Abstract
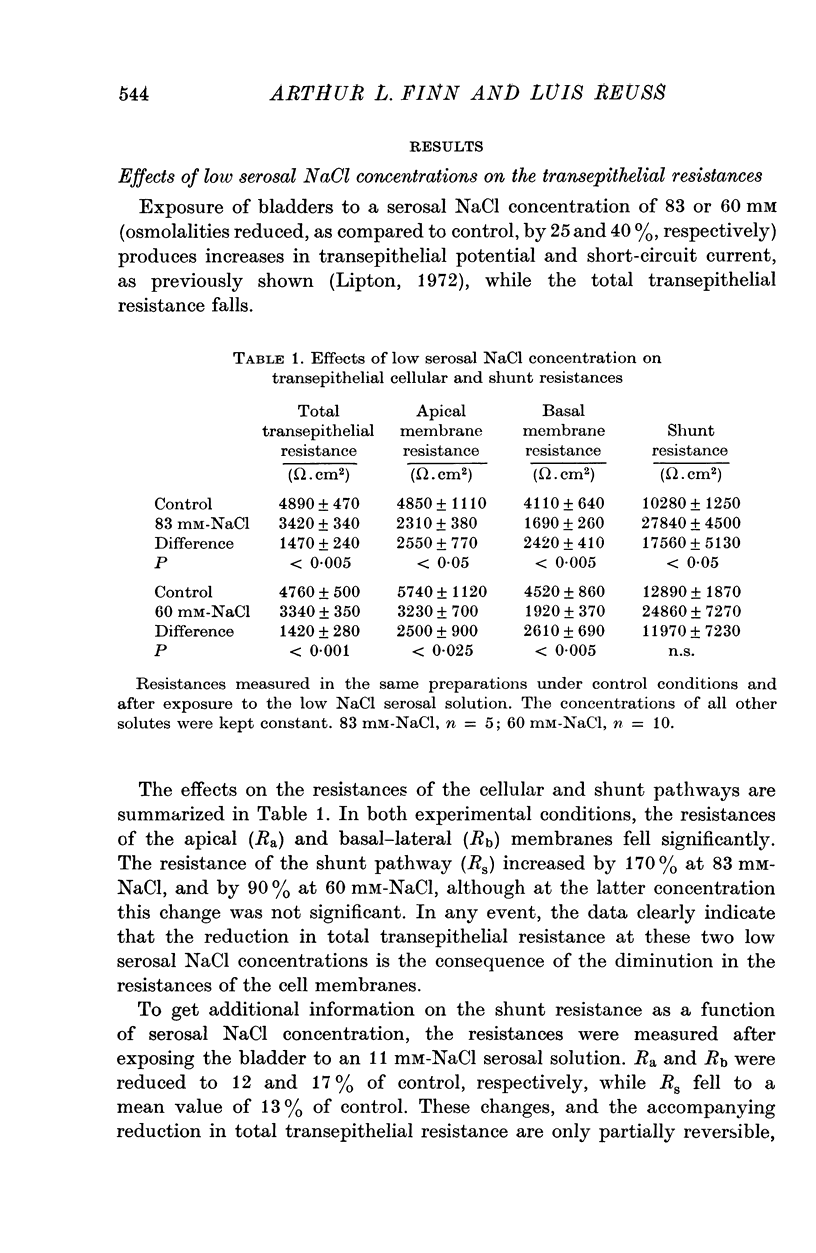
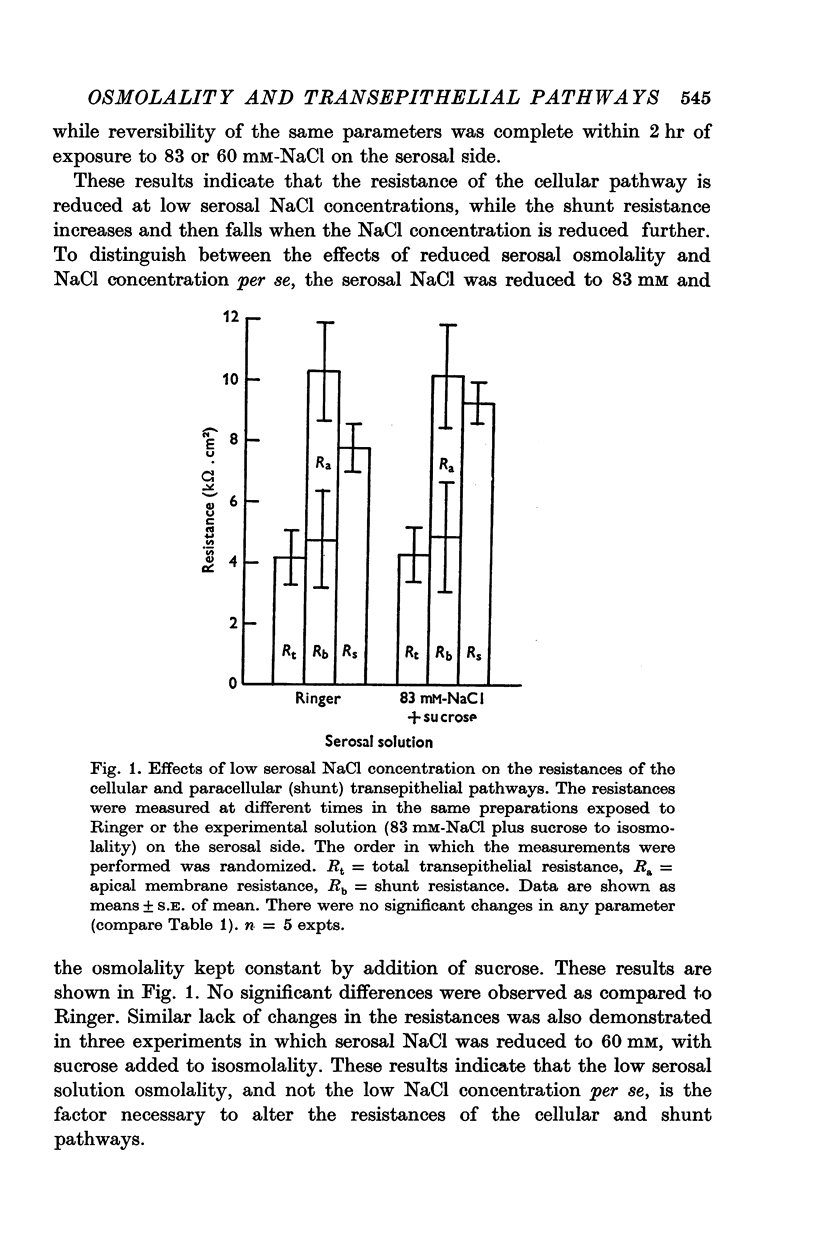
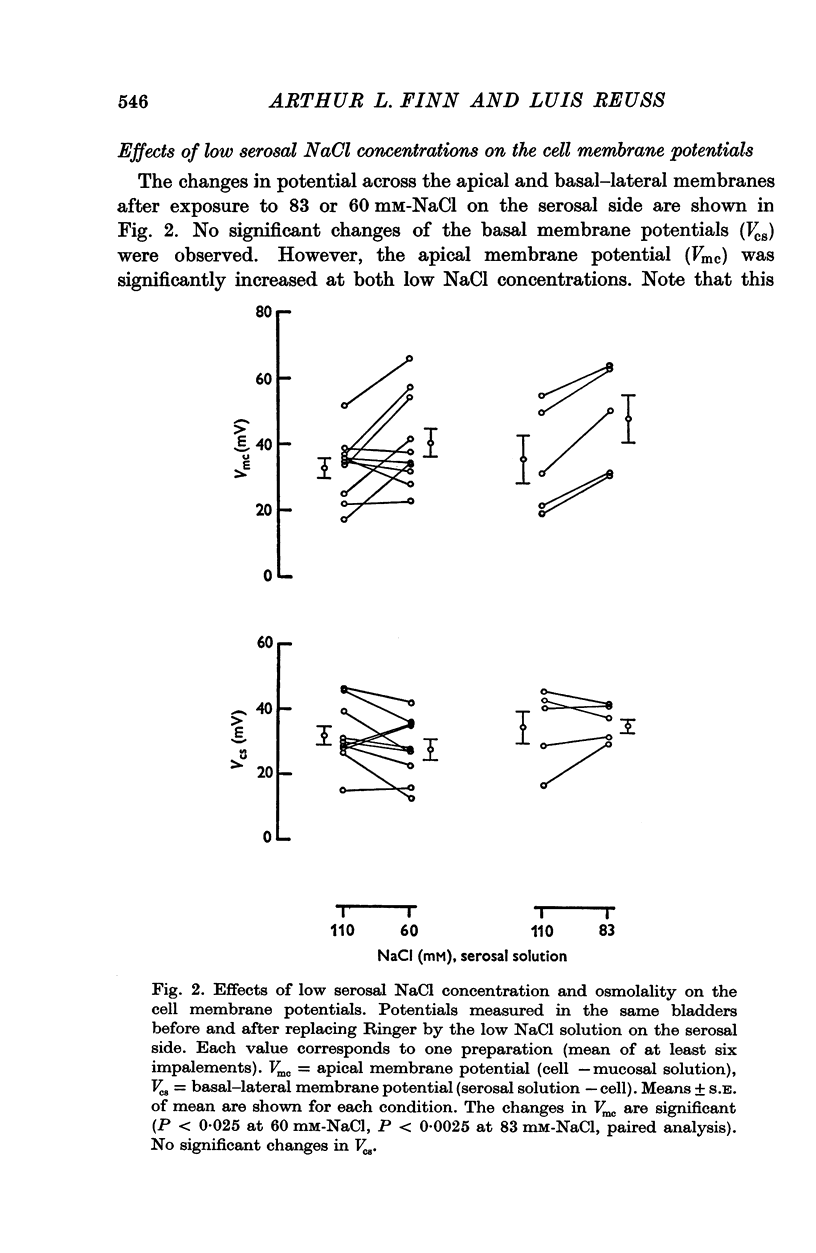
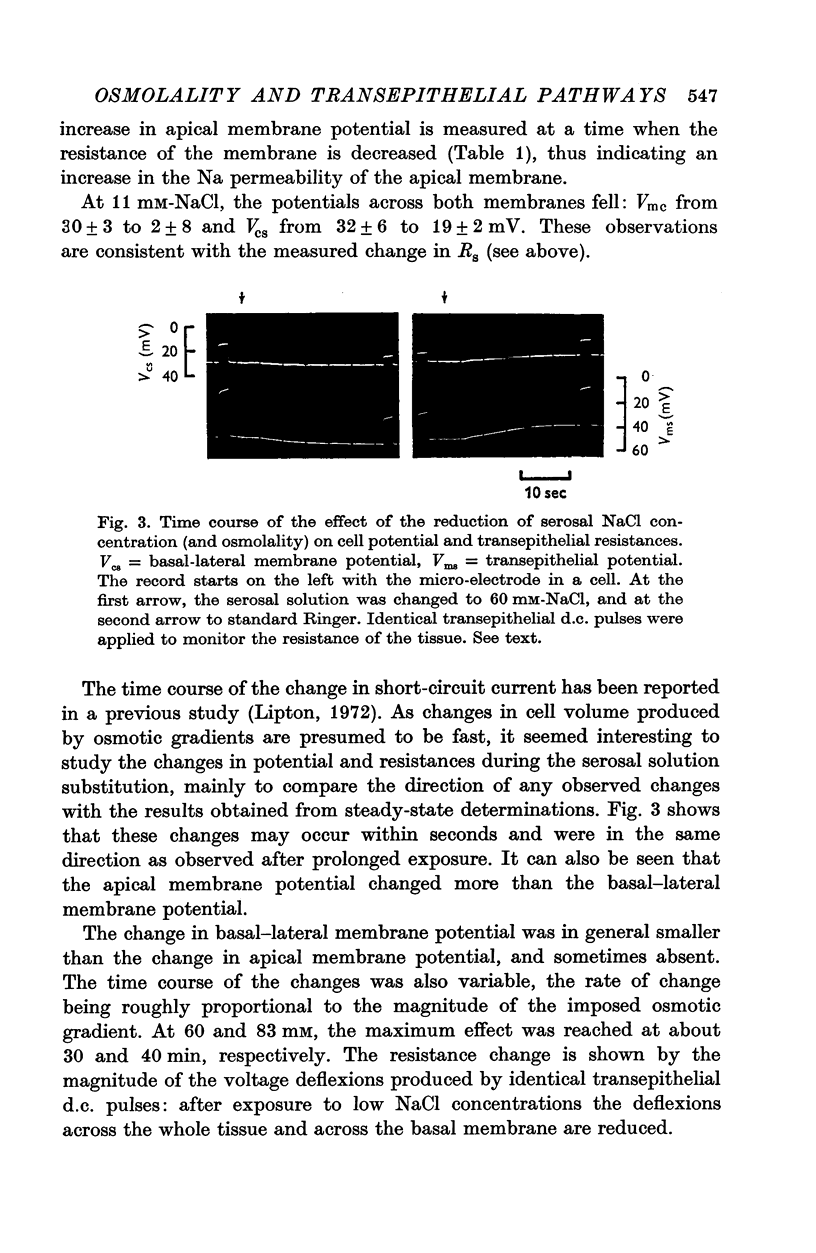
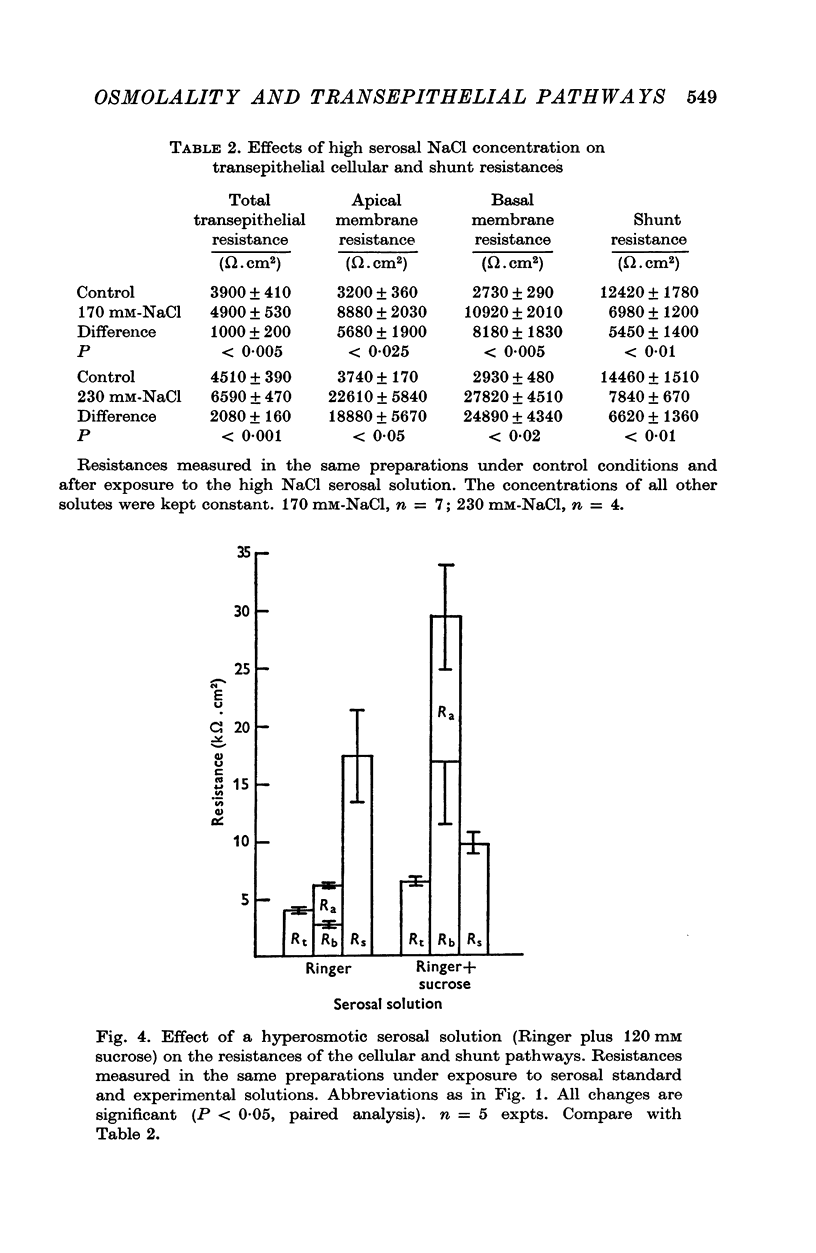
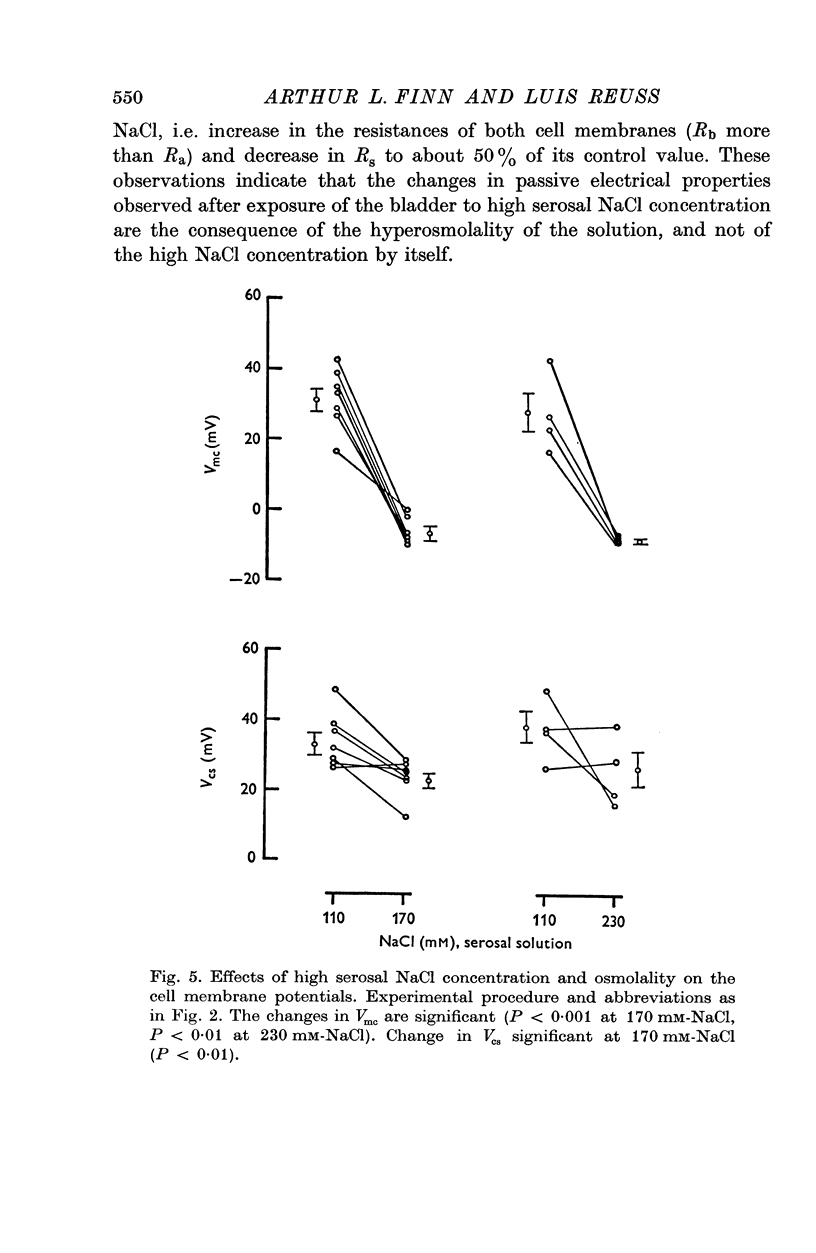
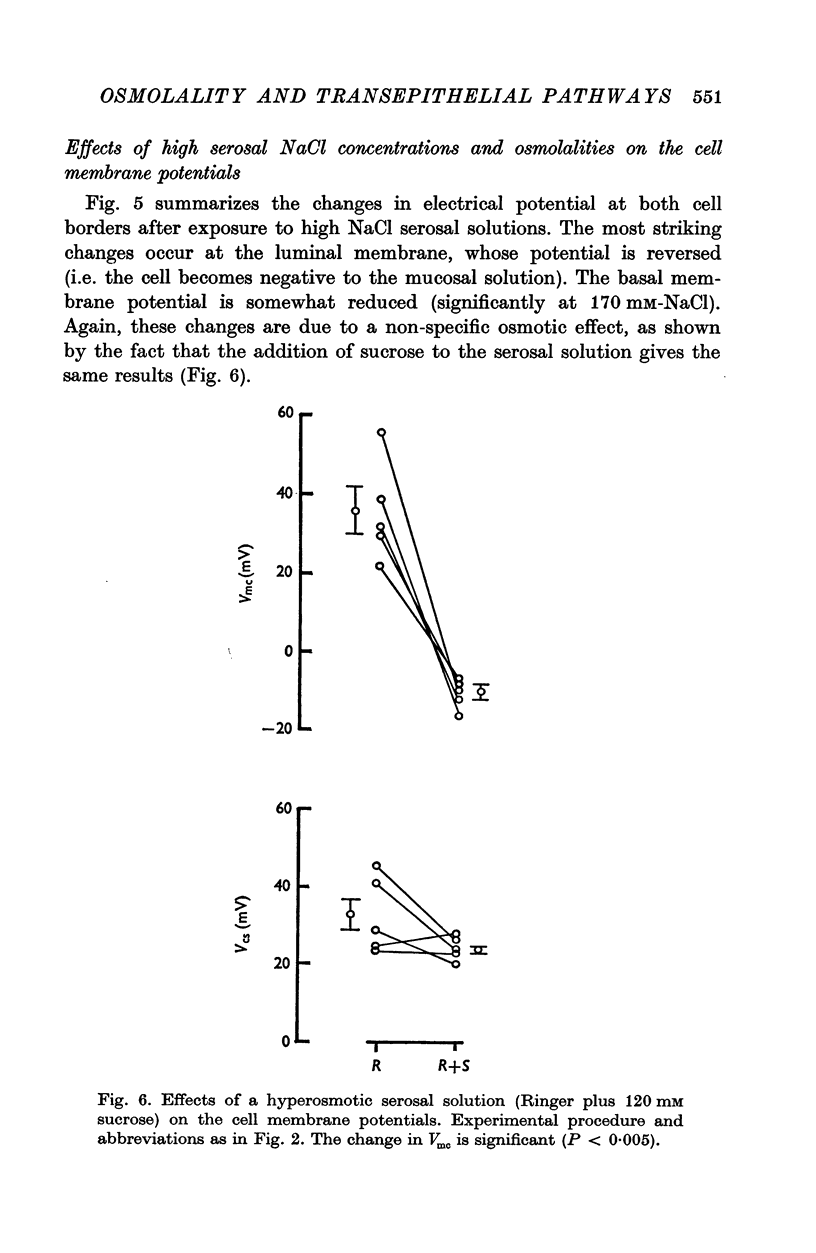
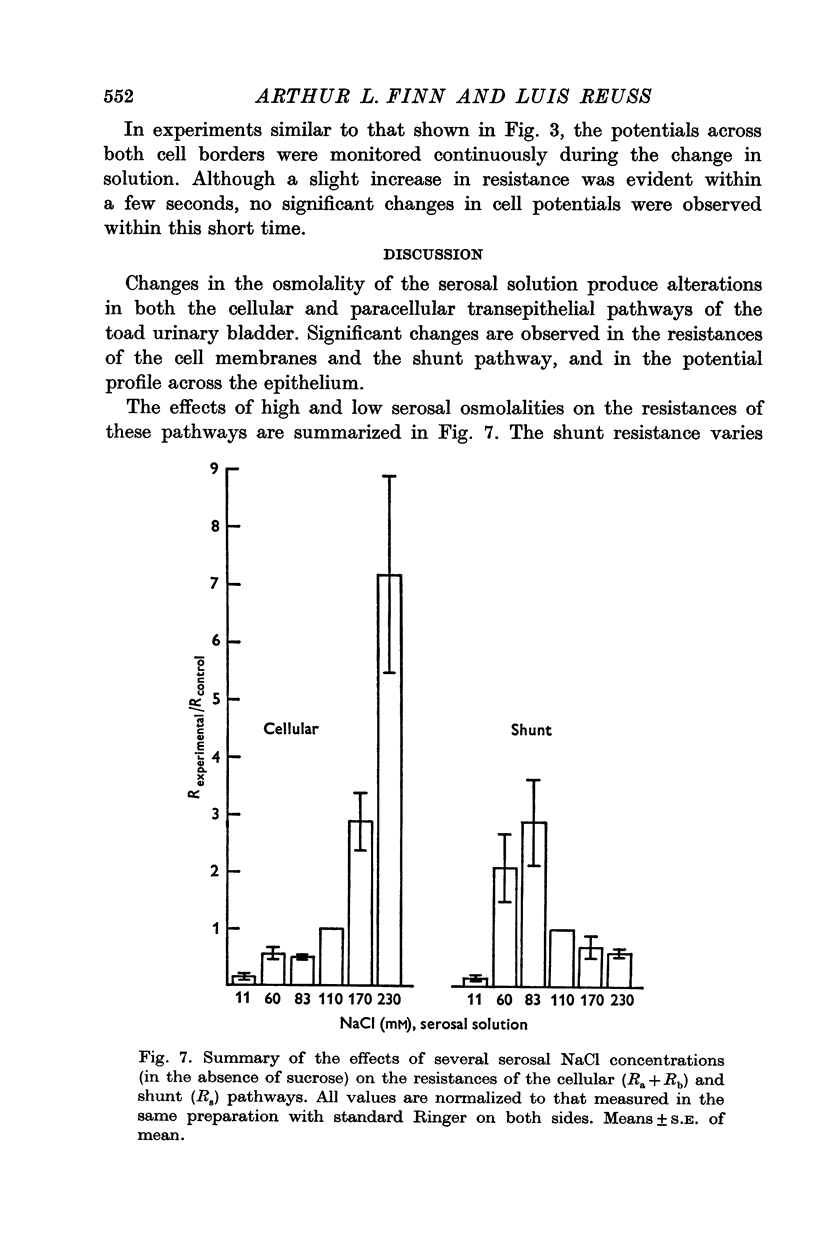
1. The potential profile and the cellular and paracellular transepithelial resistances of the toad urinary bladder were measured, by means of micro-electrode techniques, as functions of the osmolality of the serosal solution. 2. Reductions in serosal osmolality (that increase the rate of active sodium transport) produced proportional decreases in the electrical resistances of the apical and basal-lateral cell membranes, while the changes in resistance of the paracellular pathway were more complex. The apical membrane potential increased. 3. Increases in serosal osmolality (that decrease sodium transport) produced increases in the electrical resistances of both cell membranes, and moderate reduction in the paracellular resistance. The polarity of the apical membrane potential reversed. 4. These results indicate that reductions in serosal solution osmolality stimulate sodium transport by increasing both the sodium permeability of the luminal cell membrane (thus increasing sodium entry), and the electromotive force generated at the serosal border of the cell, thus enhancing the rate of sodium pumping. Conversely, increases in osmolality reduced sodium transport by reducing both the sodium permeability of the luminal membrane and the serosal membrane electromotive force.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley P. J., Candia O. A., Parisi M., Saladino A. J. Effects of hyperosmolality on transmural sodium transport in the toad bladder. Am J Physiol. 1973 Oct;225(4):818–824. doi: 10.1152/ajplegacy.1973.225.4.818. [DOI] [PubMed] [Google Scholar]
- DiBona D. R., Civan M. M. Pathways for movement of ions and water across toad urinary bladder. I. Anatomic site of transepithelial shunt pathways. J Membr Biol. 1973;12(2):101–128. doi: 10.1007/BF01869994. [DOI] [PubMed] [Google Scholar]
- FRAZIER H. S., LEAF A. The electrical characteristics of active sodium transport in the toad bladder. J Gen Physiol. 1963 Jan;46:491–503. doi: 10.1085/jgp.46.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L. Action of ouabain on sodium transport in toad urinary bladder, Evidence for two pathways for sodium entry. J Gen Physiol. 1975 Apr;65(4):503–514. doi: 10.1085/jgp.65.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L., Nellans H. The kinetics and distribution of potassium in the toad bladder. J Membr Biol. 1972;8(2):189–203. doi: 10.1007/BF01868102. [DOI] [PubMed] [Google Scholar]
- Finn A. L. The kinetics of sodium transport in the toad bladder. II. Dual effects of vasopressin. J Gen Physiol. 1971 Mar;57(3):349–362. doi: 10.1085/jgp.57.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A. L. Transepithelial potential difference in toad urinary bladder is not due to ionic diffusion. Nature. 1974 Aug 9;250(5466):495–496. doi: 10.1038/250495a0. [DOI] [PubMed] [Google Scholar]
- GATZY J. T., CLARKSON T. W. THE EFFECT OF MUCOSAL AND SEROSAL SOLUTION CATIONS ON BIOELECTRIC PROPERTIES OF THE ISOLATED TOAD BLADDER. J Gen Physiol. 1965 Mar;48:647–671. doi: 10.1085/jgp.48.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEB D. E., HOSHIKO T., LINDLEY B. D., DUGAN J. A. EFFECT OF ALKALI METAL CATIONS ON THE POTENTIAL ACROSS TOAD AND BULLFROG URINARY BLADDER. J Gen Physiol. 1965 Jan;48:527–540. doi: 10.1085/jgp.48.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Effect of changes in osmolarity on sodium transport across isolated toad bladder. Am J Physiol. 1972 Apr;222(4):821–828. doi: 10.1152/ajplegacy.1972.222.4.821. [DOI] [PubMed] [Google Scholar]
- Ludens J. H., Fanestil D. D. Acidification of urine by the isolated urinary bladder of the toad. Am J Physiol. 1972 Dec;223(6):1338–1344. doi: 10.1152/ajplegacy.1972.223.6.1338. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Dependence of serosal membrane potential on mucosal membrane potential in toad urinary bladder. Biophys J. 1975 Jan;15(1):71–75. doi: 10.1016/S0006-3495(75)85792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Passive electrical properties of toad urinary bladder epithelium. Intercellular electrical coupling and transepithelial cellular and shunt conductances. J Gen Physiol. 1974 Jul;64(1):1–25. doi: 10.1085/jgp.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. RELATIONSHIP BETWEEN OSMOTIC REACTIONS AND ACTIVE SODIUM TRANSPORT IN THE FROG SKIN EPITHELIUM. Acta Physiol Scand. 1965 Jan-Feb;63:141–155. doi: 10.1111/j.1748-1716.1965.tb04052.x. [DOI] [PubMed] [Google Scholar]
- Urakabe S., Handler J. S., Orloff J. Effect of hypertonicity on permeability properties of the toad bladder. Am J Physiol. 1970 Apr;218(4):1179–1187. doi: 10.1152/ajplegacy.1970.218.4.1179. [DOI] [PubMed] [Google Scholar]
- Wade J. B., Revel J. P., DiScala V. A. Effect of osmotic gradients on intercellular junctions of the toad bladder. Am J Physiol. 1973 Feb;224(2):407–415. doi: 10.1152/ajplegacy.1973.224.2.407. [DOI] [PubMed] [Google Scholar]


