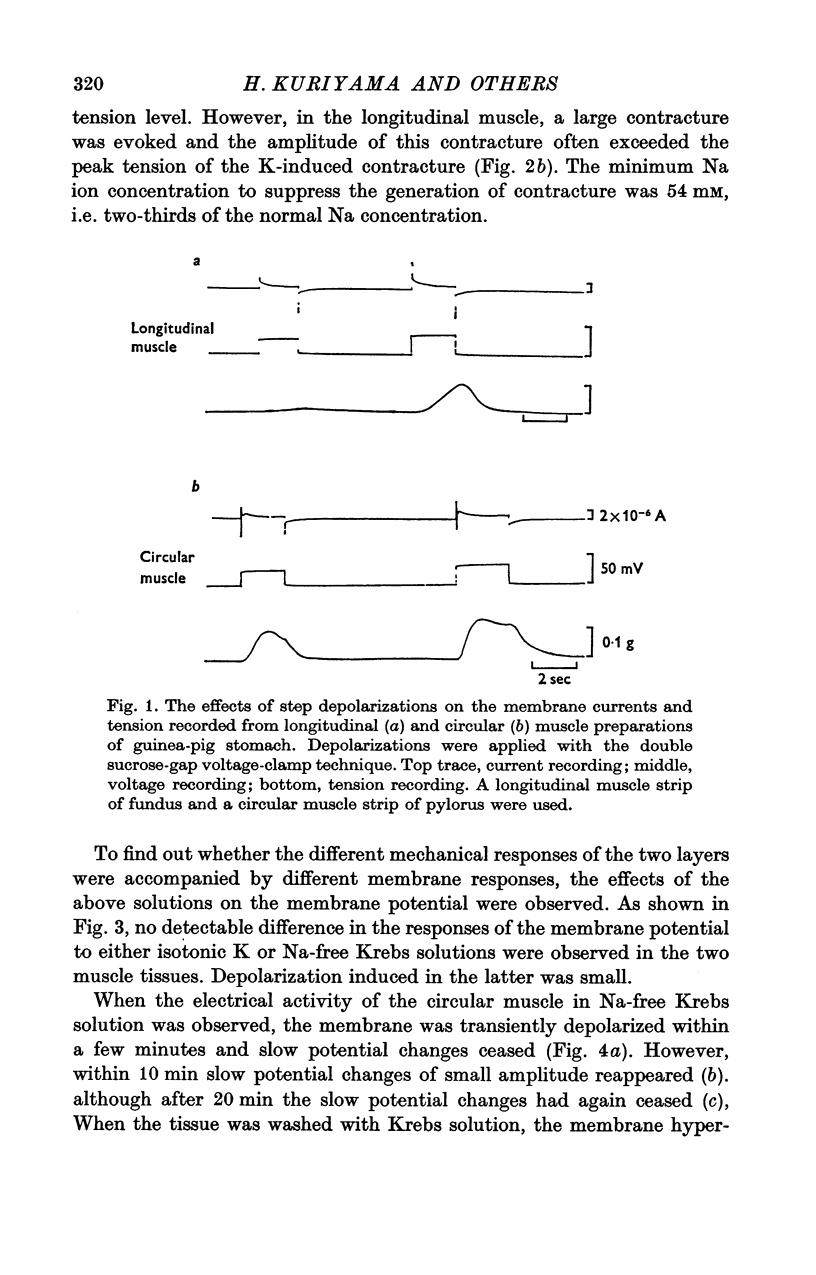
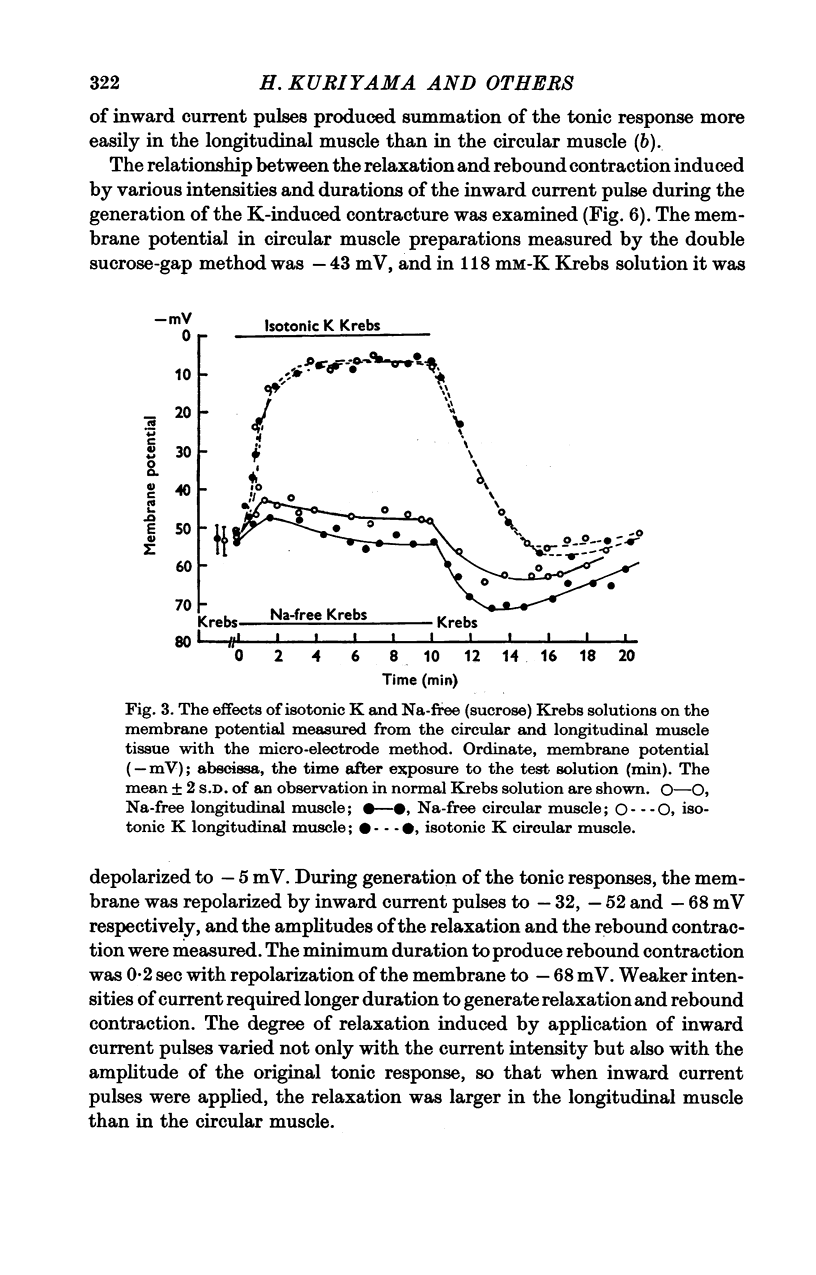
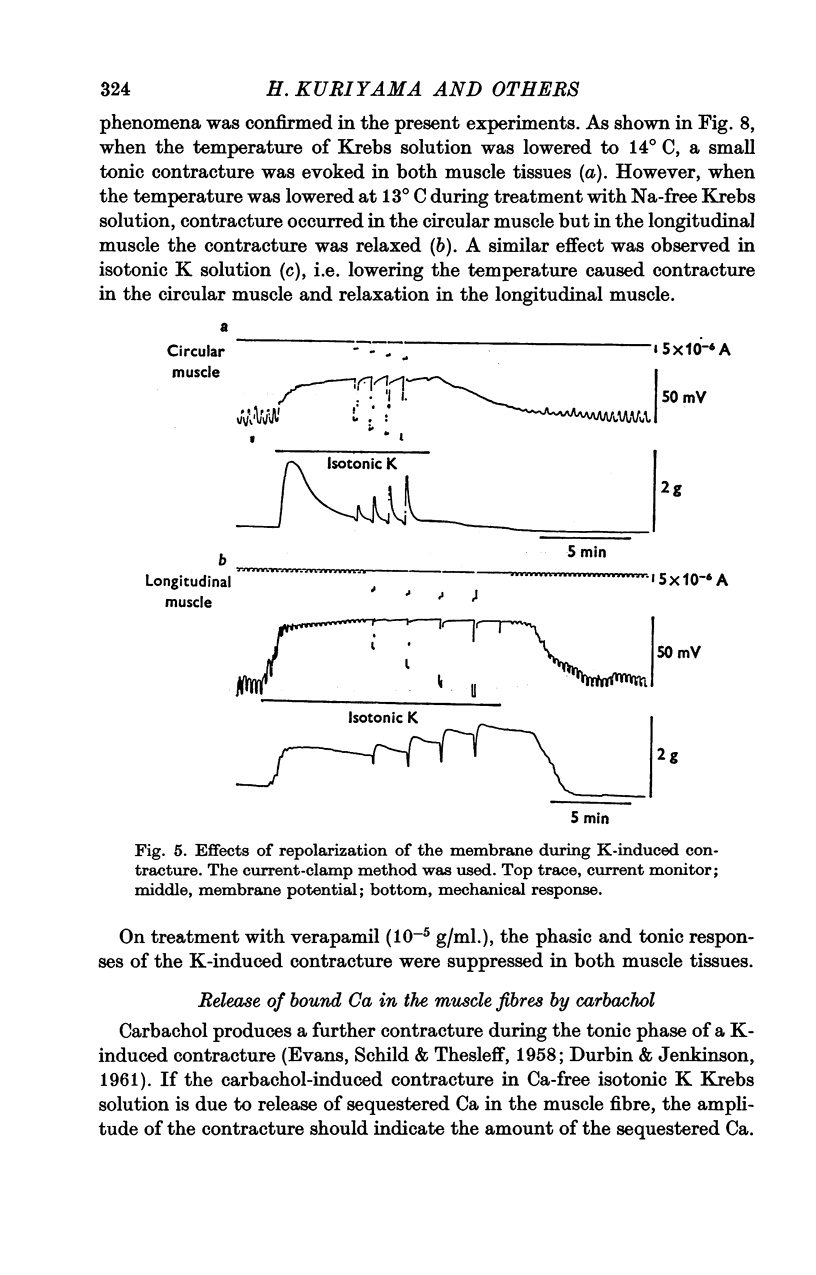
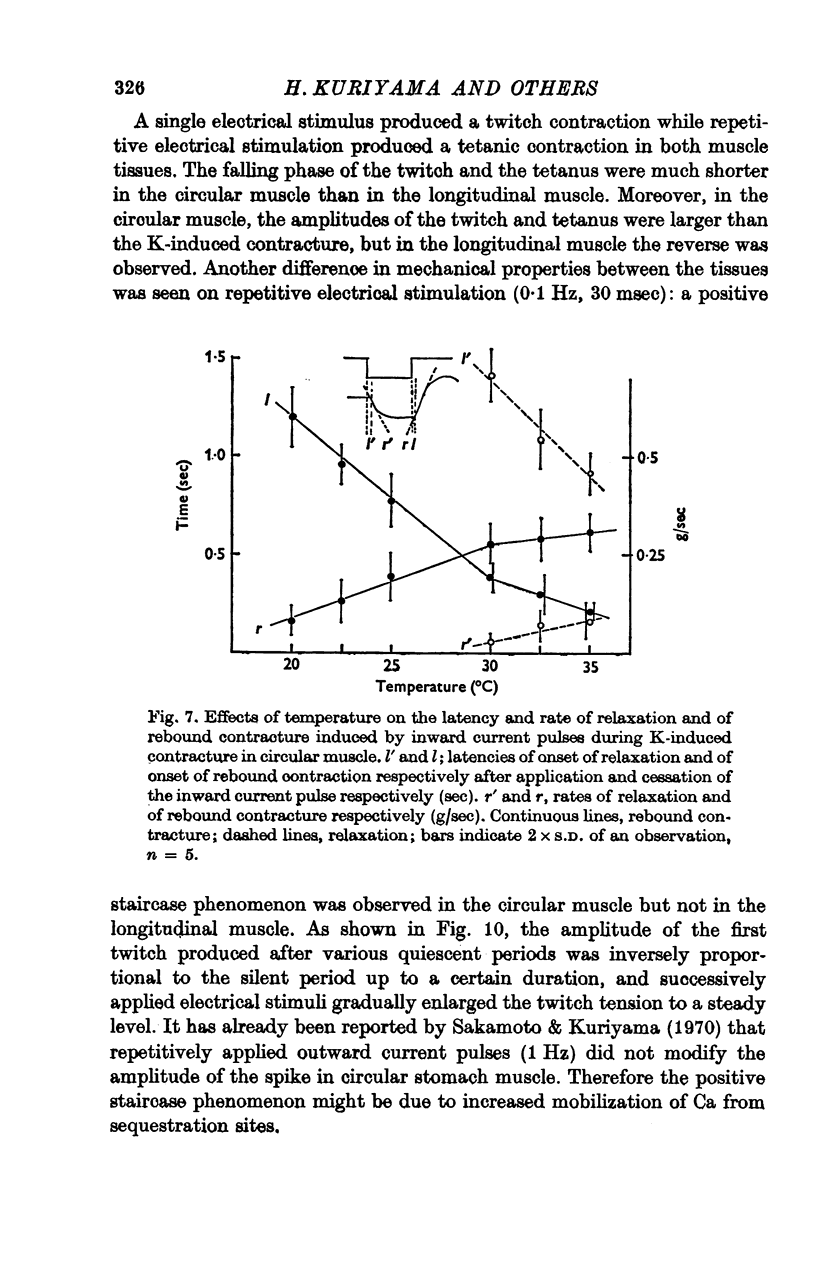
Abstract
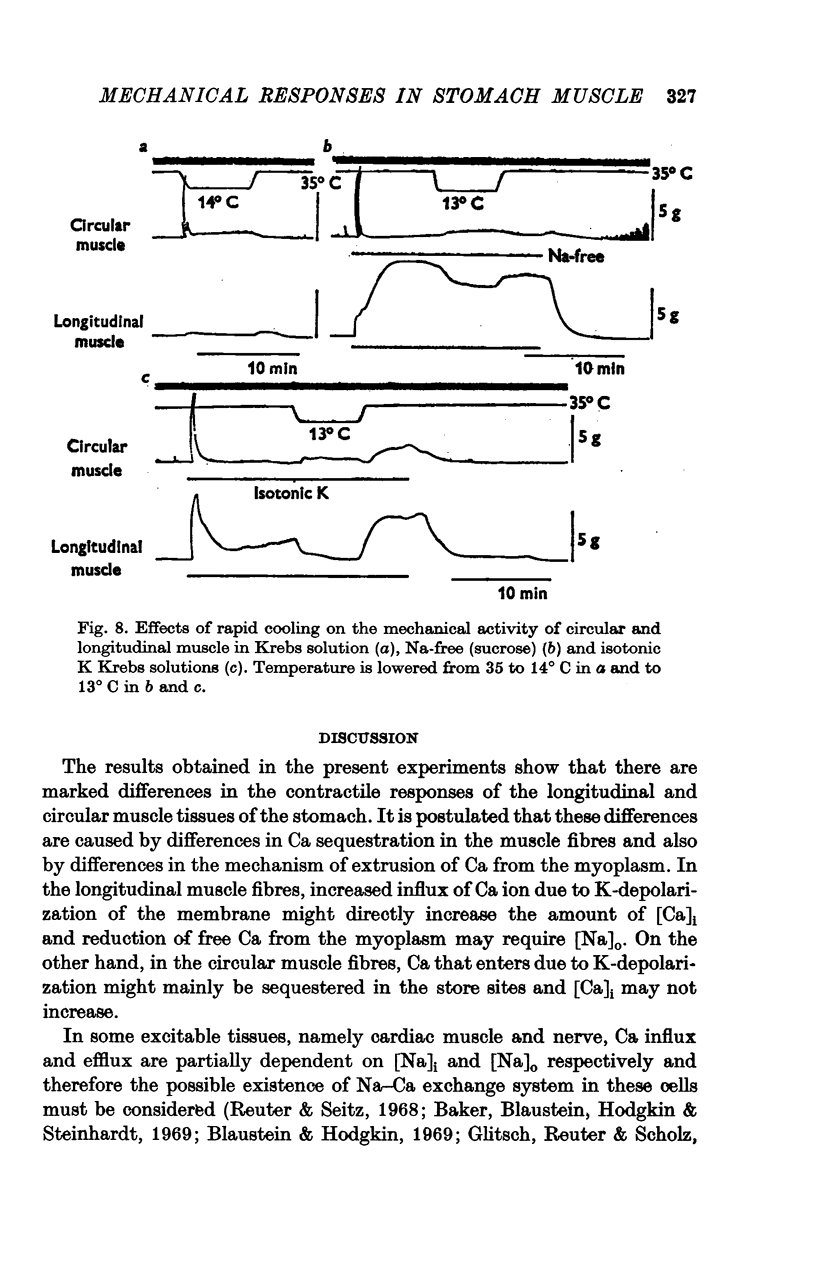
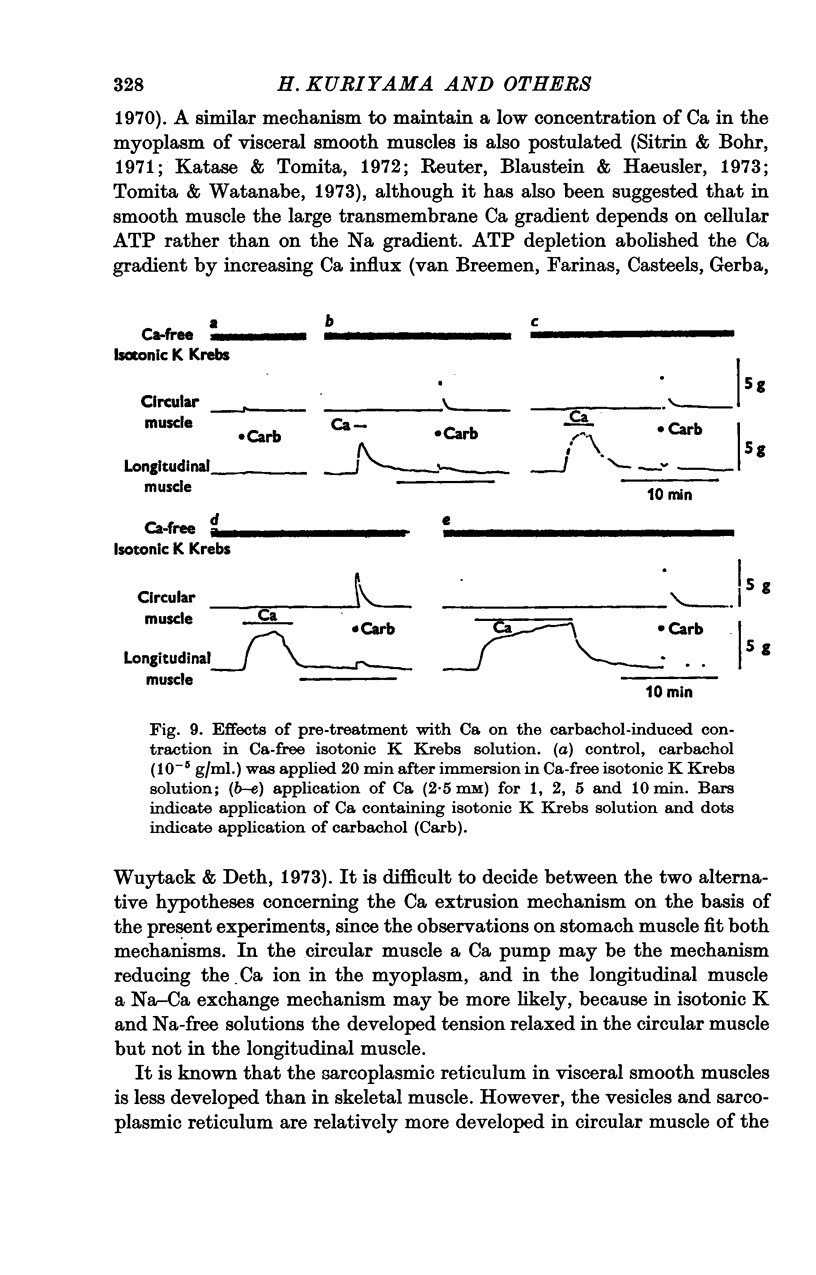
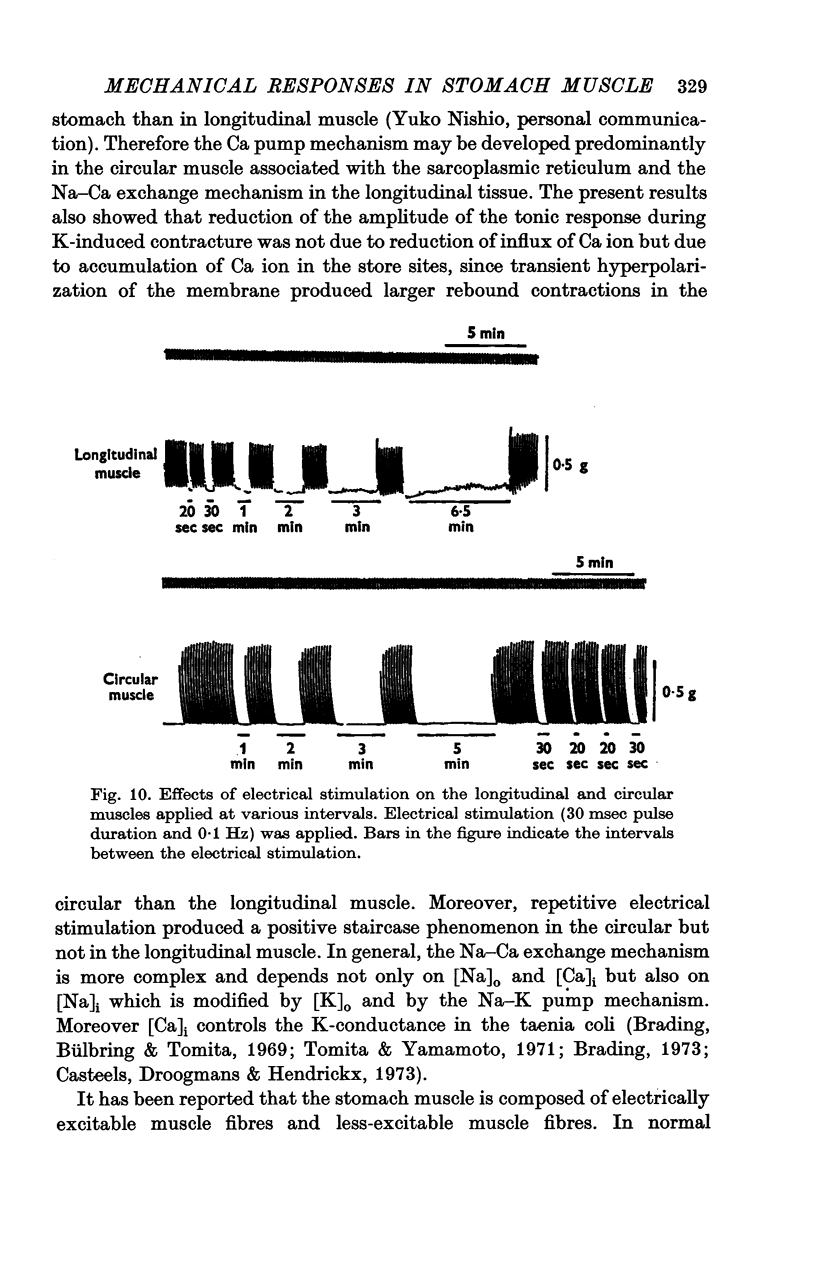
The mechanical and electrical properties of the longitudinal (fundus and corpus) and circular (antrum) muscle fibres of the guinea-pig stomach were investigated. 1. In the longitudinal but not in the circular muscle isotonic K Krebs and Na-free (sucrose) Krebs solutions produced a contracture with a tonic component. The different mechanical responses were not accompanied by different membrane responses. Verapamil abolished both phasic and tonic components of K-induced contracture. 2. During the tonic response of the K-induced contracture, repolarization of the membrane by current pulses relaxed the tissue; after cessation of the current pulse, rebound contracture occurred. In the circular muscle, the Q10 value for the rate of relaxation induced by inward current pulse was 3-1 and for the development of rebound contracture was 2-4. 3. After the tissue had been immersed in Ca-free isotonic K Krebs solution, application of Ca produced a large contracture in the longitudinal muscle, but contracture in the circular muscle was small or absent. However, the amplitude of subsequent carbachol-induced contracture in the above solution was enlarged in proportion to the durations of Ca treatment in both tissues. 4. Direct tetanic electrical stimulation could produce tension in both tissues. With low frequency of stimulation (0-1 Hz) a positive staircase was observed in the circular but not in the longitudinal muscle. 5. It is concluded from these results that topical differences of the motility in the stomach may be due not only to the activity of nervous elements, but also to differences in the properties of the muscle fibres themselves.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Effect of sodium and sodium-substitutes on the active ion transport and on the membrane potential of smooth muscle cells. J Physiol. 1973 Feb;228(3):733–748. doi: 10.1113/jphysiol.1973.sp010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURBIN R. P., JENKINSON D. H. The effect of carbachol on the permeability of depolarized smooth muscle to inorganic ions. J Physiol. 1961 Jun;157:74–89. doi: 10.1113/jphysiol.1961.sp006706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D. H., SCHILD H. O., THESLEFF S. Effects of drugs on depolarized plain muscle. J Physiol. 1958 Oct 31;143(3):474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Osa T., Kuriyama H. Topical differences of caffeine action on the smooth muscle cells of the guinea pig alimentary canal. Jpn J Physiol. 1974 Apr;24(2):217–232. doi: 10.2170/jjphysiol.24.217. [DOI] [PubMed] [Google Scholar]
- Katase T., Tomita T. Influences of sodium and calcium on the recovery process from potassium contracture in the guinea-pig taenia coli. J Physiol. 1972 Jul;224(2):489–500. doi: 10.1113/jphysiol.1972.sp009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaribuchi T., Ito Y., Kuriyama H. Effects of rapid cooling on the mechanical and electrical activities of smooth muscles of guinea pig stomach and taenia coli. J Gen Physiol. 1973 Mar;61(3):323–341. doi: 10.1085/jgp.61.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T., Kuriyama H. The membrane properties and decremental conduction of excitation in the fundus of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):626–639. doi: 10.2170/jjphysiol.20.626. [DOI] [PubMed] [Google Scholar]
- Prosser C. L. Smooth muscle. Annu Rev Physiol. 1974;36:503–535. doi: 10.1146/annurev.ph.36.030174.002443. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Kuriyama H. The relationship between the electrical and mechanical activity of the guinea-pig stomach. Jpn J Physiol. 1970 Dec 15;20(6):640–656. doi: 10.2170/jjphysiol.20.640. [DOI] [PubMed] [Google Scholar]
- Sitrin M. D., Bohr D. F. Ca and Na interaction in vascular smooth muscle contraction. Am J Physiol. 1971 Apr;220(4):1124–1128. doi: 10.1152/ajplegacy.1971.220.4.1124. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]
- Tomita T., Yamamoto T. Effects of removing the external potassium on the smooth muscle of guinea-pig taenia coli. J Physiol. 1971 Feb;212(3):851–868. doi: 10.1113/jphysiol.1971.sp009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


