Abstract
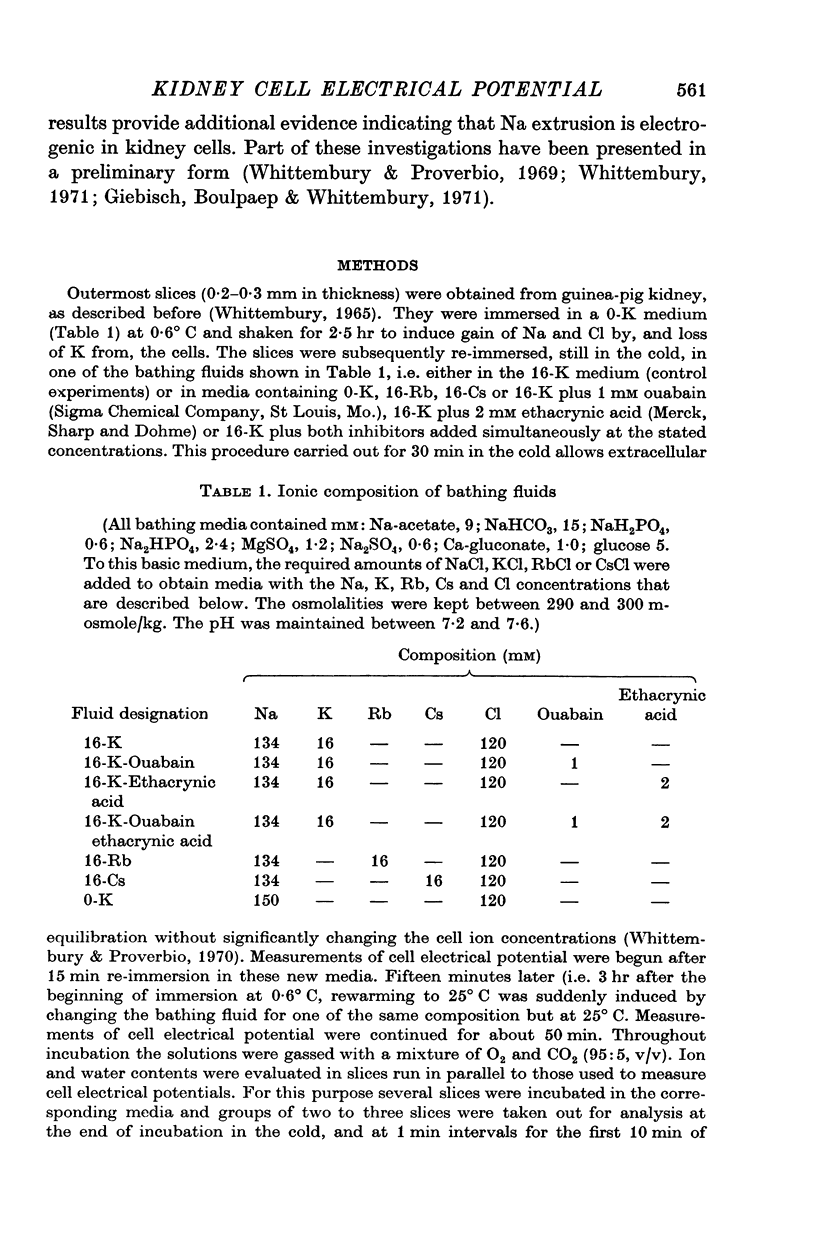
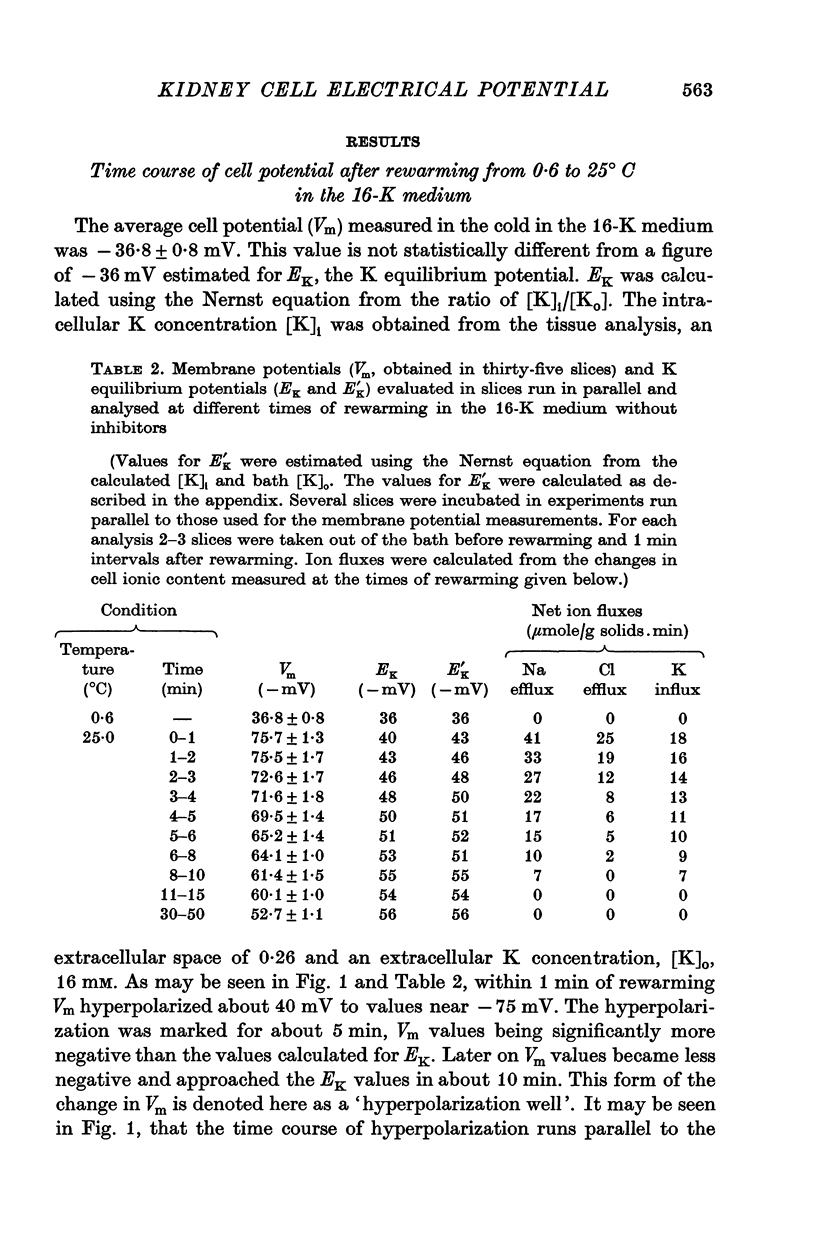
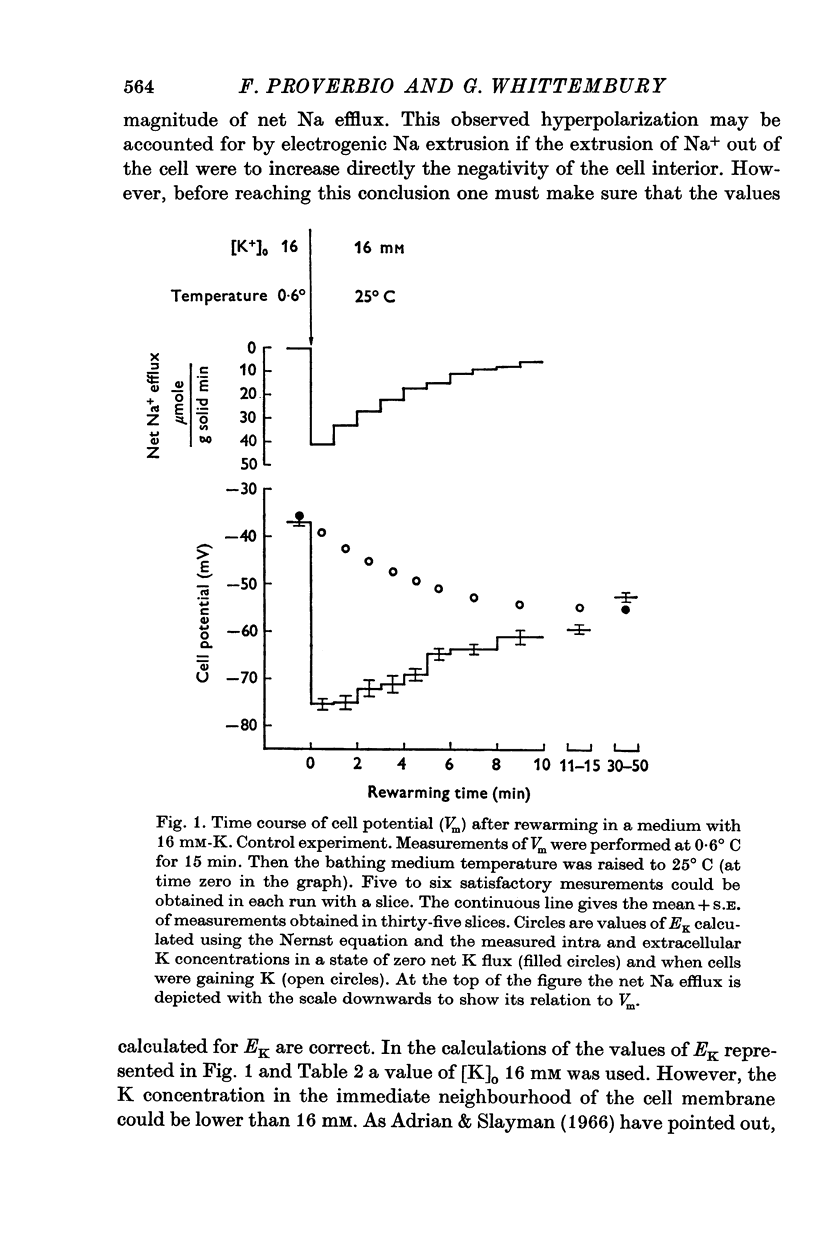
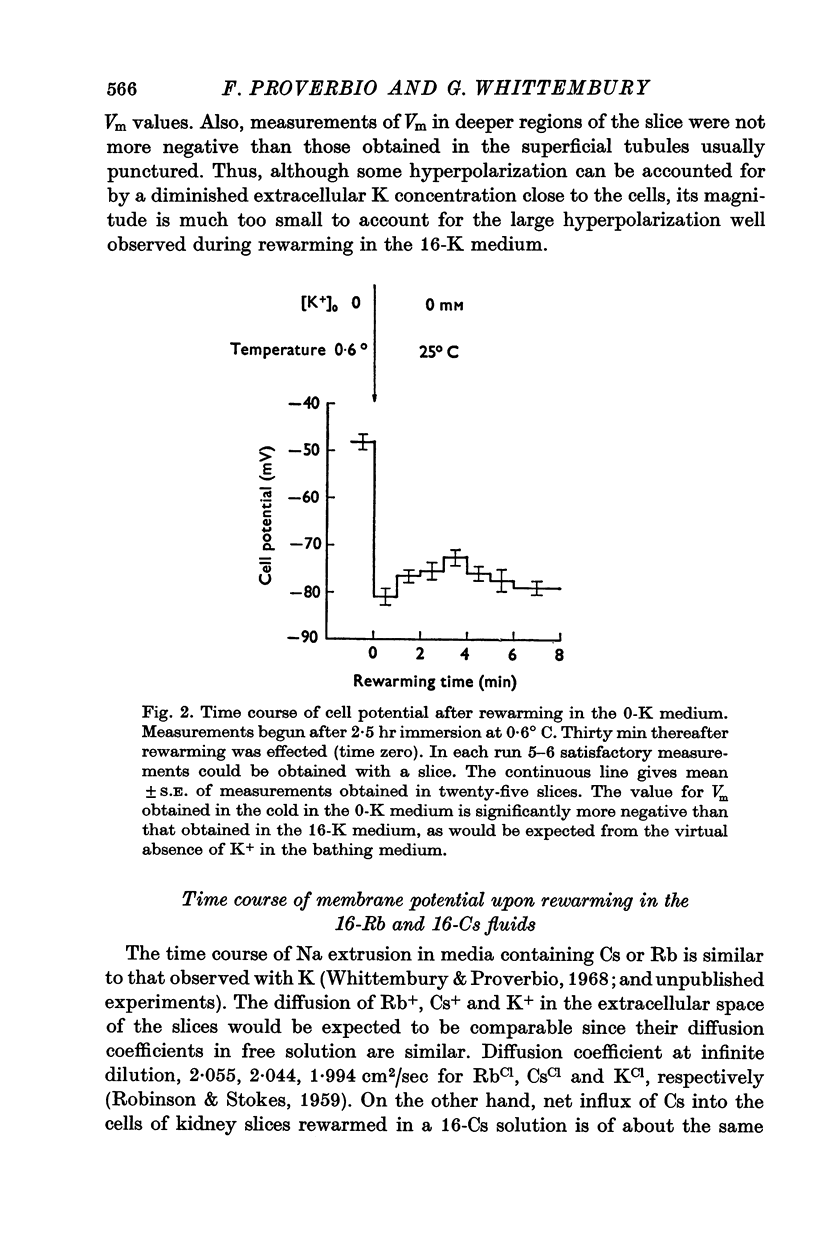
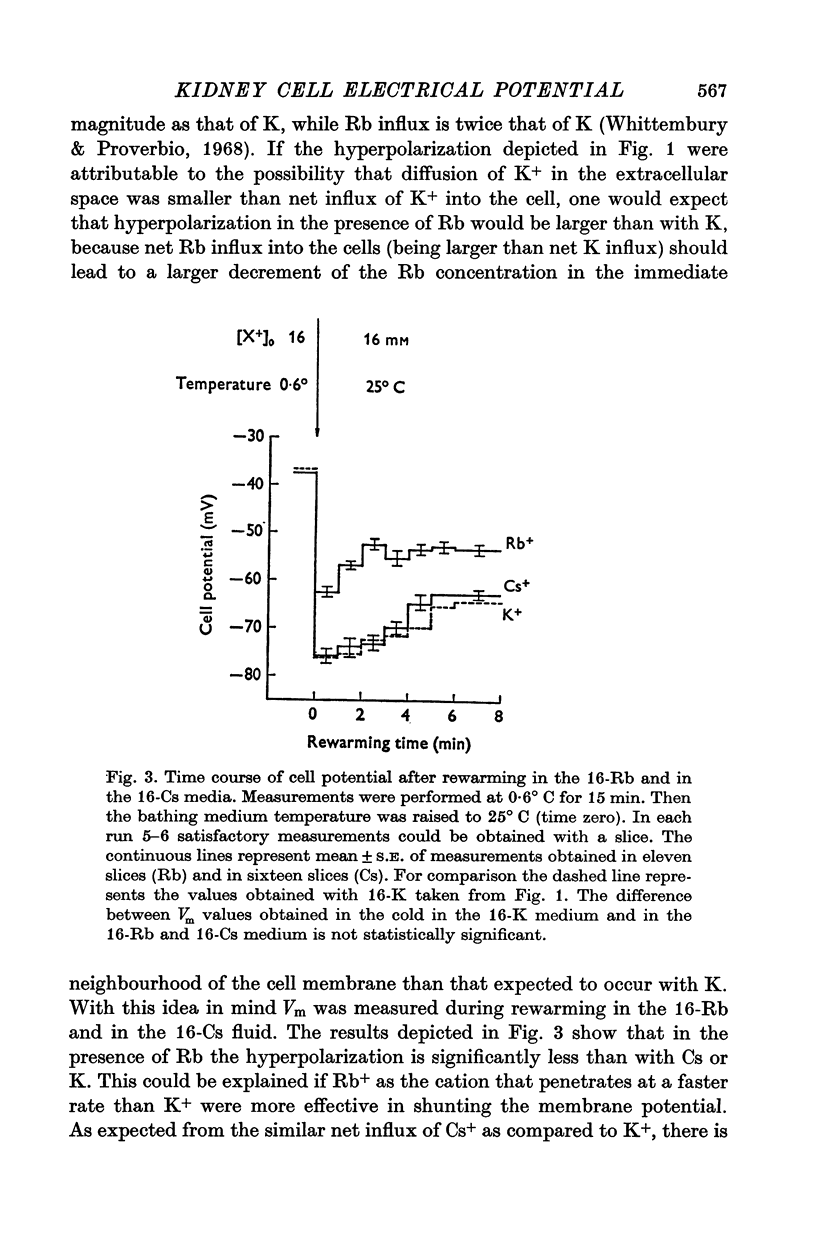
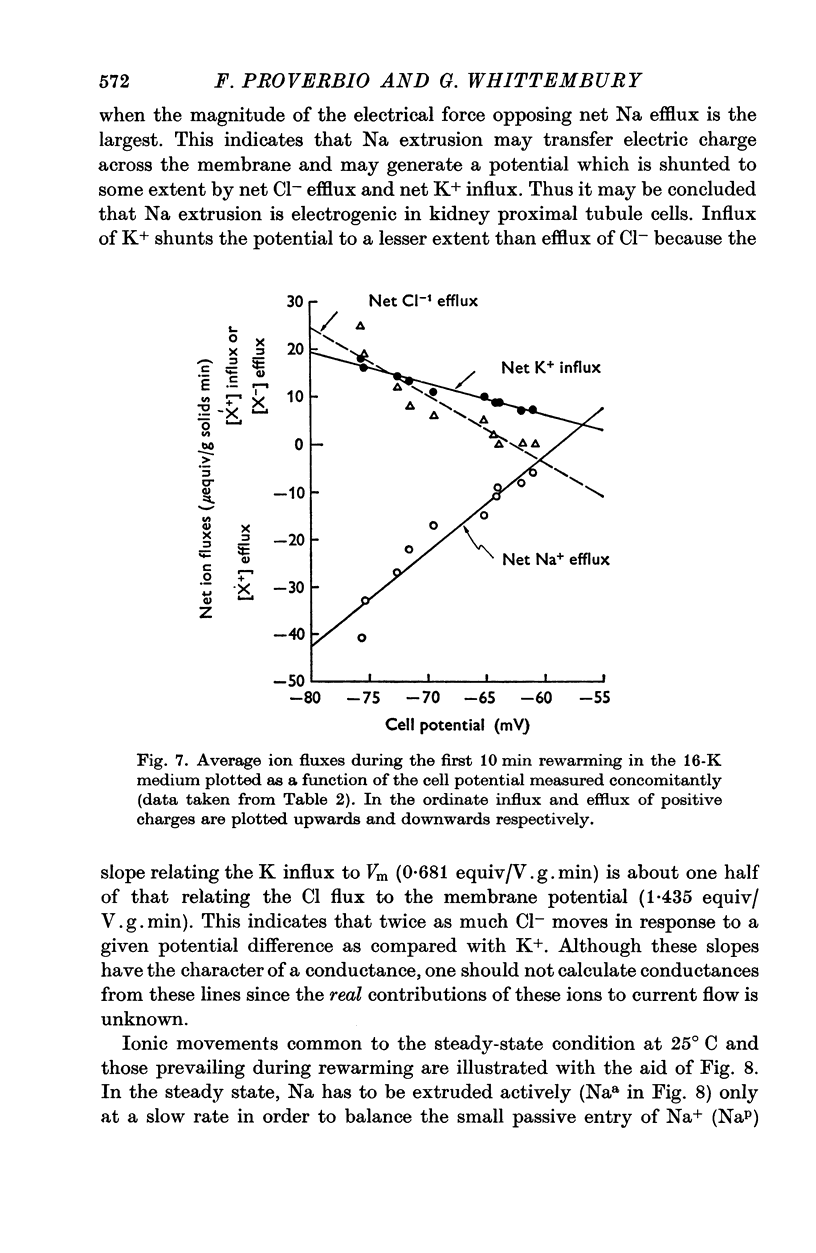
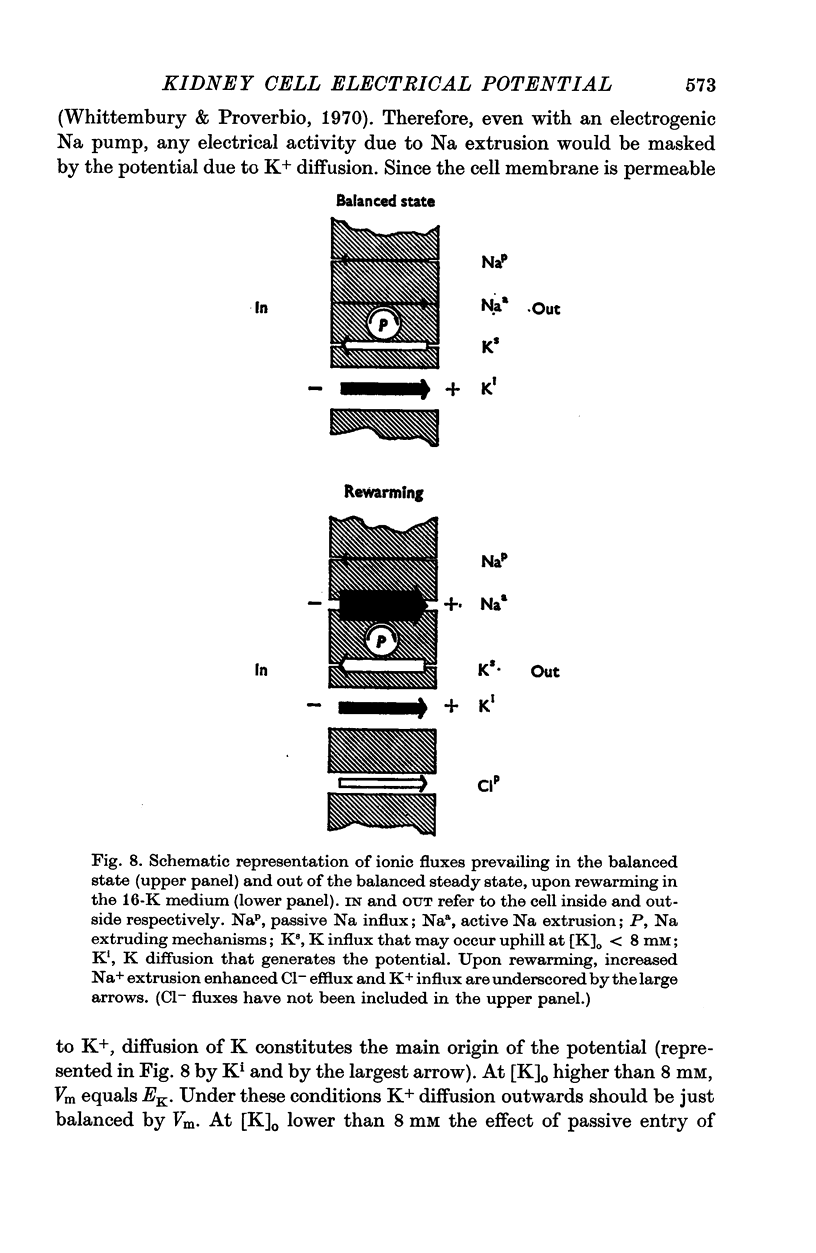
1. Experiments were performed on outermost slices of the guinea-pig kidney which are mainly made up of proximal tubular cells. 2. Kidney cells loaded with Na+ by chilling at 0.6 degrees C for 2.5 hr, when subsequently rewarmed to 25 degrees C in a medium containing 16 mM-K+ extrude Na+ at enhanced speed for about 10 min. This Na+ movement is accompanied by efflux of Cl and influx of K+. 3. Measurements of cell potential during enhanced Na+ extrusion show that cells hyperpolarize to values about 30 mV more negative than the K+ equilibrium potential. 4. This hyperpolarization is only partly inhibited by 1 mM ouabain or by 2 mM ethacrynic acid but both agents added together suppress it completely. 5. With 16 mM-Rb instead of 16 mM-K the hyperpolarization is smaller. 6. A diminished extracellular K+ concentration outside of the cells, within the slice, can account for only a small part of the hyperpolarization. 7. The hyperpolarization is proportional to the rate of Na+ pumping. 8. Cl- seems to shunt the hyperpolarization to a greater extent than K+. 9. It is concluded that Na+ extrusion is capable of transferring electric charge across the membrane.
Full text
PDF




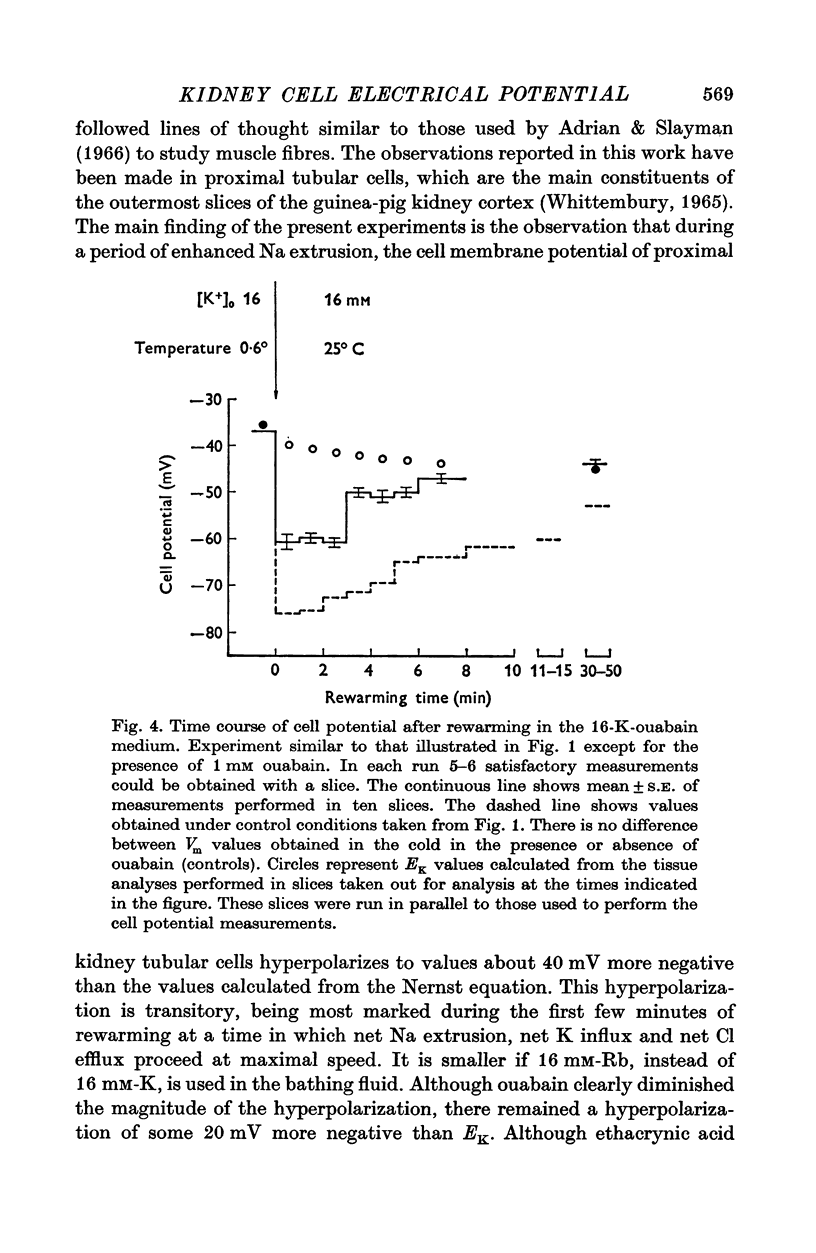
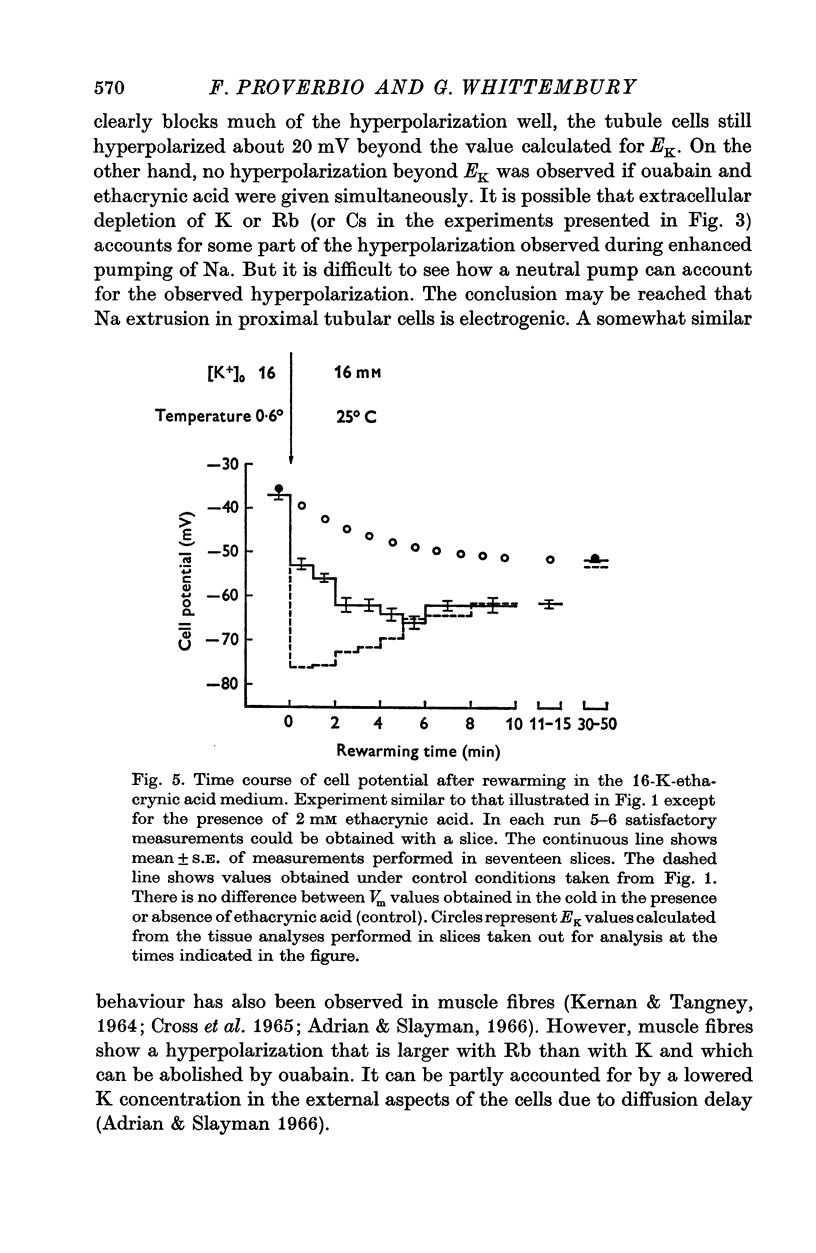
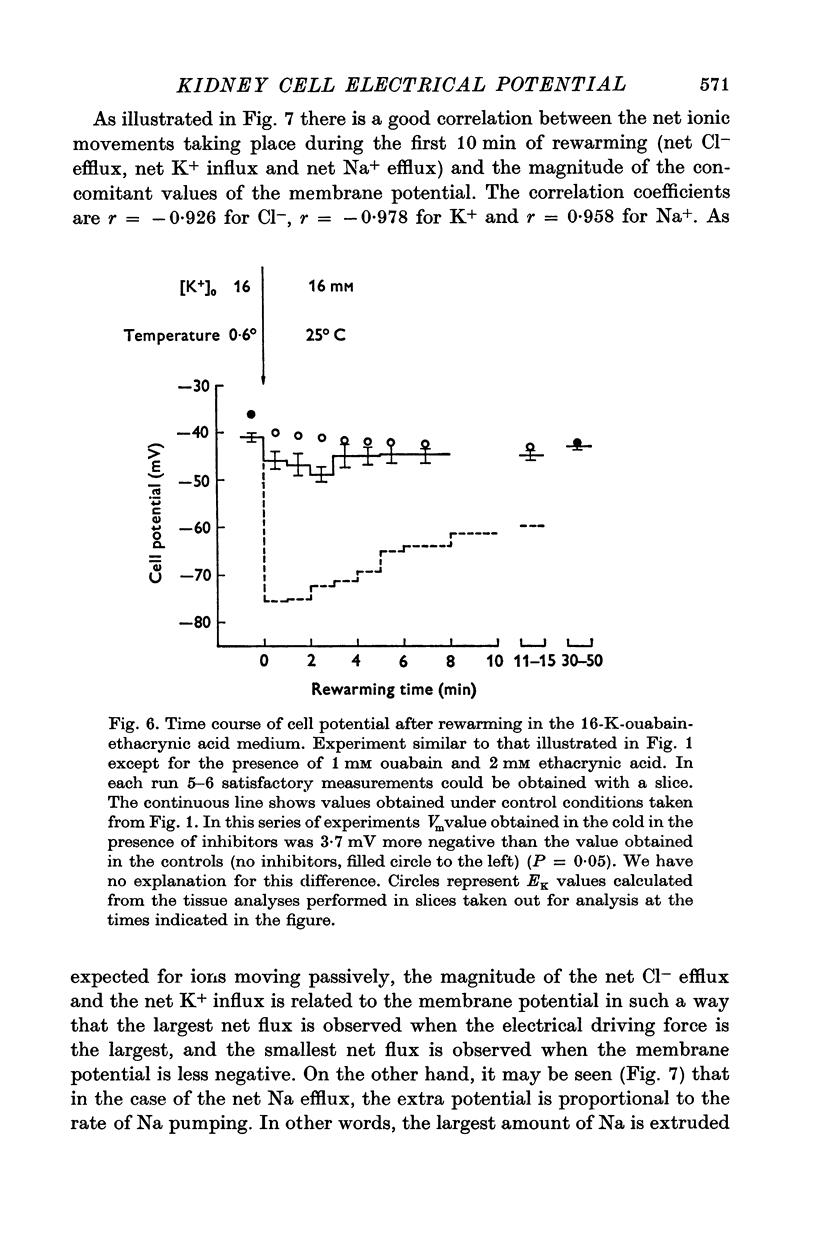





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Fitzgerald O. Diffusion relations of urea, inulin and chloride in some mammalian tissues. J Physiol. 1942 Jun 2;101(1):86–105. doi: 10.1113/jphysiol.1942.sp003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Fitzgerald O. Diffusion relations of urea, inulin and chloride in some mammalian tissues. J Physiol. 1942 Jun 2;101(1):86–105. doi: 10.1113/jphysiol.1942.sp003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. B., Keynes R. D., Rybová R. The coupling of sodium efflux and potassium influx in frog muscle. J Physiol. 1965 Dec;181(4):865–880. doi: 10.1113/jphysiol.1965.sp007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEYRUP I. J., USSING H. H. Accumulation of sulfate labelled with S35 by rat tissue in vitro. J Gen Physiol. 1955 May 20;38(5):599–612. doi: 10.1085/jgp.38.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEBISCH G. Measurements of electrical potential differences on single nephrons of the perfused Necturus kidney. J Gen Physiol. 1961 Mar;44:659–678. doi: 10.1085/jgp.44.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebisch G., Boulpaep E. L., Whittembury G. Electrolyte transport in kidney tubule cells. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):175–196. doi: 10.1098/rstb.1971.0088. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. THE EFFECT OF OUABAIN ON THE ELECTROLYTE AND WATER TRANSPORT IN KIDNEY CORTEX AND LIVER SLICES. J Physiol. 1964 Dec;175:172–192. doi: 10.1113/jphysiol.1964.sp007510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight A. D. The effects of ethacrynic acid on the electrolyte and water contents of rat renal cortical slices. Biochim Biophys Acta. 1969 Mar 11;173(2):223–233. doi: 10.1016/0005-2736(69)90106-0. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Water and electrolyte contents of rat renal cortical slices incubated in potassium-free media and media containing ouabain. Biochim Biophys Acta. 1968 Mar 1;150(2):263–270. doi: 10.1016/0005-2736(68)90169-7. [DOI] [PubMed] [Google Scholar]
- Orloff J., Burg M. Kidney. Annu Rev Physiol. 1971;33:83–130. doi: 10.1146/annurev.ph.33.030171.000503. [DOI] [PubMed] [Google Scholar]
- Podevin R. A., Boumendil-Podevin E. F. Effects of temperature, medium K+, ouabain and ethacrynic acid on transport of electrolytes and water by separated renal tubules. Biochim Biophys Acta. 1972 Sep 1;282(1):234–249. doi: 10.1016/0005-2736(72)90329-x. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WILLIS J. S. ION MOVEMENTS AND OXYGEN CONSUMPTION IN KIDNEY CORTEX SLICES. J Physiol. 1963 Aug;168:158–177. doi: 10.1113/jphysiol.1963.sp007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTEMBURY G., SUGINO N., SOLOMON A. K. Ionic permeability and electrical potential differences in Necturus kidney cells. J Gen Physiol. 1961 Mar;44:689–712. doi: 10.1085/jgp.44.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]
- Willis J. S. The interaction of K+, ouabain and Na+ on the cation transport and respiration of renal cortical cells of hamsters and ground squirrels. Biochim Biophys Acta. 1968 Dec 10;163(4):516–530. doi: 10.1016/0005-2736(68)90081-3. [DOI] [PubMed] [Google Scholar]


