Abstract
The archaeal plasma membrane consists mainly of diether lipids and tetraether lipids instead of the usual ester lipids found in other organisms. Although a molecule of tetraether lipid is thought to be synthesized from two molecules of diether lipids, there is no direct information about the biosynthetic pathway(s) or intermediates of tetraether lipid biosynthesis. In this study, we examined the effects of the fungal squalene epoxidase inhibitor terbinafine on the growth and ether lipid biosyntheses in the thermoacidophilic archaeon Thermoplasma acidophilum. Terbinafine was found to inhibit the growth of T. acidophilum in a concentration-dependent manner. When growing T. acidophilum cells were pulse-labeled with [2-14C]mevalonic acid in the presence of terbinafine, incorporation of radioactivity into the tetraether lipid fraction was strongly suppressed, while accumulation of radioactivity was noted at the position corresponding to diether lipids, depending on the concentration of terbinafine. After the cells were washed with fresh medium and incubated further without the radiolabeled substrate and the inhibitor, the accumulated radioactivity in the diether lipid fraction decreased quickly while that in the tetraether lipids increased simultaneously, without significant changes in the total radioactivity of ether lipids. These results strongly suggest that terbinafine inhibits the biosynthesis of tetraether lipids from a diether-type precursor lipid(s). The terbinafine treatment will be a tool for dissecting tetraether lipid biosynthesis in T. acidophilum.
One of the fundamental differences between members of the domain Archaea (archaebacteria) and other organisms is the molecular structure of the lipids in their plasma membranes (7). The membrane lipids of Archaea consist mainly of ether-linked lipids instead of the usual ester-linked lipids found in the domain Bacteria and eukaryotes. The ether-linked lipids are made of a glycerol group (or more-complex polyols) with various polar head groups and saturated isopranyl hydrophobic chains with 20, 25, or 40 carbon atoms. They are classified as diether (archaeol) and tetraether (caldarchaeol) lipids depending on the hydrophobic core (Fig. 1). A molecule of tetraether lipid is apparently made of two molecules of diether lipids in a structure, and tetraether lipids are thought to constitute a stable monolayer in the membrane (reviewed in reference 8). Although detailed studies of the structural properties of these ether-linked lipids have been made, their biosynthetic pathways are still only partially understood, especially that of tetraether lipids.
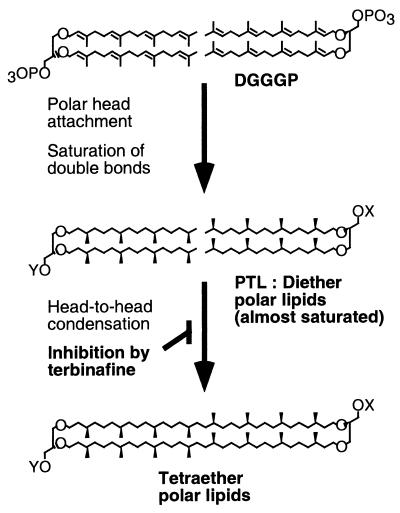
FIG. 1.
Hypothetical pathway of tetraether polar lipid biosynthesis and its inhibition by terbinafine in T. acidophilum. X and Y, complex polar head groups other than a simple phosphate group. PTL is expected to be a fully saturated diether polar lipid(s). However, the presence of a few double bonds in the precursor of diether polar lipids cannot be denied.
The hydrophobic chains of the diether lipids are synthesized from mevalonate through an isoprenoid biosynthetic pathway. Some enzymes and genes involved in the pathway have been isolated from Archaea (2, 4-6, 21, 27). Two molecules of geranylgeranyl diphosphate (C20) or geranylfarnesyl diphosphate (C25) are successively transferred to a glycerol group. Ether bond formation between geranylgeranyl diphosphate and sn-glycerol-1-phosphate in cell-free preparations from Methanobacterium thermoautotrophicum and Halobacterium halobium has been reported (29, 30). The resulting di-geranylgeranylglyceryl phosphate (DGGGP; an unsaturated form of diether lipid; Fig. 1) is thought to be a common precursor of most diether and tetraether lipids in Archaea. One molecule of the tetraether polar lipid is believed to be synthesized from two molecules of the corresponding unsaturated forms of diether polar lipids by head-to-head condensation (we followed the terminology in reference 15), followed by saturation of the isoprenyl moieties without changes of lipid arrangement in the membrane (20) (Fig. 1). This hypothesis is based on (i) the finding that the structure and asymmetric distribution of polar head groups of the tetraether lipids are identical to those of diether lipids in the cellular membrane of M. thermoautotrophicum with the exception of the ethanolamine group (18) and (ii) in vivo pulse-labeling and chase experiments with [32P]orthophosphate which showed that orthophosphate was incorporated into diether polar lipids faster than into tetraether polar lipids and that the incorporated radioactivity in the diether polar lipids quickly decreased, while that in the tetraether polar lipids increased, during chase experiments (20). However, there is no direct information about the biosynthetic pathway(s) or intermediates of the tetraether lipids.
The isoprenoid biosynthetic pathway is also quite important for eukaryotes and eubacteria. Squalene is a common product synthesized through this pathway. In eukaryotes, squalene is oxidized by squalene epoxidase and then enzymatically cyclized in the first step of steroid biosynthesis. Specific inhibitors of squalene epoxidase such as terbinafine have been reported. Terbinafine SF 86-327 is one of the allylamines, which were developed as synthetic antifungal drugs (22). Terbinafine has been studied in detail and has been shown to perform its antifungal activity by inhibiting squalene epoxidase (23, 24). The exact mechanism of the inhibition of squalene epoxidase by terbinafine is not yet clear. However, terbinafine may inhibit enzyme activity by disrupting a specific lipid-binding domain of the enzyme (25).
In this study, we examined the effects of terbinafine on the growth and lipid biosynthesis of the archaeon Thermoplasma acidophilum although steroids have not been found in Archaea. We found that terbinafine effectively inhibited the growth and the biosynthesis of tetraether lipids of T. acidophilum cells without inhibiting the biosynthesis of diether-type lipids. Furthermore, pulse-labeling and chase experiments with terbinafine strongly suggested that the tetraether polar lipids are synthesized from a diether-type polar lipid(s).
MATERIALS AND METHODS
Materials.
[2-14C]mevalonic acid dibenzylethylenediamine salt was purchased from NEN Life Science Products, Inc. Silica Gel 60 thin-layer chromatography (TLC) and RP-18 high-performance TLC (HPTLC) plates were purchased from Whatman or Merck. AgNO3-Silica Gel 60 TLC plates were prepared in the dark according to the method described by Kito et al. (12). Terbinafine was kindly provided by Banyu Pharmaceutical Co., Ltd., Tokyo, Japan.
Microorganisms and culture conditions.
T. acidophilum HO-62 (28) was grown at 57°C in medium composed of the following compounds in the indicated amounts (per liter) (28): Bacto Casamino Acids (Difco), 1.0 g; Bacto yeast extracts (Difco), 1.0 g; (NH4)2SO4, 1.3 g; KH2PO4, 0.3 g; NaCl, 0.2 g; MgSO4 · 7H2O, 0.25 g; CaCl2 · 2H2O, 0.05 g. The pH was adjusted to 1.8 with H2SO4. H. halobium strain L-33 was kindly provided by T. Yamanaka and Y. Fukumori (Tokyo Institute of Technology, Yokohama, Japan) and was grown at 37oC in the medium reported previously (10). Escherichia coli HB101 was grown at 37oC in Luria-Bertani medium.
Analysis of growth inhibition
Five milliliters of each fresh medium was inoculated with 0.5 ml of mid-log-phase culture of each organism. Five microliters of ethanol solution containing a suitable amount of terbinafine was added to the media at the time of inoculation. E. coli and H. halobium were cultured with shaking. T. acidophilum was incubated without shaking. The cell densities of E. coli and H. halobium were optically monitored at 600 nm with a spectrophotometer (DU640; Beckman). The cell density of T. acidophilum was determined with a counting chamber under a microscope.
Pulse-labeling and chase experiments.
For pulse-labeling ether lipids, 5 μCi of [2-14C]mevalonic acid dibenzylethylenediamine salt and the indicated amount of terbinafine were added to 5 ml of a mid-log-phase culture of T. acidophilum in a 15-ml test tube and the culture was incubated at 57oC for various times. For chase experiments, the pulse-labeled cells were washed repeatedly with fresh medium to remove the inhibitor and the radiolabeled substrate until the total radioactivity in the supernatant dropped below 100 dpm, usually twice, and then the cells were resuspended and incubated in fresh medium at 57oC.
Extraction and analyses of ether lipids.
After the pulse-labeling or pulse-labeling and chase experiment, the cells were immediately harvested by centrifugation at 1,650 × g for 20 min with a swing rotor at 4oC. Lipids were extracted according to the method of Bligh and Dyer (3). The cells harvested from the 5-ml culture were suspended in 200 μl of distilled water. Five hundred microliters of methanol and 250 μl of chloroform were added to the suspension sequentially, and then the suspension was stirred for 3 h at room temperature. Another 250 μl of chloroform and 250 μl of distilled water were added to the suspension. The suspension was separated into two phases by centrifugation. The lower chloroform phase was collected, and the solvent was evaporated with a stream of nitrogen gas. The residual total lipids were used for further analyses.
Ether core lipids were prepared by acid methanolysis of total lipids essentially as described previously (13, 14). The total lipids were treated with 500 μl of 5% methanolic HCl at 110oC for 10 h in a test tube with a Teflon-sealed screw cap. Then the core lipids were extracted with 500 μl of petroleum ether. Suitable aliquots of the petroleum ether phase of the hydrolyzed lipids were dried in a stream of nitrogen gas. The residual lipids were immediately dissolved in a small amount of chloroform-methanol (2:1 [vol/vol]) and analyzed by TLC. TLC was done on a 0.25-mm-thick Silica Gel 60 plate that had been activated by heat treatment at 150oC for 1 h. A chloroform-diethylether mixture (9:1 [vol/vol]) was used for the TLC development.
For further analysis of the radiolabeled ether lipids, hydrolyzed ether lipids were converted to their corresponding acetylated derivatives (12). A small amount of the sample which had been hydrolyzed by acid methanolysis was treated with 600 μl of pyridine and 300 μl of acetic anhydride with stirring at room temperature for 16 h. The solvent was evaporated with nitrogen gas. The hydrolyzed lipids and their acetylated derivatives were developed on an AgNO3-Silica Gel 60 TLC plate with a solvent system of benzene-chloroform-methanol (98:2:0.1 [vol/vol]) or developed on an RP-18 HPTLC plate with a solvent system of acetone. Radioactive spots on the TLC plates were recorded, and the radioactivity of each spot was estimated by a BAS 2000 bioimaging analyzer (Fuji film) with an imaging plate. A known amount of radioactive compound was spotted on the same plate as a standard for quantitative analysis.
Standard lipids.
Standards of archaeol and caldarchaeol were prepared from stationary-phase cultures of H. halobium and T. acidophilum, respectively, labeled with [2-14C]mevalonic acid as described above.
RESULTS
Effects of terbinafine on the growth of Archaea.
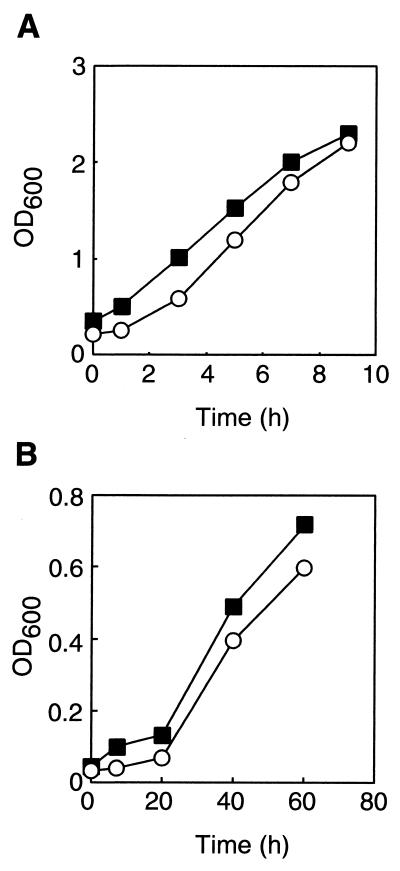
Terbinafine is one of the potent inhibitors of squalene epoxidase in fungi. To investigate the effect of the compound on the growth of Bacteria and Archaea, it was added to the cultures of E. coli HB101, extremely halophilic archaeon H. halobium L-33 (10), and thermoacidophilic archaeon T. acidophilum HO-62 (28). Terbinafine showed little effect on the growth rate and saturation cell density of E. coli and those of H. halobium (Fig. 2). On the other hand, the growth of T. acidophilum was strongly inhibited by the compound in a concentration-dependent manner (Fig. 3A). The MICs of terbinafine against various species of fungi and pathogenic yeasts range from 0.001 to 128 μg/ml (1). Therefore, the concentrations of terbinafine used in our experiments (1 to 100 μg/ml) were not much higher than those required to inhibit the growth of various fungi and yeasts.
FIG. 2.
Effect of terbinafine on the growth of E. coli (A) and H. halobium (B). The concentration of terbinafine was 0 (circles) or 100 μg/ml (squares). OD600, optical density at 600 nm.
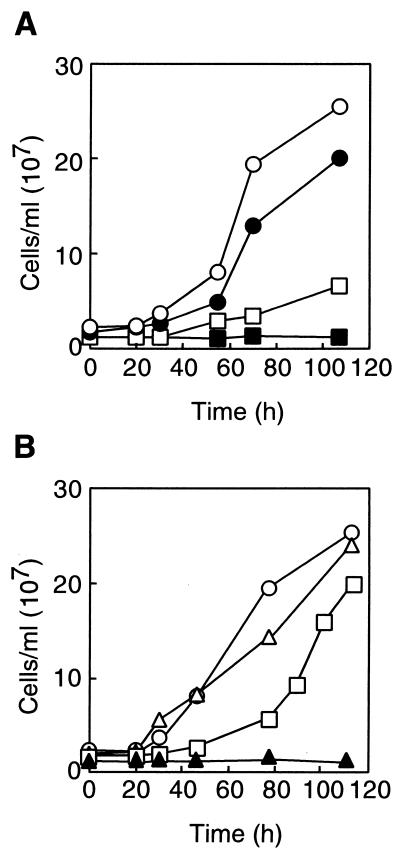
FIG. 3.
(A) Effect of terbinafine on the growth of T. acidophilum. Concentration of inhibitors: 0 (open circles), 1 (solid circles), 10 (open squares), and 100 μg/ml (solid squares). (B) Effect of terbinafine after washing the cells with fresh medium. T. acidophilum cells were incubated in the presence of 0 (circles), 100 (open and solid triangles), or 600 (open and solid squares) μg of terbinafine/ml at 57°C for 1 h. Then, the cells were washed with fresh medium and further cultivated for the times indicated in the absence (open symbols) or presence (solid triangles) of the compound.
The inhibitory effect of terbinafine on the growth of T. acidophilum was reversible (Fig. 3B). A growth rate comparable to that of the organism in the absence of the inhibitor could be attained by washing and resuspending the cells in the medium without the inhibitor after incubating the cells with 100 or 600 μg of terbinafine/ml.
Squalene epoxidase (EC 1.14.99.7) catalyzes the epoxidation of squalene, which is the first step in steroid biosynthesis. Steroids are unique lipids found only in eukaryotes and have not been found in either Bacteria or Archaea. Some members of Bacteria have steroid analogs called hopanoids, but neither epoxidation nor epoxidase participates in their biosynthesis. Accordingly, it is reasonable that the inhibitor of squalene epoxidase showed no effect on the growth of the bacterium E. coli and archaeon H. halobium. However, terbinafine inhibited the growth of the thermoacidophilic archaeon T. acidophilum, implying that the inhibitor affected at least a biological reaction in T. acidophilum.
Effects of terbinafine on ether lipid biosynthesis in T. acidophilum.
One of the major differences between halophilic archaea and thermoacidophilic archaea is the composition of ether lipids in their cellular membranes. All of the membrane ether lipids of H. halobium are fully saturated diether lipids, while those of T. acidophilum are a mixture of diether lipids (5 to 10%) and tetraether lipids (90 to 95%) (reviewed in reference 16). Considering the difference, we examined the effect of terbinafine on the biosynthesis of diether and tetraether lipids in T. acidophilum.
To monitor ether lipid biosynthesis in T. acidophilum cells, we utilized a pulse-labeling and chase experiment, in which the cells were labeled with [2-14C]mevalonic acid for a short time and were further incubated after removal of the radiolabeled substrate by washing the cells with fresh medium. T. acidophilum cells were pulse-labeled for short periods (15 or 60 min), which are much shorter than the doubling time of the archaeon (10 to 11 h). After the pulse-labeling or the pulse-labeling and chase periods, lipids were extracted from the cells and the polar head groups of the lipids were removed by acid methanolysis. The resulting 14C-labeled ether core lipids were analyzed by TLC (Fig. 4 to 6).
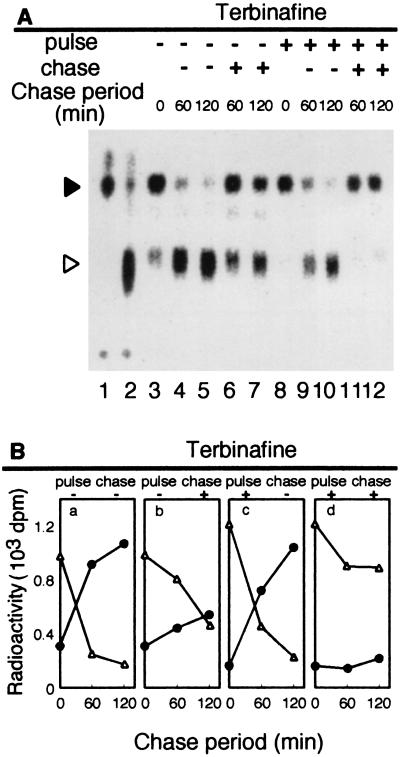
FIG. 4.
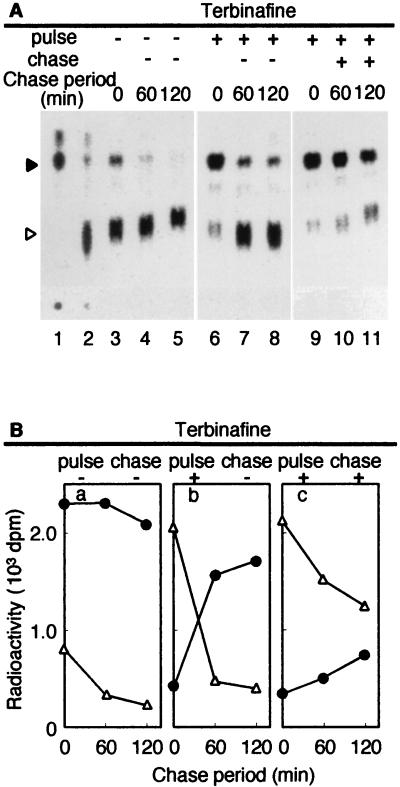
Effect of terbinafine on ether lipid biosynthesis in T. acidophilum after 15 min of pulse-labeling. T. acidophilum cells were labeled with 5 μCi of [2-14C]mevalonic acid for 15 min (pulse-labeling) in the absence or presence of 600 μg of terbinafine/ml. Then, the cells were washed with fresh medium and incubated without the radiolabeled substrate in the absence or presence of terbinafine (600 μg/ml) for the times indicated (chase). After the pulse-labeling or the pulse-labeling and chase period, the lipids were extracted from the cells, and the core lipids were analyzed by TLC as described in Materials and Methods. (A) Autoradiogram of the TLC plate. Solid and open arrowheads, diether and tetraether core lipids, respectively. Authentic [14C]diether core lipid prepared from H. halobium and [14C]tetraether core lipid prepared from T. acidophilum were spotted at lanes 1 and 2, respectively. (B) Radioactivities incorporated in diether (triangles) and tetraether (circles) core lipid fractions.
FIG. 6.
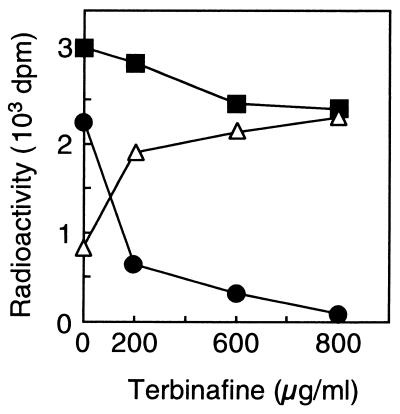
Concentration dependence of the effect of terbinafine on ether lipid biosynthesis in T. acidophilum. Five microcuries of [2-14C]mevalonic acid and the indicated concentration of terbinafine were added to a mid-log-phase culture of T. acidophilum. After a 60-min incubation, the lipids were extracted, and [14C]diether (triangles) and [14C]tetraether (circles) core lipids were isolated and quantified as described in Materials and Methods. Squares, radioactivity in the total ether lipids.
When growing cells were pulse-labeled for 15 min, 76.2 and 23.8% of the total radioactivity in ether lipids were incorporated into diether and tetraether lipids, respectively (Fig. 4A, lane 3). Lanes 1 and 2 show ether lipids prepared from stationary-phase cultures of H. halobium and T. acidophilum, respectively. The positions of diether and tetraether lipids are shown. They are major lipids in H. halobium and T. acidophilum, respectively (16). The lipids that migrated at the top are expected to be the neutral lipids, which did not appear in short labeling experiments (11). During the chase period, the radioactivity incorporated in the diether lipids quickly decreased while that in the tetraether lipids increased simultaneously (Fig. 4A, lanes 4 and 5) without significant changes in the total radioactivity of the ether lipids (Fig. 4B, a). After 120 min, 13.8 and 86.2% of the total radioactivity in ether lipids were incorporated into diether and tetraether lipids, respectively. The ratio is similar to that in the membrane of T. acidophilum. The changes in the radioactivities suggest a precursor-product relationship between the diether and tetraether lipids in T. acidophilum. The results also indicate that we could monitor the biosynthesis of diether lipids after a 15-min pulse-labeling and that of tetraether lipids during the chase period.
Under the same conditions, we examined the effect of terbinafine on ether lipid biosynthesis by adding the compound to the cell culture during the pulse-labeling and/or the chase period (Fig. 4A, lanes 6 to 12, and 4B, b to d). The incorporation of radioactivity into diether lipids during the pulse period was little affected by terbinafine (Fig. 4A, lane 8). However, the changes of radioactivity in diether and tetraether lipids during the chase period were strongly inhibited by the presence of terbinafine (Fig. 4A, lanes 6, 7, 11, and 12, and 4B, b and d). These results suggest that terbinafine inhibits tetraether lipid biosynthesis from the diether lipids without significantly affecting diether lipid biosynthesis.
To test the possible inhibition of tetraether lipid biosynthesis by terbinafine, the cells were pulse-labeled for a longer period and the radioactivity in the ether lipids was chased. When the cells were labeled for 60 min without terbinafine, radioactivity was incorporated mainly into tetraether lipids (73.0%; Fig. 5A, lane 3). The ratio of the radioactivities in diether and tetraether lipids is close to the composition of the ether lipids in the membrane of T. acidophilum. However, the radioactivity in the tetraether lipids significantly decreased when terbinafine was added to the medium during the pulse-labeling, while the accumulation of radioactivity at the position corresponding to diether lipids was noted (Fig. 5A, lane 6). The effect of the inhibitor on the radioactivities in tetra- and diether lipids depended on the concentration of the inhibitor (Fig. 6), while the radioactivity in the total ether lipids was not significantly affected by the presence of terbinafine.
FIG. 5.
Effect of terbinafine on ether lipid biosynthesis in T. acidophilum after 60 min of pulse-labeling. The pulse-labeling, the pulse-labeling and chase experiments, and analysis of the lipids were carried out as described in the legend to Fig. 4 except that the cells were labeled for 60 min instead of 15 min. (A) Autoradiograms of the TLC plates. Solid and open arrowheads, diether and tetraether core lipids, respectively. Authentic [14C]diether core lipid prepared from H. halobium and [14C]tetraether core lipid prepared from T. acidophilum were spotted at lanes 1 and 2, respectively. (B) Radioactivities in diether (triangles) and tetraether (circles) core lipid fractions.
A small spot moving between the diether and tetraether-type lipid cores and another just above the diether-type lipid core were noted in some lanes in Fig. 4A and 5A. Swain et al. have shown that T. acidophilum contains a small amount of hydroxydiether lipids (26). The lipids are sensitive to methanolic HCl and are converted to degradation artifacts appearing in TLC as the methoxyl (between caldarchaeol and archaeol) and cis/trans forms of archaeol (9). The small spots that appeared in some lanes may correspond to these degraded products. However, because the amount was small and showed no clear relation to the treatment with terbinafine, we did not analyze the spots further in this report.
When the chase experiment was performed in the absence of terbinafine after the 60-min pulse-labeling in the presence of the compound, the accumulated radioactivity in the diether lipid fraction quickly decreased, with a parallel increment of radioactivity in tetraether lipids (Fig. 5A, lanes 7 and 8, and 5B, b). In contrast, during the chase period in the presence of terbinafine, the changes of the radioactivities in the diether and tetraether lipids were significantly suppressed (Fig. 5A, lanes 10 and 11, and 5B, c). These results strongly suggest that (i) the tetraether lipids are synthesized from the lipid(s) detected at the position corresponding to the diether lipid that accumulated in the presence of terbinafine, which is designated PTL [for precursor(s) of tetraether lipids], and that (ii) terbinafine inhibits the reaction(s) converting PTL to the tetraether lipids.
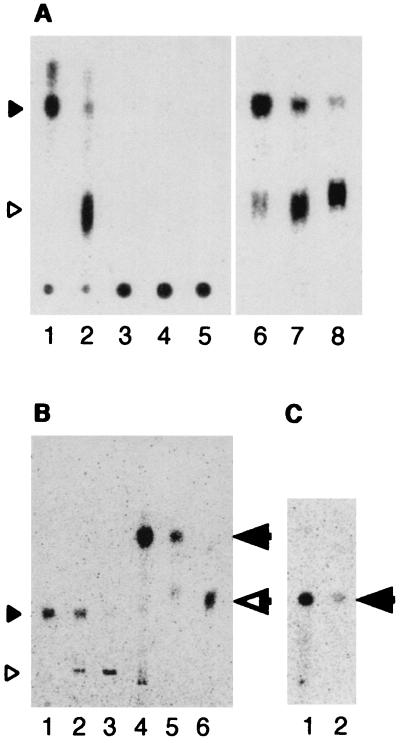
The PTL that accumulated in the presence of terbinafine was examined by several TLC analyses (Fig. 7). When PTL was developed in a normal-phase TLC without acid methanolysis, it remained at the origin of TLC development (Fig. 7A, left), while the lipid was detected at the position of the diether core lipid after the removal of polar head groups by acid methanolysis (Fig. 7A, right). The Rf values for hydrolyzed PTL and its acetylated derivative were the same as those for the authentic diether core lipid and acetylated diether core lipid in two additional TLCs: AgNO3-TLC and reverse-phase HPTLC (Fig. 7B and C). The absence of a double bond in the diether core lipid of H. halobium has been well documented (11). The results suggest that PTL is a diether-type polar lipid(s).
FIG. 7.
Analyses of the PTL by normal-phase TLC (A), AgNO3-TLC (B), and reverse-phase HPTLC (C). Autoradiograms of the TLC plates are shown. Positions of diether core lipid (solid arrowhead), tetraether core lipid (open arrowhead), acetylated diether core lipid (solid arrow), and acetylated tetraether core lipid (open arrow) are indicated. (A) The radiolabeled lipids were analyzed by normal-phase TLC before (lanes 3 to 5) or after acid methanolysis (lanes 6 to 8). Lane 1, authentic [14C]diether core lipid prepared from H. halobium; lane 2, authentic [14C]tetraether core lipid prepared from T. acidophilum; lanes 3 and 6, ether lipids prepared from T. acidophilum cells pulse-labeled in the presence of 600 μg of terbinafine/ml for 60 min; lanes 4 and 7, ether lipids prepared from the cells pulse-labeled in the presence of 600 μg of terbinafine/ml for 60 min followed by the chase for 60 min without the compound; lanes 5 and 8, ether lipids prepared from the cells pulse-labeled in the presence of 600 μg of terbinafine/ml for 60 min followed by the chase without the compound for 120 min. (B and C) The radiolabeled lipids were analyzed by AgNO3-TLC (B) or reverse-phase HPTLC (C) after acid methanolysis (lanes 1 to 3) followed by acetylation (lanes 4 to 6). Lanes 1 and 4, authentic diether core lipid prepared from H. halobium; lanes 2 and 5, ether lipids prepared from T. acidophilum pulse-labeled in the presence of 600 μg of terbinafine/ml for 60 min; lanes 3 and 6, authentic [14C]tetraether core lipid prepared from T. acidophilum.
DISCUSSION
In this study, we provided evidence that terbinafine, a potent inhibitor of squalene epoxidase in eukaryotes, inhibits tetraether lipid biosynthesis from a diether lipid(s) in the thermoacidophilic archaeon T. acidophilum. The inhibitory effect of terbinafine on tetraether lipid biosynthesis was reversible, at least under the conditions used in this study. The phenomenon is consistent with the observation that the growth rate was recovered by washing the cells after the inhibition by terbinafine (Fig. 3B).
Tetraether lipids have been thought to be synthesized from unsaturated diether lipid DGGGP (Fig. 1), which contains four double bonds per hydrophobic chain and a phosphate group as a polar head group. At least three reactions, head-to-head condensation, saturation of hydrophobic chains, and modification of a polar head group, must occur during the biosynthesis of tetraether polar lipids from DGGGP (Fig. 1). Our results suggest that PTL is different from DGGGP in two characteristics. First, the hydrophobic cores of unsaturated ether lipids including DGGGP are acid labile and yield degraded hydrophobic chains and a glycerol group after acid methanolysis treatment (17, 19), while that of PTL was acid stable and yielded diether core lipid after the treatment. The results suggest that PTL contains fewer unsaturated C-C bonds on its hydrophobic chains than DGGGP. Second, DGGGP contains a simple phosphate group as a polar head group, which can be removed by alkaline phosphatase treatment (30), while the polar head group of PTL could not be removed by the treatment (unpublished result), suggesting that PTL has a modified polar head group different from a simple phosphate group. Accordingly, PTL is likely to be an intermediate at a stage of tetraether lipid biosynthesis later than that at which DGGGP is an intermediate. Based on the characteristics of PTL, we propose that unsaturated diether lipids can be converted to tetraether lipids after (i) the saturation of some double bonds on their hydrophobic chains and (ii) the modification of the polar head group (Fig. 1) Morii and Koga (18) and Nishihara et al. (20) suggested the possibility that the polar head groups attach to the diether lipids before the head-to-head condensation to form tetraether polar lipids. Our proposals are consistent with their model. More-detailed analyses of PTL will provide clues for further dissection of the tetraether biosynthetic pathway and for identification of the enzymes responsible for the reaction(s); these analyses are in progress.
It is widely accepted that molecular biology and some biochemical reactions in Eucarya are more closely related to those of Archaea than to those of Bacteria. Nevertheless, membrane lipids for these groups are totally different. Most of the membrane lipids of Archaea are isopranyl ether lipids, while eukaryotes have ester lipids as the main component of the membranes and a very small quantity of ether lipids and isoprenoids as molecules with specific functions. We found that the inhibitor of steroid biosynthesis, which is unique to Eucarya, inhibited tetraether lipid biosynthesis in one of the thermoacidophilic archaea. This implies that the enzyme that catalyzes the head-to-head condensation of Archaea may be structurally similar to squalene epoxidase, in a sense that both can be inhibited by terbinafine. If the enzymes are genetically related, this shows the linkage between tetraether lipid biosynthesis in thermophilic Archaea and steroid biosynthesis in Eucarya.
Acknowledgments
We thank Banyu Pharmaceutical Co., Ltd., for providing terbinafine.
This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (09839033).
REFERENCES
- 1.Balfour, J. A., and D. Faulds. 1992. Terbinafine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial mycoses. Drugs 43:259-284. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, K. M., and V. W. Rodwell. 1996. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase from Haloferax volcanii: purification, characterization, and expression in Escherichia coli. J. Bacteriol. 178:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Bochar, D. A., J. R. Brown, W. F. Doolittle, H. P. Klenk, W. Lam, M. E. Schenk, C. V. Stauffacher, and V. W. Rodwell. 1997. 3-Hydroxy-3-methylglutaryl coenzyme A reductase of Sulfolobus solfataricus: DNA sequence, phylogeny, expression in Escherichia coli of the hmgA gene, and purification and kinetic characterization of the gene product. J. Bacteriol. 179:3632-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, A., and C. D. Poulter. 1994. Isolation and characterization of idsA: the gene for the short chain isoprenyl diphosphate synthase from Methanobacterium thermoautotrophicum. Arch. Biochem. Biophys. 314:399-404. [DOI] [PubMed] [Google Scholar]
- 6.Chen, A., and C. D. Poulter. 1993. Purification and characterization of farnesyl diphosphate/geranylgeranyl diphosphate synthase. J. Biol. Chem. 268:11002-11007. [PubMed] [Google Scholar]
- 7.De Rosa, M., and A. Gambacorta. 1988. The lipids of archaebacteria. Prog. Lipid Res. 27:153-175. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa, M., A. Gambacorta, and A. Gliozzi. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekiel, I., and G. D. Sprott. 1992. Identification of degradation artifacts formed upon treatment of hydroxydiether lipids from methanogens with methanolic HCl. Can. J. Microbiol. 38:764-768. [Google Scholar]
- 10.Fukumori, Y., T. Fujiwara, Y. Okada-Takahashi, Y. Mukohata, and T. Yamanaka. 1985. Purification and properties of a peroxidase from Halobacterium halobium L-33. J. Biochem. (Tokyo) 98:1055-1061. [DOI] [PubMed] [Google Scholar]
- 11.Kates, M. 1978. The phytanyl ether-linked polar lipids and isoprenoid neutral lipids of extremely halophilic bacteria. Prog. Chem. Fats Other Lipids 15:301-342. [DOI] [PubMed] [Google Scholar]
- 12.Kito, M., M. Ishinaga, M. Nishihara, M. Kato, S. Sawada, and T. Hata. 1975. Metabolism of the phosphatidylglycerol molecular species in Escherichia coli. Eur. J. Biochem. 54:55-63. [DOI] [PubMed] [Google Scholar]
- 13.Kushwaha, S. C., M. Kates, G. D. Sprott, and I. C. P. Smith. 1981. Novel polar lipids from the methanogen Methanospirillum hungatei GP1. Biochim. Biophys. Acta 664:156-173. [DOI] [PubMed] [Google Scholar]
- 14.Langworthy, T. A. 1982. Lipids of Thermoplasma. Methods Enzymol. 88:396-406. [Google Scholar]
- 15.Langworthy, T. A. 1985. Lipids of archaebacteria, p. 459-497. In C. R. Woese and R. S. Wolfe (ed.), The bacteria, vol. VIII. Academic Press Inc., London, United Kingdom. [Google Scholar]
- 16.Langworthy, T. A., and J. L. Pond. 1986. Archaebacterial ether lipids and chemotaxonomy. Syst. Appl. Microbiol. 7:253-257. [Google Scholar]
- 17.Moldoveanu, N., and M. Kates. 1988. Biosynthetic studies of the polar lipids of Halobacterium cutirubrum. Formation of isoprenyl ether intermediates. Biochim. Biophys. Acta 960:164-182. [Google Scholar]
- 18.Morii, H., and Y. Koga. 1994. Asymmetrical topology of diether- and tetraether-type polar lipids in membranes of Methanobacterium thermoautotrophicum cells. J. Biol. Chem. 269:10492-10497. [PubMed] [Google Scholar]
- 19.Morii, H., M. Nishihara, and Y. Koga. 2000. CTP:2,3-di-O-geranylgeranyl-sn-glycero-1-phosphate cytidyltransferase in the methanogenic archaeon Methanothermobacter thermoautotrophicus. J. Biol. Chem. 275:36568-36574. [DOI] [PubMed] [Google Scholar]
- 20.Nishihara, M., H. Morii, and Y. Koga. 1989. Heptads of polar ether lipids of an archaebacterium, Methanobacterium thermoautotrophicum: structure and biosynthetic relationship. Biochemistry 28:95-102. [Google Scholar]
- 21.Ohnuma, S., M. Suzuki, and T. Nishino. 1994. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J. Biol. Chem. 269:14792-14797. [PubMed] [Google Scholar]
- 22.Petranyi, G., N. S. Ryder, and A. Stutz. 1984. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science 224:1239-1241. [DOI] [PubMed] [Google Scholar]
- 23.Ryder, N. S. 1984. Selective inhibition of squalene epoxidation by allylamine antimycotic agents, p. 313-321. In C. Nombela (ed.), Microbial cell wall synthesis and autolysis. Elsevier, Amsterdam, The Netherlands.
- 24.Ryder, N. S. 1985. Specific inhibition of fungal sterol biosynthesis by SF 86-327, a new allylamine antimycotic agent. Antimicrob. Agents Chemother. 27:252-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryder, N. S. 1990. Squalene epoxidase-enzymology and inhibition, p. 189-203. In P. J. Kuhn, A. P. J. Trinci, M. J. Jung, M. W. Goosey, and L. G. Copping (ed.), Biochemistry of cell walls and membranes in fungi. Springer-Verlag, Berlin, Germany.
- 26.Swain, M., J.-R. Brisson, G. D. Sprott, F. P. Cooper, and G. B. Patel. 1997. Identification of β-L-gulose as the sugar moiety of the main polar lipid Thermoplasma acidophilum. Biochim. Biophys. Acta 1345:56-64. [DOI] [PubMed] [Google Scholar]
- 27.Tachibana, A. 1994. A novel prenyltransferase, farnesylgeranyl diphosphate synthase, from the haloalkaliphilic archaeon Natronobacterium pharaonis. FEBS Lett. 341:291-294. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda, M., H. Oyaizu, A. Yamagishi, and T. Oshima. 1995. Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs. Appl. Environ. Microbiol. 61:3482-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial lipids in Halobacterium halobium and Methanobacterium thermoautotrophicum. J. Org. Chem. 58:3919-3922. [Google Scholar]
- 30.Zhang, D., and C. D. Poulter. 1993. Biosynthesis of archaebacterial ether lipids. Formation of ether linkages by prenyltransferases. J. Am. Chem. Soc. 115:1270-1277. [Google Scholar]