Abstract
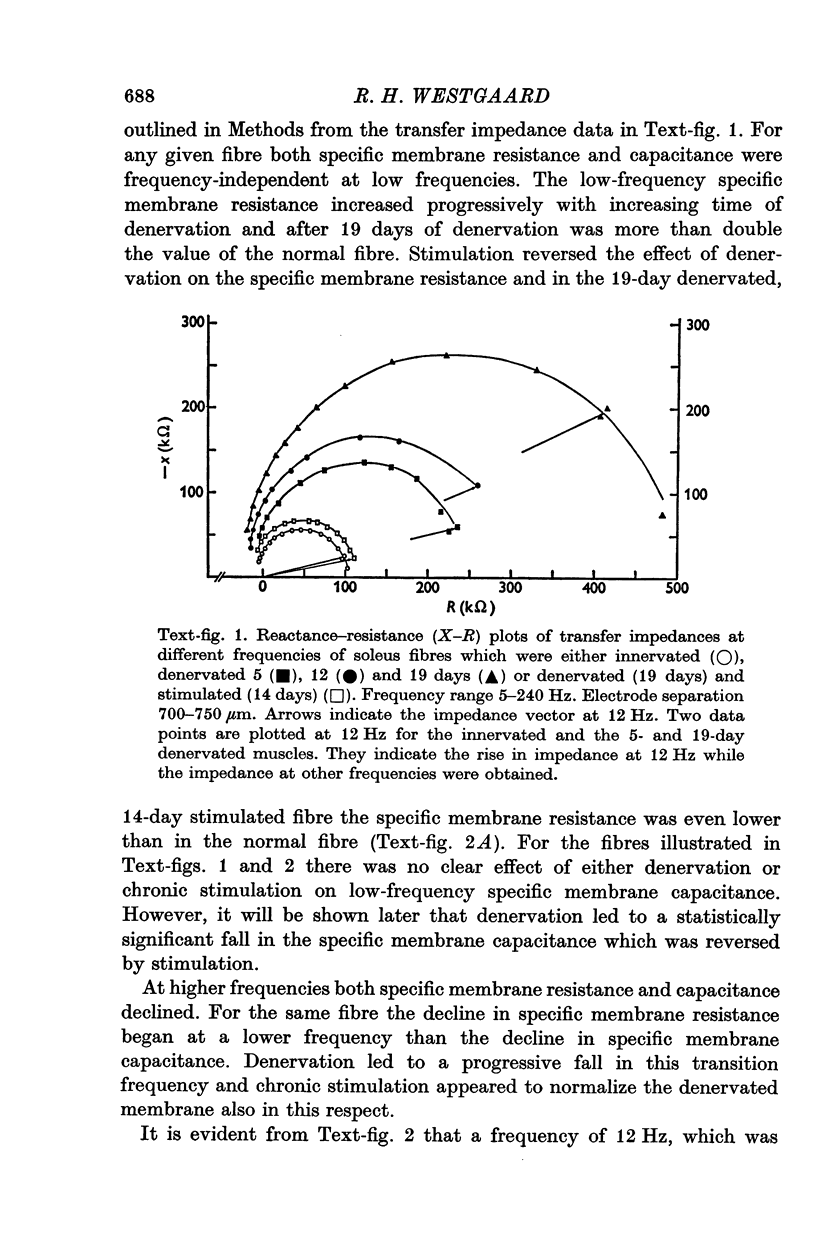
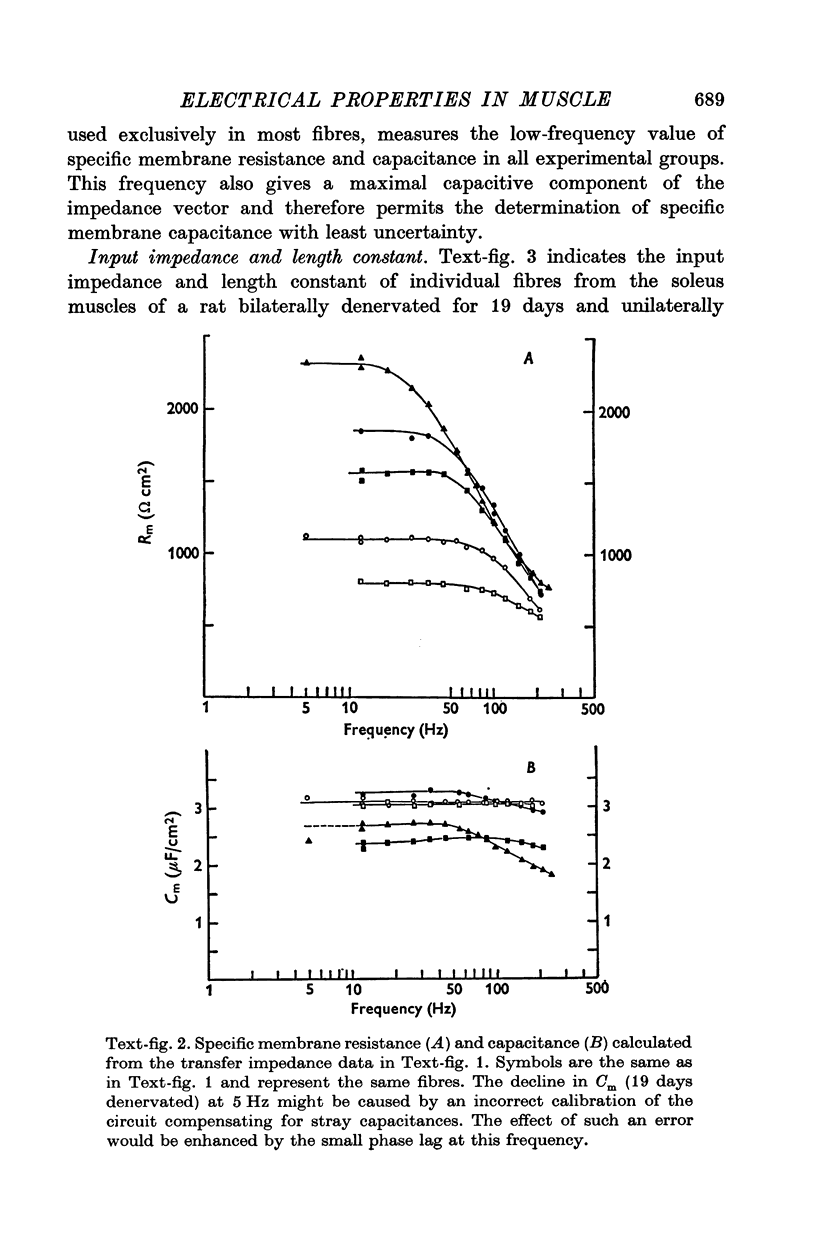
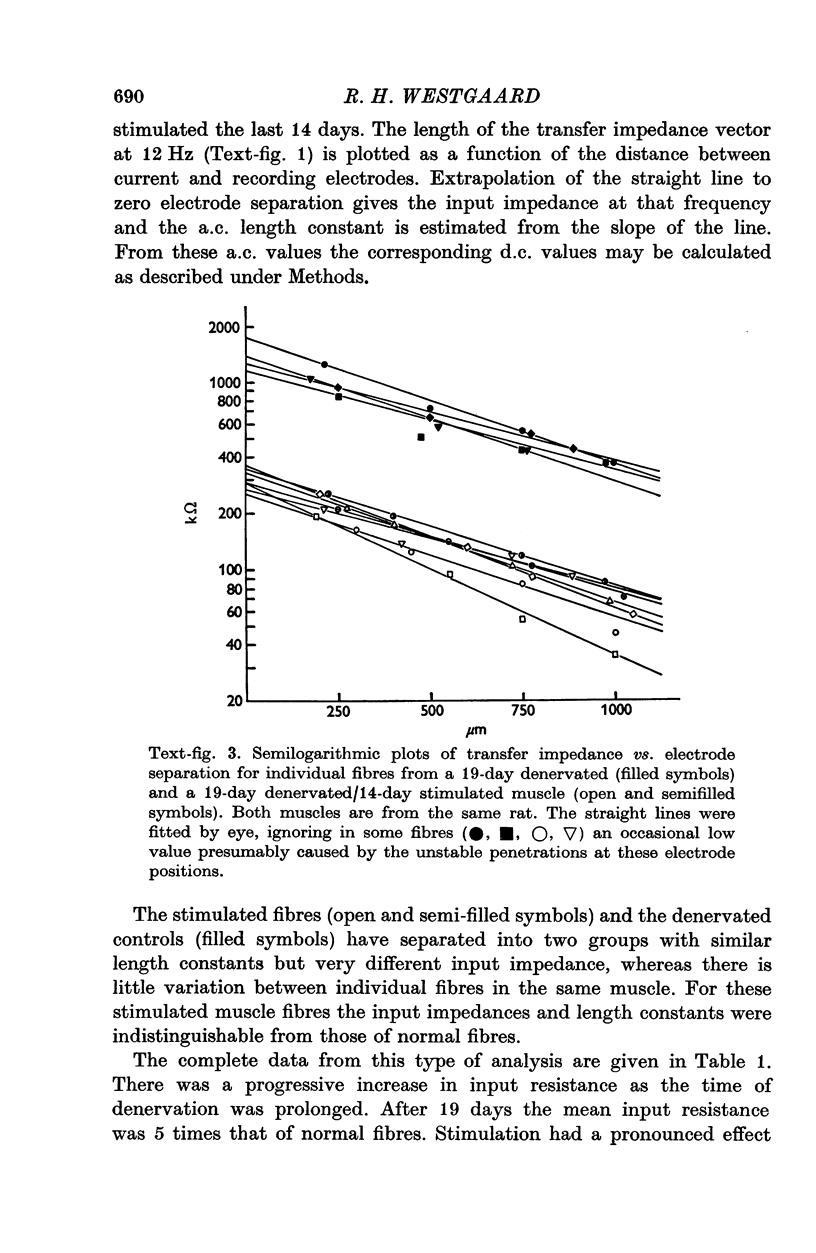
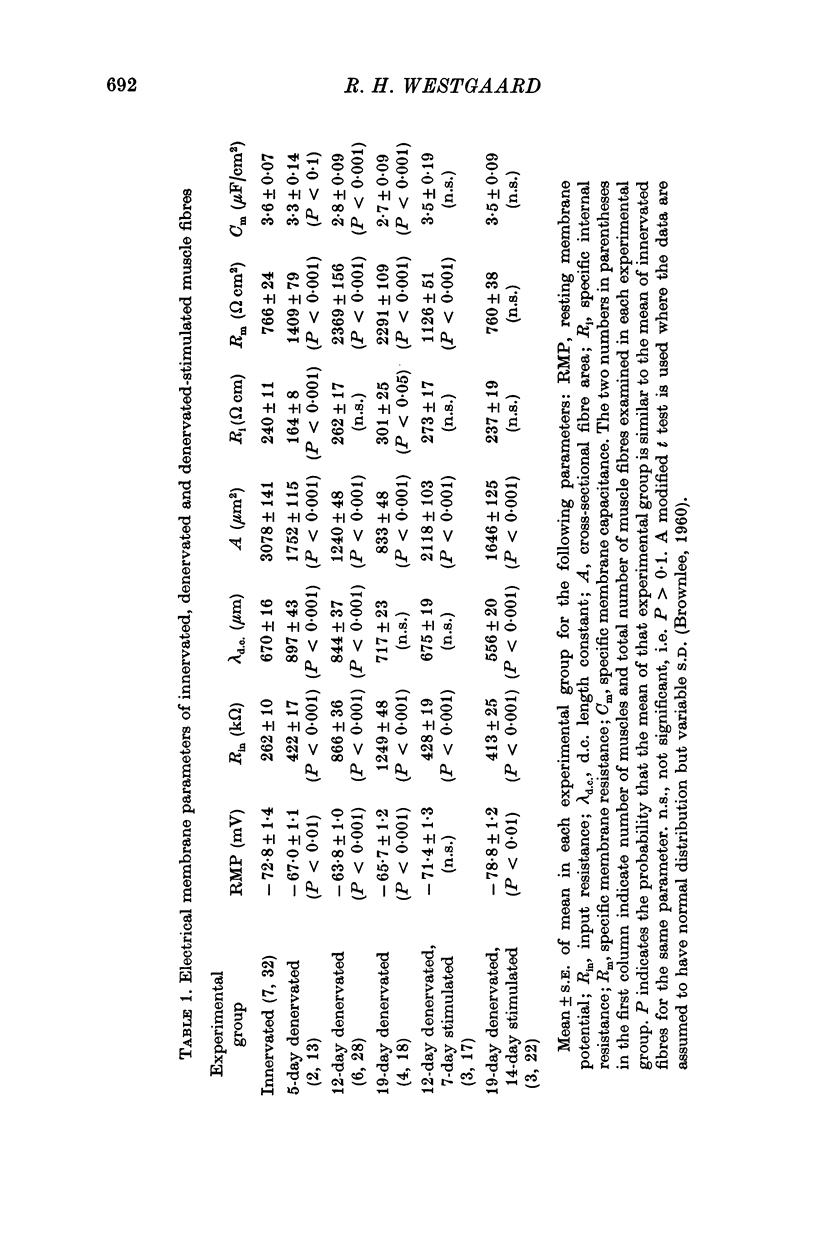
The technique of direct electrical stimulation of denervated muscle was used to study the role of muscle activity per se in controlling the passive electrical properties of muscle fibres. 2. Specific membrane resistance and capacitance of the denervated and the denervated-stimulated muscle fibres were measured by a sinewave technique at frequencies between 5 and 240 Hz. The parameter values were constant at low frequencies up to a variable transition frequency and declined rapidly at higher frequencies. 3. Following denervation the low-frequency value of specific membrane resistance increased (2291 omega cm2 for 19-day denervated fibres vs. 766 omega cm2 for innervated fibres), the specific membrane capacitance declined (2-7 muF/cm2 vs. 3-6 muF/cm2) and the transition frequency shifted towards lower frequencies. The specific internal resistance was higher in denervated fibres (301 omega cm for 19-day denervated fibres vs. 240 omega cm in innervated fibres) apart from a transient decline after 5 days of denervation (164 omega cm). 4. Direct electrical stimulation for 2 weeks beginning on the 5th day after denervation restored all parameters listed above to their original values before denervation. 5. Stimulation arrested in most cases further atrophy from the time of stimulation but did not restore normal fibre size.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Almers W. Measurement of membrane capacity in skeletal muscle. Nat New Biol. 1973 Mar 14;242(115):62–64. doi: 10.1038/newbio242062a0. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. The kinetics of mechanical activation in frog muscle. J Physiol. 1969 Sep;204(1):207–230. doi: 10.1113/jphysiol.1969.sp008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., McIsaac R. J. Fast and slow mammalian muscles after denervation. Exp Neurol. 1970 Jan;26(1):183–202. doi: 10.1016/0014-4886(70)90099-3. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Membrane sealing in frog skeletal-muscle fibers. Proc Natl Acad Sci U S A. 1973 Apr;70(4):982–984. doi: 10.1073/pnas.70.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Electrical properties of toad sartorius muscle fibres in summer and winter. J Physiol. 1973 May;230(3):619–641. doi: 10.1113/jphysiol.1973.sp010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELIN N., MALM L. DEVELOPMENT OF SUPERSENSITIVITY AS DEPENDENT ON THE LENGTH OF DEGENERATING NERVE FIBRES. Q J Exp Physiol Cogn Med Sci. 1965 Apr;50:142–145. doi: 10.1113/expphysiol.1965.sp001776. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S., Howell J. N., Vaughan P. C. The maintenance of resting potentials in glycerol-treated muscle fibres. J Physiol. 1971 May;215(1):95–102. doi: 10.1113/jphysiol.1971.sp009459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsberg C. A. EXPERIMENTS ON MOTOR NERVE REGENERATION AND THE DIRECT NEUROTIZATION OF PARALYZED MUSCLES BY THEIR OWN AND BY FOREIGN NERVES. Science. 1917 Mar 30;45(1161):318–320. doi: 10.1126/science.45.1161.318. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Stonnington H. H. Trophic functions of the neuron. II. Denervation and regulation of muscle. Morphological effects of denervation of muscle. A quantitative ultrastructural study. Ann N Y Acad Sci. 1974 Mar 22;228(0):68–88. doi: 10.1111/j.1749-6632.1974.tb20503.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Robbins N. Changes in contractile properties of disused soleus muscles. J Physiol. 1969 Apr;201(2):305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTH L., ZALEWSKI A. A. Disposition of cholinesterase following implantation of nerve into innervated and denervated muscle. Exp Neurol. 1963 Apr;7:316–326. doi: 10.1016/0014-4886(63)90078-5. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L. Work-induced growth of skeletal muscle in normal and hypophysectomized rats. Am J Physiol. 1967 Nov;213(5):1193–1198. doi: 10.1152/ajplegacy.1967.213.5.1193. [DOI] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Hájek I., Horský P. Effect of excessive use on contraction and metabolic properties of cross-striated muscle. J Physiol. 1969 Jul;203(1):46P–47P. [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Lomo T., Nicolaysen K., Westgaard R. H. Hyperinnervation of skeletal muscle fibers: dependence on muscle activity. Science. 1973 Aug 10;181(4099):559–561. doi: 10.1126/science.181.4099.559. [DOI] [PubMed] [Google Scholar]
- Lomo T. Neurotrophic control of colchicine effects on muscle? Nature. 1974 May 31;249(456):473–474. doi: 10.1038/249473a0. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Induced innervation of end-plate free muscle segments. Nature. 1962 Jan 20;193:281–282. doi: 10.1038/193281a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J. G. The electrical properties of denervated skeletal muscle. J Physiol. 1956 Jan 27;131(1):1–12. doi: 10.1113/jphysiol.1956.sp005440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G. Functional consequences of tenotomy in hind limb muscles of the cat. J Physiol. 1969 Apr;201(2):321–333. doi: 10.1113/jphysiol.1969.sp008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. Membrane properties underlying spontaneous activity of denervated muscle fibres. J Physiol. 1974 May;239(1):125–153. doi: 10.1113/jphysiol.1974.sp010559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]