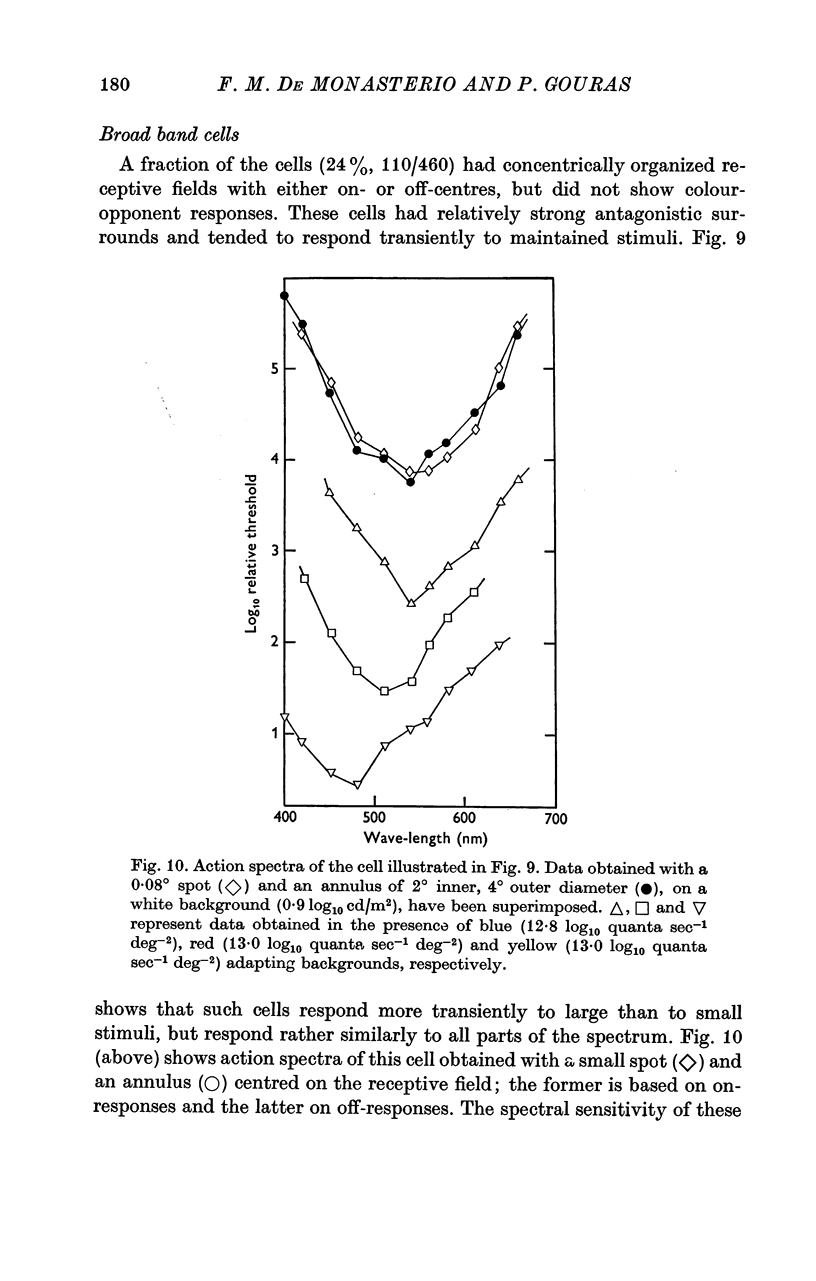
Abstract
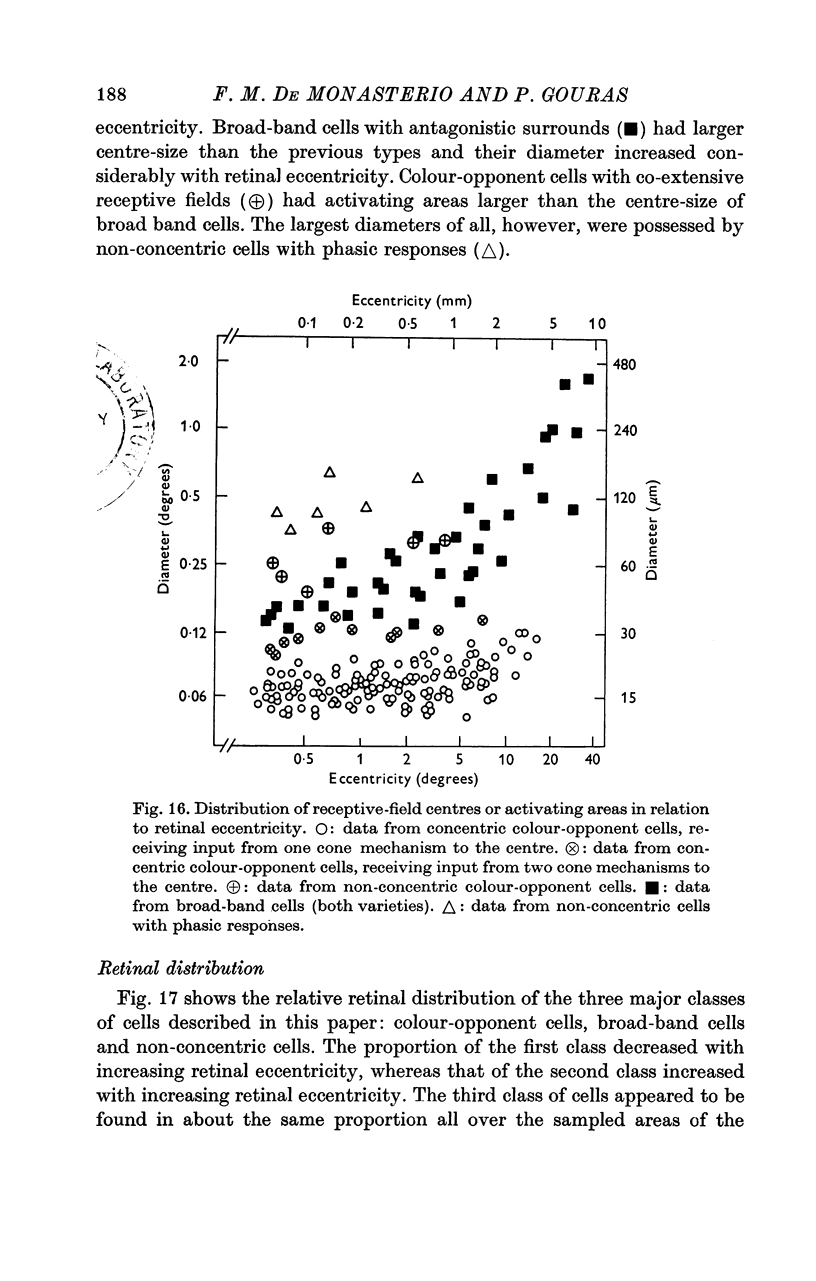
Three general classes of cells were identified in a sample of 460 cells recorded from all areas of the retina subserving the central 40 degrees of vision in the rhesus monkey. 2. One class (colour-opponent) had sustained colour-opponent responses and concentrically organized receptive fields, in which usually one cone mechanism mediated the centre response and one or two different cone mechanisms mediated the antagonistic surround. A few cells of this class had non-concentric (co-extensive) receptive field organization. 3. A second class (broad-band) had transient responses and concentrically organized receptive fields, in which usually two cone mechanisms mediated the centre response. In most cells, the surround had the same spectral sensitivity as the centre and the cells had non-colour opponent responses. In other cells, the surround had a spectral sensitivity different to that of the centre and the cells had colour-opponent responses. 4. The third class (non-concentric) did not have concentrically organized receptive fields. One group of cells had extremely phasic on-, off- or on-off responses and no spontaneous activity, another group had characteristically regular spontaneous activity and was responsive only to moving stimuli. 5. Cells of the colour-opponent class with concentric receptive fields had the smallest centre-sizes, which did not vary markedly from cell to cell (mean 15 mum); cells of the non-concentric class with phasic responses had the largest centre-sizes, which varied from cell to cell. 6. Colour-opponent cells comprised the highest proportion of cells near the foveola; broad-band cells comprised the highest proportion in the more peripheral areas of the retina; non-concentric cells were equally represented in all areas.
Full text
PDF


























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. K., Perez J. M., Hawthorne M. N. Rod and cone densities in the Rhesus. Invest Ophthalmol. 1974 Nov;13(11):885–888. [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M., LEVICK W. R. RETINAL GANGLION CELLS RESPONDING SELECTIVELY TO DIRECTION AND SPEED OF IMAGE MOTION IN THE RABBIT. J Physiol. 1964 Oct;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. D., Lovasik J. V. Blue mechanism response of single goldfish optic fibers. J Neurophysiol. 1973 Sep;36(5):925–939. doi: 10.1152/jn.1973.36.5.925. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Proximal negative response of frog retina. J Neurophysiol. 1970 May;33(3):405–420. doi: 10.1152/jn.1970.33.3.405. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974 Jul;240(2):457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Neurophysiology of color vision. Physiol Rev. 1973 Jul;53(3):571–611. doi: 10.1152/physrev.1973.53.3.571. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Wyatt H. J. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J Physiol. 1974 Jul;240(2):309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P., Tolhurst D. J. Concealed colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):217–229. doi: 10.1113/jphysiol.1975.sp011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P., Tolhurst D. J. Trichromatic colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow B. M., Gouras P. Color and spatial specificity of single units in Rhesus monkey foveal striate cortex. J Neurophysiol. 1973 Jan;36(1):79–100. doi: 10.1152/jn.1973.36.1.79. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Simon E. J. Interactions leading to horizontal cell responses in the turtle retina. J Physiol. 1974 Jul;240(1):177–198. doi: 10.1113/jphysiol.1974.sp010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. E., Wurtz R. H. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J Neurophysiol. 1972 Jul;35(4):542–559. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- Gouras P. Antidromic responses of orthodromically identified ganglion cells in monkey retina. J Physiol. 1969 Oct;204(2):407–419. doi: 10.1113/jphysiol.1969.sp008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Padmos P. Identification of cone mechanisms in graded responses of foveal striate cortex. J Physiol. 1974 May;238(3):569–581. doi: 10.1113/jphysiol.1974.sp010544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Spatial organization of receptive fields of LGN neurones. J Physiol. 1972 Apr;222(1):53P–54P. [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. The outer disinhibitory surround of the retinal ganglion cell receptive field. J Physiol. 1972 Oct;226(2):511–544. doi: 10.1113/jphysiol.1972.sp009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., FRENK S. DIRECTIONAL MOVEMENT AND HORIZONTAL EDGE DETECTORS IN THE PIGEON RETINA. Science. 1963 Nov 15;142(3594):977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Retinogeniculate convergence and analysis of contrast. J Neurophysiol. 1972 Jan;35(1):65–72. doi: 10.1152/jn.1972.35.1.65. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. II: directionally selective units. J Neurophysiol. 1968 Mar;31(2):257–267. doi: 10.1152/jn.1968.31.2.257. [DOI] [PubMed] [Google Scholar]
- Ogden T. E. The proximal negative response of the primate retina. Vision Res. 1973 Apr;13(4):797–807. doi: 10.1016/0042-6989(73)90044-8. [DOI] [PubMed] [Google Scholar]
- Pasik T., Pasik P., Hámori J., Szentágothai J. Nucleus of the accessory optic tract: electron microscopic analysis in normal and eye-enucleated monkeys. Trans Am Neurol Assoc. 1970;95:302–305. [PubMed] [Google Scholar]
- Raviola E., Gilula N. B. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E. T., Cowey A. Topography of the retina and striate cortex and its relationship to visual acuity in rhesus monkeys and squirrel monkeys. Exp Brain Res. 1970;10(3):298–310. doi: 10.1007/BF00235053. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Stryker M., Cynader M., Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J Neurophysiol. 1974 Jan;37(1):181–194. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Stone J., Fukuda Y. Properties of cat retinal ganglion cells: a comparison of W-cells with X- and Y-cells. J Neurophysiol. 1974 Jul;37(4):722–748. doi: 10.1152/jn.1974.37.4.722. [DOI] [PubMed] [Google Scholar]
- Stone J., Hoffmann K. P. Very slow-conducting ganglion cells in the cat's retina: a major, new functional type? Brain Res. 1972 Aug 25;43(2):610–616. doi: 10.1016/0006-8993(72)90416-7. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Unit activity in accessory optic system in alert monkeys. Invest Ophthalmol. 1974 Jul;13(7):533–534. [PubMed] [Google Scholar]
- Westheimer G., Wiley R. W. Distance effects in human scotopic retinal interaction. J Physiol. 1970 Jan;206(1):129–143. doi: 10.1113/jphysiol.1970.sp009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Toyne M. J. Retino-tectal and cortico-tectal projections in Macaca mulatta. Brain Res. 1970 Dec 18;24(3):395–406. doi: 10.1016/0006-8993(70)90181-2. [DOI] [PubMed] [Google Scholar]


