phenotypes caused by pdxH insertion mutations. J. Bacteriol. 174:6033-6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam, H.-L., and M. E. Winkler. 1990. Metabolic relationships between pyridoxine (vitamin B6) and serine biosynthesis in Escherichia coli K-12. J. Bacteriol. 172:6518-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lam, H.-L., E. Tancula, W. B. Dempsey, and M. E. Winkler. 1992. Suppression of insertions in the complex pdxJ operon of Escherichia coli K-12 by lon and other mutations. J. Bacteriol. 174:1554-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazar, S. W., M. Almiron, A. Tormo, and R. Kolter. 1998. Role of the Escherichia coli SurA protein in stationary-phase survival. J. Bacteriol. 180:5704-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Man, T.-K., A. J. Pease, and M. E. Winkler. 1997. Maximization of transcription of the serC (pdxF)-aroA multifunctional operon by antagonistic effects of the cyclic AMP (cAMP) receptor protein-cAMP complex and Lrp global regulators of Escherichia coli K-12. J. Bacteriol. 179:3458-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Man, T.-K., G. Zhao, and M. E. Winkler. 1996. Isolation of a pdxJ point mutation that bypasses the requirement for the PdxH oxidase in pyridoxal 5"-phosphate coenzyme biosynthesis in Escherichia coli K-12. J. Bacteriol. 178:2445-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsunaga, J., M. Dyer, E. L. Simons, and R. W. Simons. 1996. Expression and regulation of the rnc and pdxJ operons of Escherichia coli. Mol. Microbiol. 22:977-989. [DOI] [PubMed] [Google Scholar]
- 46.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Misra, R., and P. R. Reeves. 1987. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J. Bacteriol. 169:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittenhuber, G. 2001. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J. Mol. Microbiol. Biotechnol. 3:1-20. [PubMed] [Google Scholar]
- 49.Morris, T. W., K. E. Reed, and J. E. Cronan. 1995. Lipoic acid metabolism in Escherichia coli: the lplA and lipB genes define redundant pathways for ligation of lipoyl groups to apoprotein. J. Bacteriol. 177:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsson, G., J. G. Belasco, S. N. Cohen, and A. von Gabain. 1984. Growth rate dependent regulation of mRNA stability in Escherichia coli. Nature 312:75-77. [DOI] [PubMed] [Google Scholar]
- 51.Osmani, A. H., G. S. May, and S. A. Osmani. 1999. The extremely conserved pyroA gene of Aspergillus nidulans is required for pyridoxine synthesis and is required indirectly for resistance to photosensitizers. J. Biol. Chem. 274:23565-23569. [DOI] [PubMed] [Google Scholar]
- 52.Pedersen, S., P. L. Bloch, S. Reeh, and F. C. Neidhardt. 1978. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell 14:179-190. [DOI] [PubMed] [Google Scholar]
- 53.Petersen, C., and L. B. Moller. 2000. Invariance of the nucleoside triphosphate pools of Escherichia coli with growth rate. J. Biol. Chem. 275:3931-3935. [DOI] [PubMed] [Google Scholar]
- 54.Rasmussen, L. J., M. G. Marinus, and A. Lobner-Olesen. 1994. Novel growth rate control of dam gene expression in Escherichia coli. Mol. Microbiol. 12:631-638. [DOI] [PubMed] [Google Scholar]
- 55.Roa, B. B., D. M. Connolly, and M. E. Winkler. 1989. Overlap between pdxA and ksgA in the complex pdxA-ksgA-apaG-apaH operon of Escherichia coli K-12. J. Bacteriol. 171:4767-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rose, J. K., and C. Yanofsky. 1972. Metabolic regulation of the tryptophan operon of Escherichia coli: repressor-independent regulation of transcription initiation frequency. J. Mol. Biol. 69:103-118. [DOI] [PubMed] [Google Scholar]
- 57.Rouviere, P. E., and C. A. Gross. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170-3182. [DOI] [PubMed] [Google Scholar]
- 58.Rowley, K. B., R. M. Elford, I. Roberts, and W. M. Holmes. 1993. In vivo regulatory responses of four Escherichia coli operons which encode leucyl-tRNAs. J. Bacteriol. 175:1309-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoenlein, P. V., B. B. Roa, and M. E. Winkler. 1989. Divergent transcription of pdxB and homology between the pdxB and serA gene products in Escherichia coli K-12. J. Bacteriol. 171:6084-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiber, G., S. Metzger, E. Aizenman, S. Roza, M. Cashel, and G. Glaser. 1991. Overexpression of the relA gene in Escherichia coli. J. Biol. Chem. 266:3760-3767. [PubMed] [Google Scholar]
- 61.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 62.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugimoto, E., and L. I. Pizer. 1968. The mechanism of end product inhibition of serine biosynthesis. I. Purification and kinetics of phosphoglycerate dehydrogenase. J. Biol. Chem. 243:2081-2089. [PubMed] [Google Scholar]
- 64.Svitil, A. L., M. Cashel, and J. W. Zyskind. 1993. Guanosine tetraphosphate inhibits protein synthesis in vivo. J. Biol. Chem. 268:2307-2311. [PubMed] [Google Scholar]
- 65.Takiff, H. E., T. Baker, T. Copeland, S. M. Chen, and D. L. Court. 1992. Locating essential Escherichia coli genes by using mini-Tn10 transposons: the pdxJ operon. J. Bacteriol. 174:1544-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tormo, A., M. Almiron, and R. Kolter. 1990. surA, an Escherichia coli gene essential for survival in stationary phase. J. Bacteriol. 172:4339-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tosa, T., and L. I. Pizer. 1971. Effect of serine hydroxamate on the growth of Escherichia coli. J. Bacteriol. 106:966-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tribe, D. E., H. Camakaris, and J. Pittard. 1976. Constitutive and repressible enzymes of the common pathway of aromatic biosynthesis in Escherichia coli K-12: regulation of enzyme synthesis at different growth rates. J. Bacteriol. 127:1085-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsui, H.-C., P. J. Arps, D. M. Connnoly, and M. E. Winkler. 1991. Absence of hisT-mediated pseudouridylation results in a uracil requirement that interferes with Escherichia coli K-12 cell division. J. Bacteriol. 173:7395-7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsui, H.-C. T., A. J. Pease, T. M. Koehler, and M. E. Winkler. 1994. Detection and quantitation of RNA transcribed from bacterial chromosomes and plasmids, p. 179-204. In K. Adolph (ed.), Methods in molecular genetics: molecular microbiology, vol. 3. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 71.Vaisvila, R., L. J. Rasmussen, A. Lobner-Olesen, U. von Freiesleben, and M. G. Marinus. 2000. The LipB protein is a negative regulator of dam gene expression in Escherichia coli. Biochim. Biophys. Acta 1494:43-53. [DOI] [PubMed] [Google Scholar]
- 72.van Gemen, B., J. Twisk, and P. H. van Knippenberg. 1989. Autogenous regulation of the Escherichia coli ksgA gene at the level of translation. J. Bacteriol. 171:4002-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Gemen, B., H. J. Koets, C. A. Plooy, J. Bodlaender, and P. H. van Knippenberg. 1987. Characterization of the ksgA gene of Escherichia coli determining kasugamycin sensitivity. Biochimie (Paris) 69:841-848. [DOI] [PubMed]
- 74.Winkler, M. E. 2000. Genetic and genomic approaches for delineating the pathway of pyridoxal 5"-phosphate coenzyme biosynthesis in Escherichia coli, p. 3-10. In A. Iriarte, H. M. Kagen, and M. Martinez-Carrion (ed.), Biochemistry and molecular biology of vitamin B6 and PQQ-dependent proteins. Birkhäuser Verlag, Basel, Switzerland.
- 75.Wolf, R. E., Jr., D. M. Prather, and F. M. Shea. 1979. Growth rate-dependent alteration of 6-phosphogluconate dehydrogenase and glucose 6-phosphate dehydrogenase levels in Escherichia coli K-12. J. Bacteriol. 139:1039-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu, J., and R. C. Johnson. 1995. Identification of genes negatively regulated by Fis: Fis and RpoS comodulate growth-phase-dependent gene expression in Escherichia coli. J. Bacteriol. 177:938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao, G., A. J. Pease, N. Bharani, and M. E. Winkler. 1995. Biochemical characterization of gapB-encoded erythrose 4-phosphate dehydrogenase of Escherichia coli K-12 and its possible role in pyridoxal 5"-phosphate biosynthesis. J. Bacteriol. 177:2804-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao, G., and M. E. Winkler. 1996. 4-Phospho-hydroxy-l-threonine is an obligatory intermediate in pyridoxal 5"-phosphate coenzyme biosynthesis in Escherichia coli K-12. FEMS Microbiol. Lett. 135:275-280. [DOI] [PubMed] [Google Scholar]