Abstract
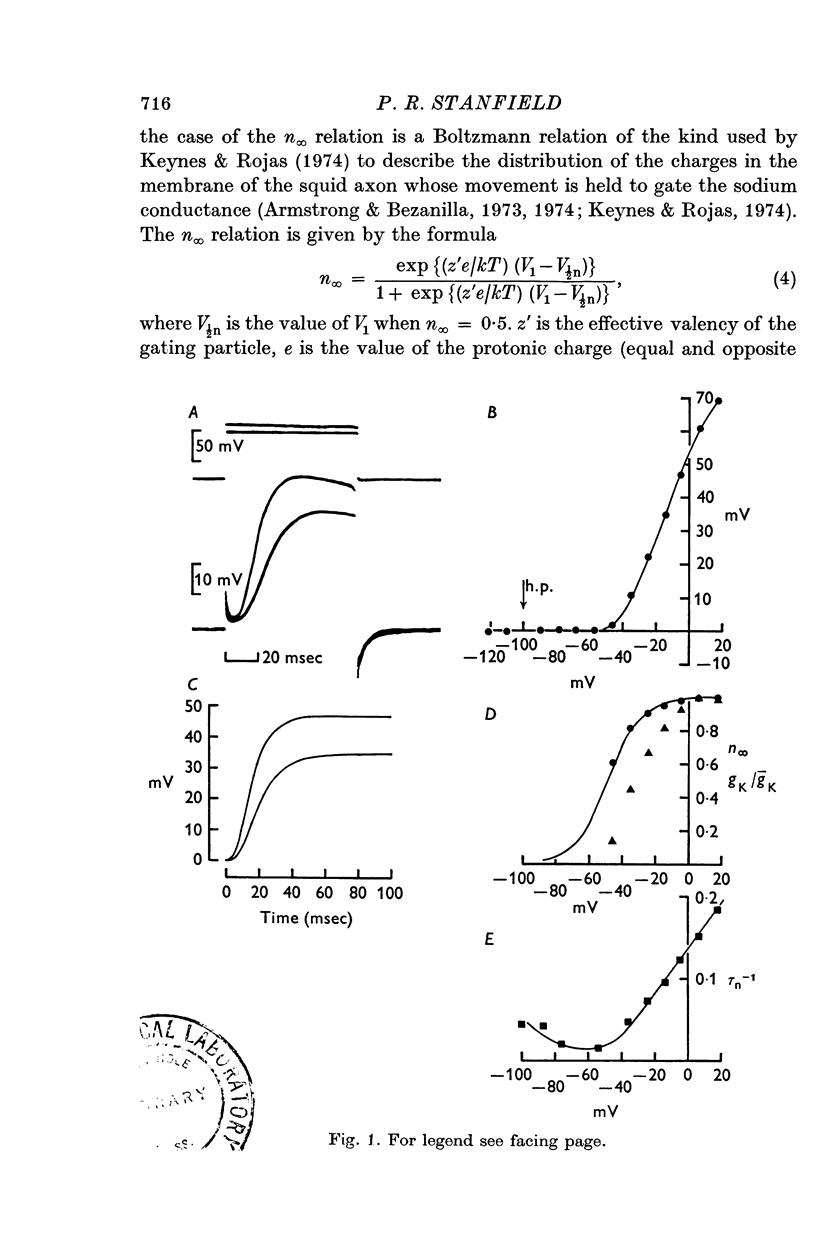
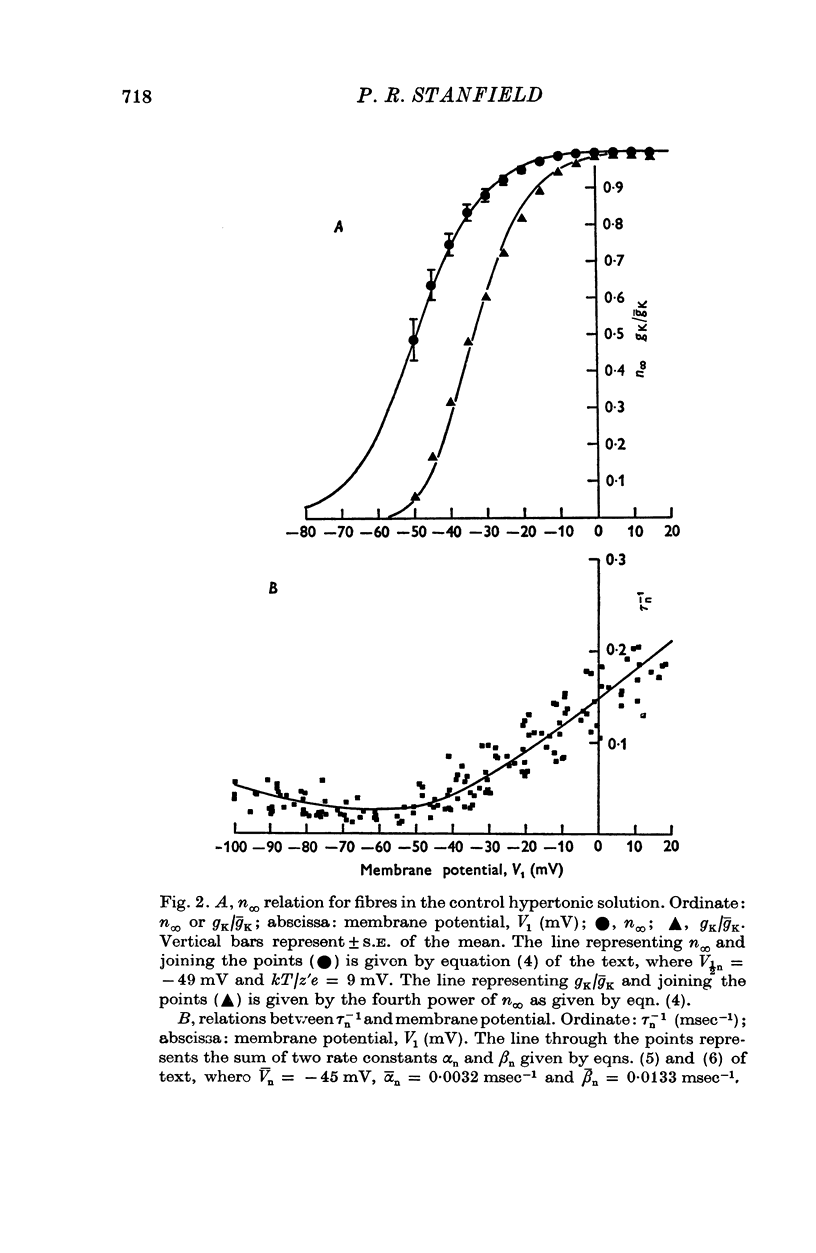
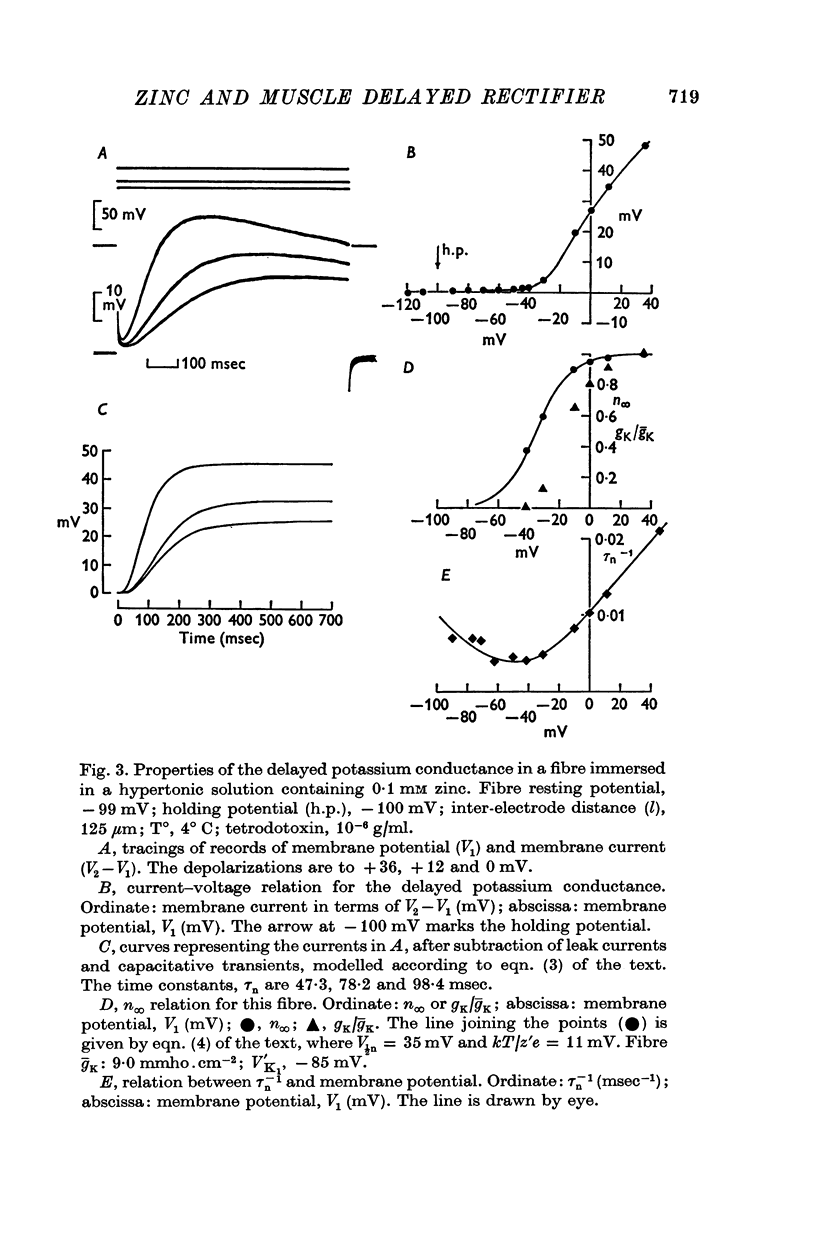
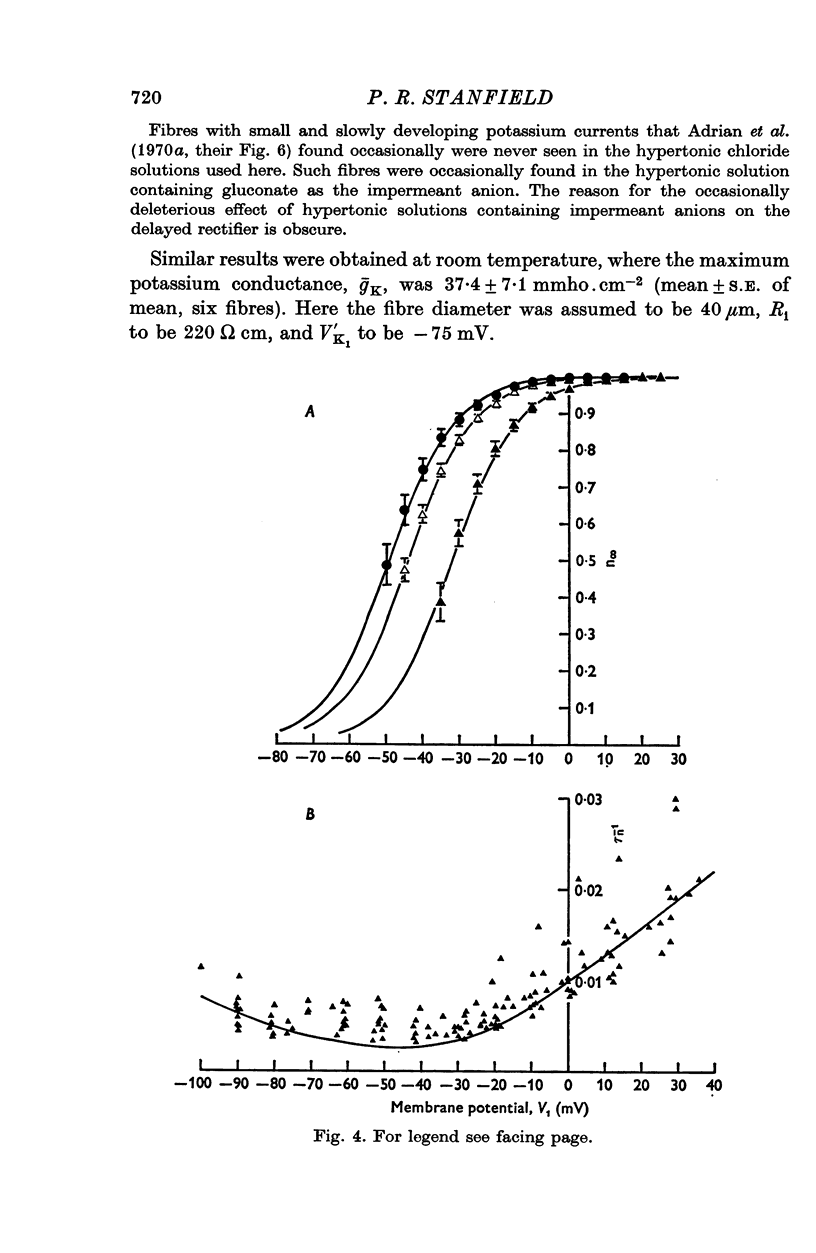
1. A voltage-clamp method was used to examine the effects of zinc ions on the delayed potassium currents and also the slowly activating potassium currents that are turned on by depolarizing the membrane of frog sartorius muscle fibres. 2. In a control solution, the delayed potassium conductance had a maximum value of 17-8 +/- 2-5 mmho.cm-2. The reversal potential for the currents was -76-9 +/- 2-5 mV. The membrane potential where ninfinity had the value 0-5 was -49 mV. 3. The major effect of zinc ions was to slow the delayed potassium currents. The value of taun was increased approximately tenfold in 0-1 mM zinc. Zinc does not alter the effective valency of the gating particles of the potassium channel, but the conductance was shifted to more positive membrane potentials: in 0-1 mM zinc, the membrane potential where ninfinity had the value 0-5 was -32 mV. 4. Zinc ions, at a concentration of 0-1 mM, also reduced the maximum potassium conductance by about 60% to 7-3 +/- 0-8 mmho, cm-2; they did not alter the reversal potential of the currents, which had a value in 0-1 mM zinc of -74-6 +/- 1-5 mV. 5. Zinc ions had little or no effect on the rate of inactivation of the potassium currents. 6. Zinc ions had little effect on the conductance attributable to the slowly activating potassium system. In 0-01 mM zinc this conductance had a value at 0 to +10 mV of 1-25 +/- 0-29 mmho.cm-2. Zinc did not alter the reversal potential of the slow potassium currents from the value of -85 +/- 1-6 mV in the absence of zinc and had no effect on the time course of the turn-off of these currents at -60 mV. 7. The delayed potassium currents obtained in 0-002 and 0-01 mM zinc could not be fitted exactly with a simple fourth order equation, but were well fitted by a model proposing that zinc ions slow the opening and closing of the gating mechanism to one tenth the normal rate when they bind to the gating molecule. If the binding sites are not saturated, those gating molecules that do not bind zinc are assumed to be quite unaltered in their properties, though the potential dependence of their rate constants alphan and betan was assumed to be shifted to more positive levels. In one fibre in 0-01 mM zinc, the model fitted the currents best if 50% of the gating molecules bound zinc.
Full text
PDF







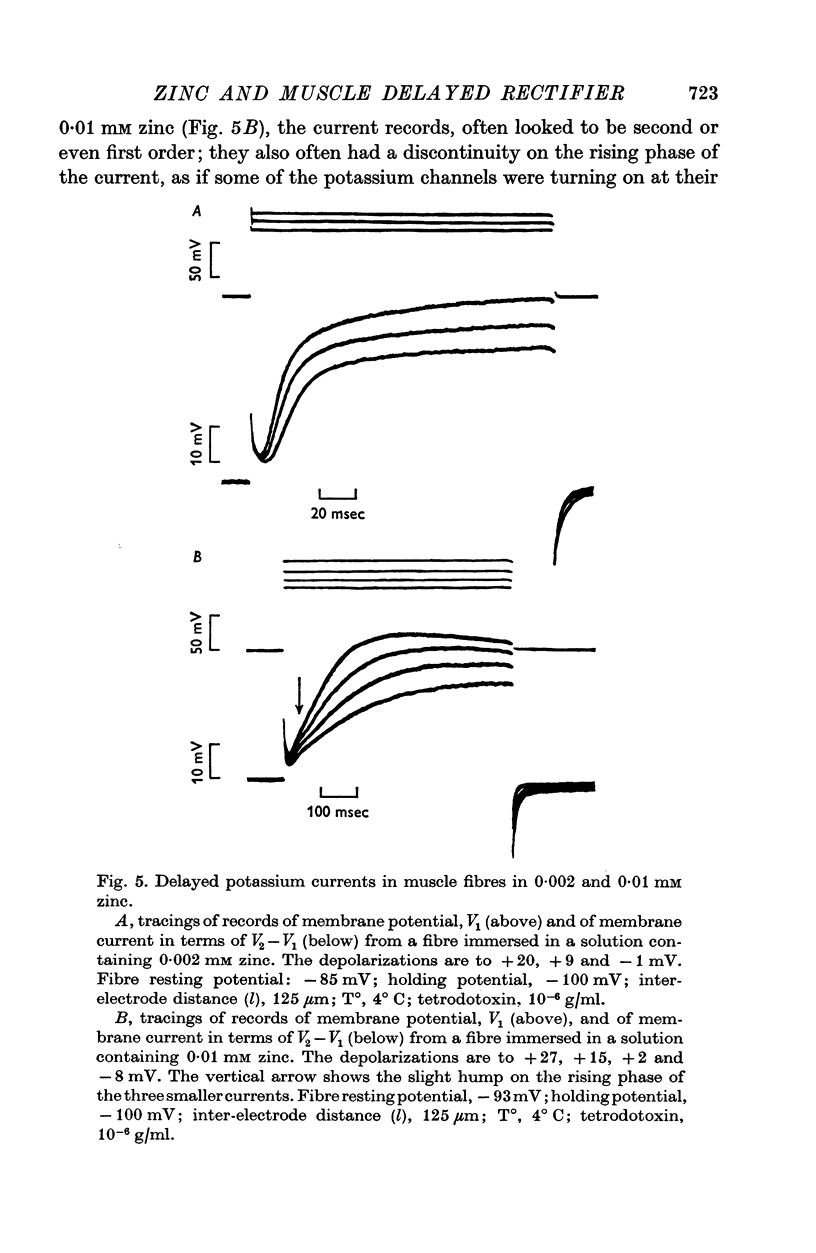

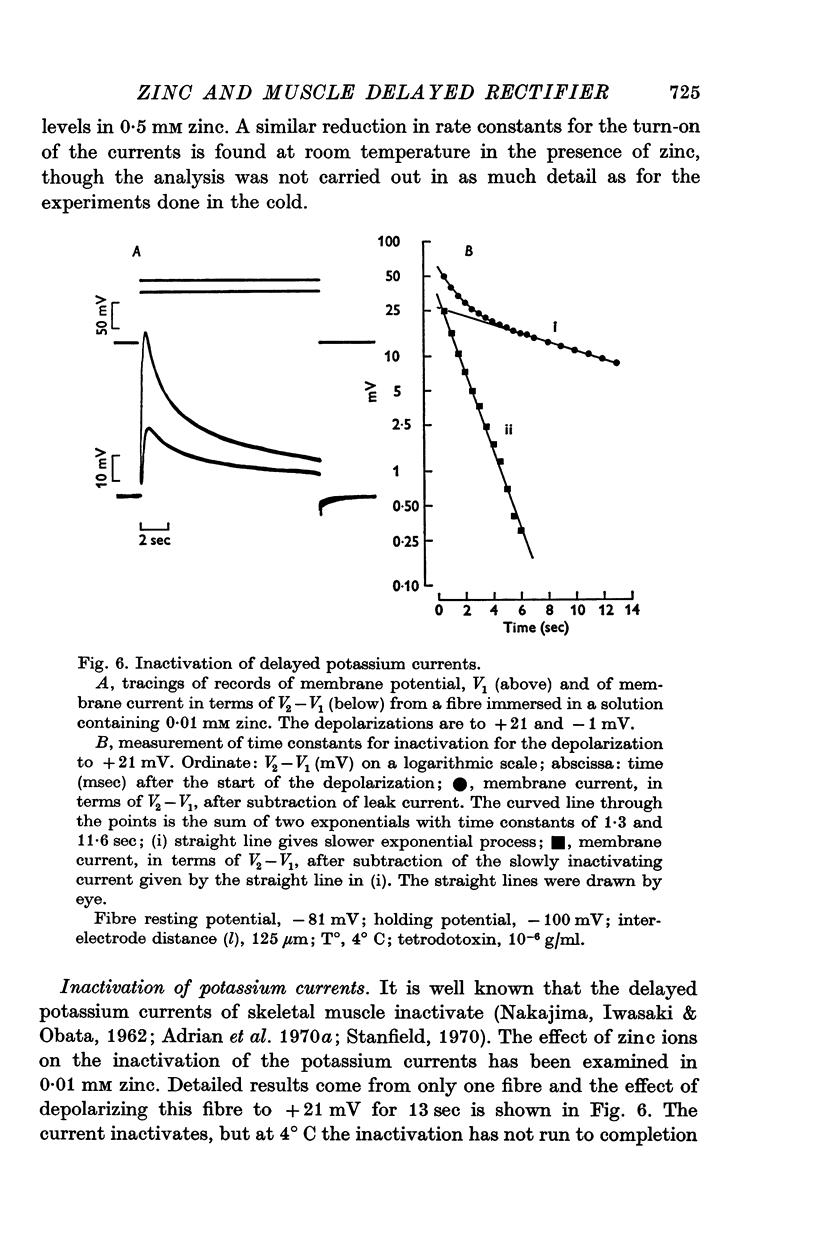

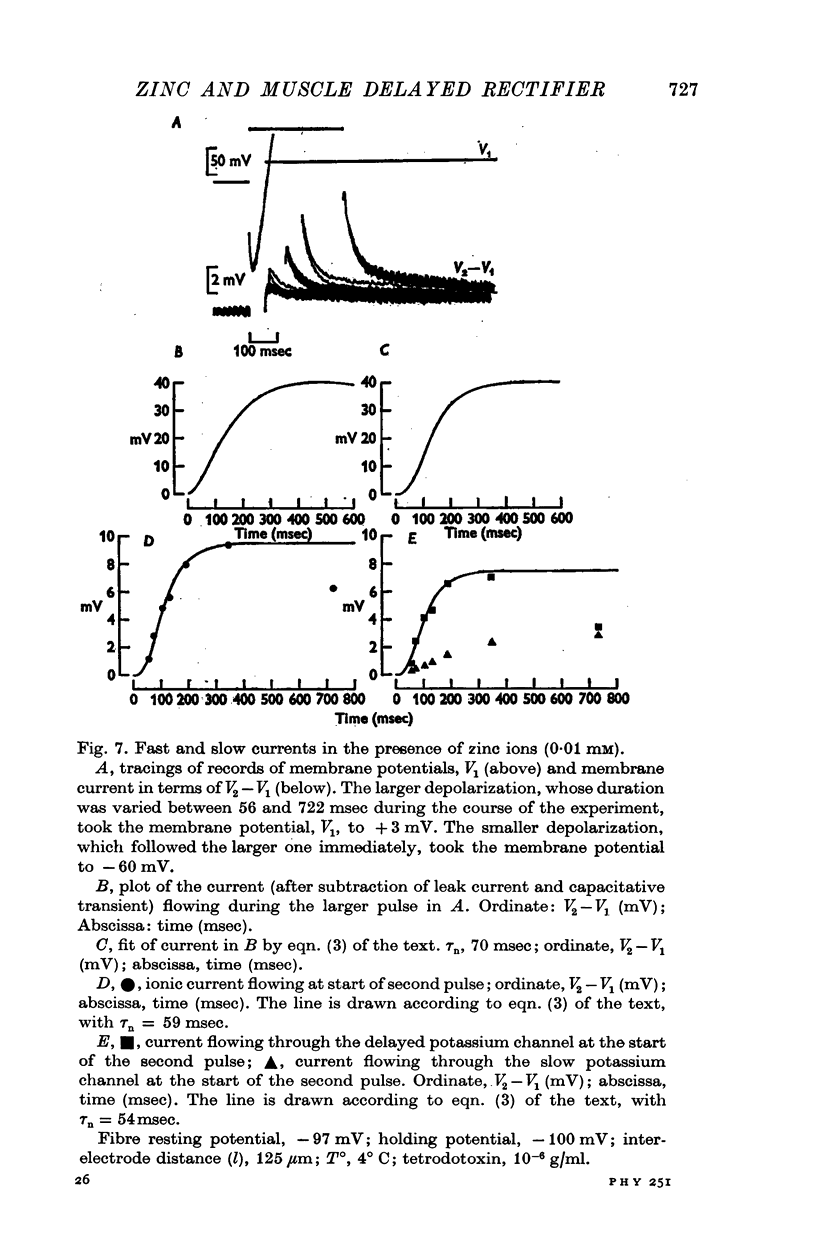




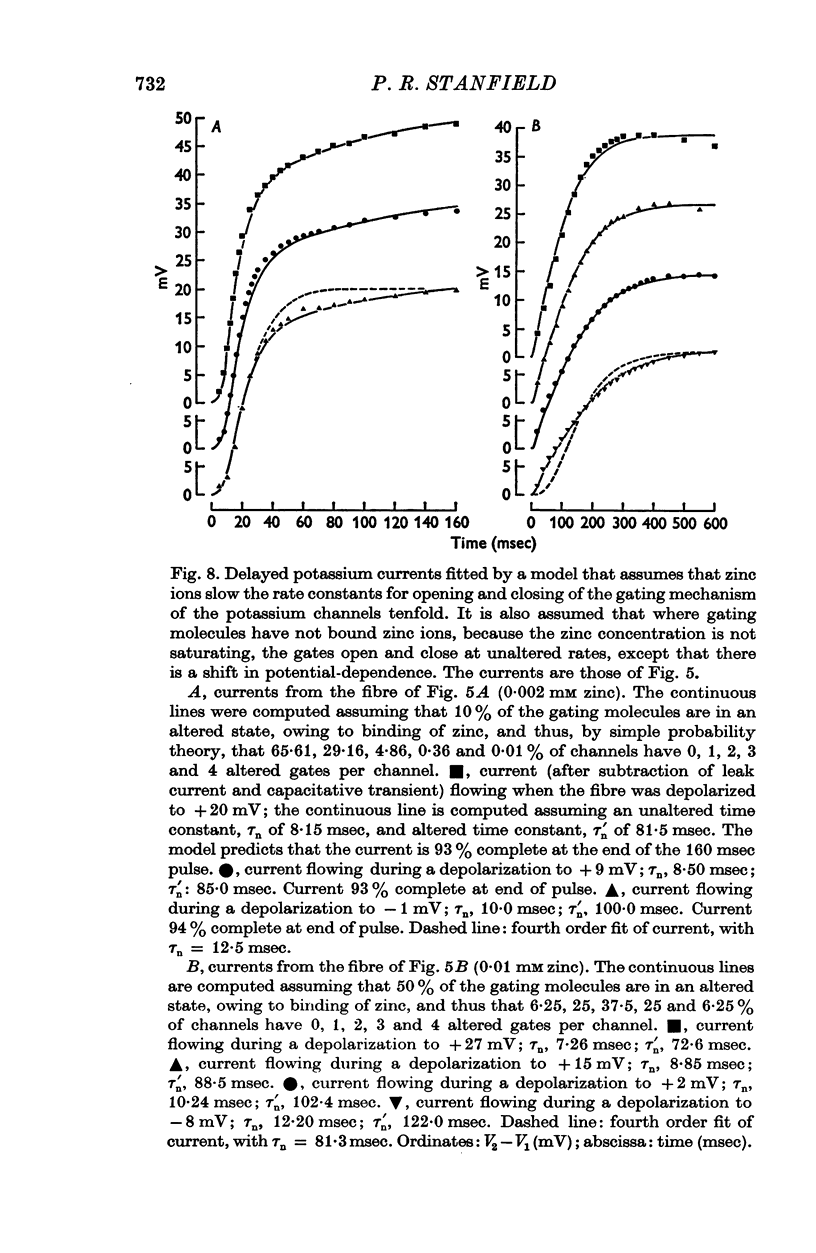



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974 May;63(5):533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Hille B. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J Gen Physiol. 1972 Apr;59(4):388–400. doi: 10.1085/jgp.59.4.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Lynch C. Effects of internal divalent cations on voltage-clamped squid axons. J Gen Physiol. 1974 Jun;63(6):675–689. doi: 10.1085/jgp.63.6.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Pharmacological modifications of the sodium channels of frog nerve. J Gen Physiol. 1968 Feb;51(2):199–219. doi: 10.1085/jgp.51.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A. L., Nakajima S. The effect of diameter on the electrical constants of frog skeletal muscle fibres. J Physiol. 1972 Feb;221(1):105–120. doi: 10.1113/jphysiol.1972.sp009742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACSON A., SANDOW A. Effects of zinc on responses of skeletal muscle. J Gen Physiol. 1963 Mar;46:655–677. doi: 10.1085/jgp.46.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974 Jun;239(2):393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASHIMA H., WASHIO H. THE EFFECT OF ZINC ON THE ELECTRICAL PROPERTIES OF MEMBRANE AND THE TWITCH TENSION IN FROG MUSCLE FIBRES. Jpn J Physiol. 1964 Oct 15;14:538–550. doi: 10.2170/jjphysiol.14.538. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA S., IWASAKI S., OBATA K. Delayed rectification and anomalous rectification in frog's skeletal muscle membrane. J Gen Physiol. 1962 Sep;46:97–115. doi: 10.1085/jgp.46.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Sandow A., Isaacson A. Topochemical factors in potentiation of contraction by heavy metal cations. J Gen Physiol. 1966 May;49(5):937–961. doi: 10.1085/jgp.49.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A., Taylor S. R., Preiser H. Role of the action potential in excitation-contraction coupling. Fed Proc. 1965 Sep-Oct;24(5):1116–1123. [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The onset of the effects of zinc and tetraethylammonium ions on action potential duration and twitch amplitude of single muscle fibres. J Physiol. 1973 Dec;235(3):639–654. doi: 10.1113/jphysiol.1973.sp010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., MURAI T., SASAKI T. Some chemical aspect of plateau formation in the action current of the myelinated nerve fibre. Jpn J Physiol. 1960 Jun 29;10:280–291. doi: 10.2170/jjphysiol.10.280. [DOI] [PubMed] [Google Scholar]


