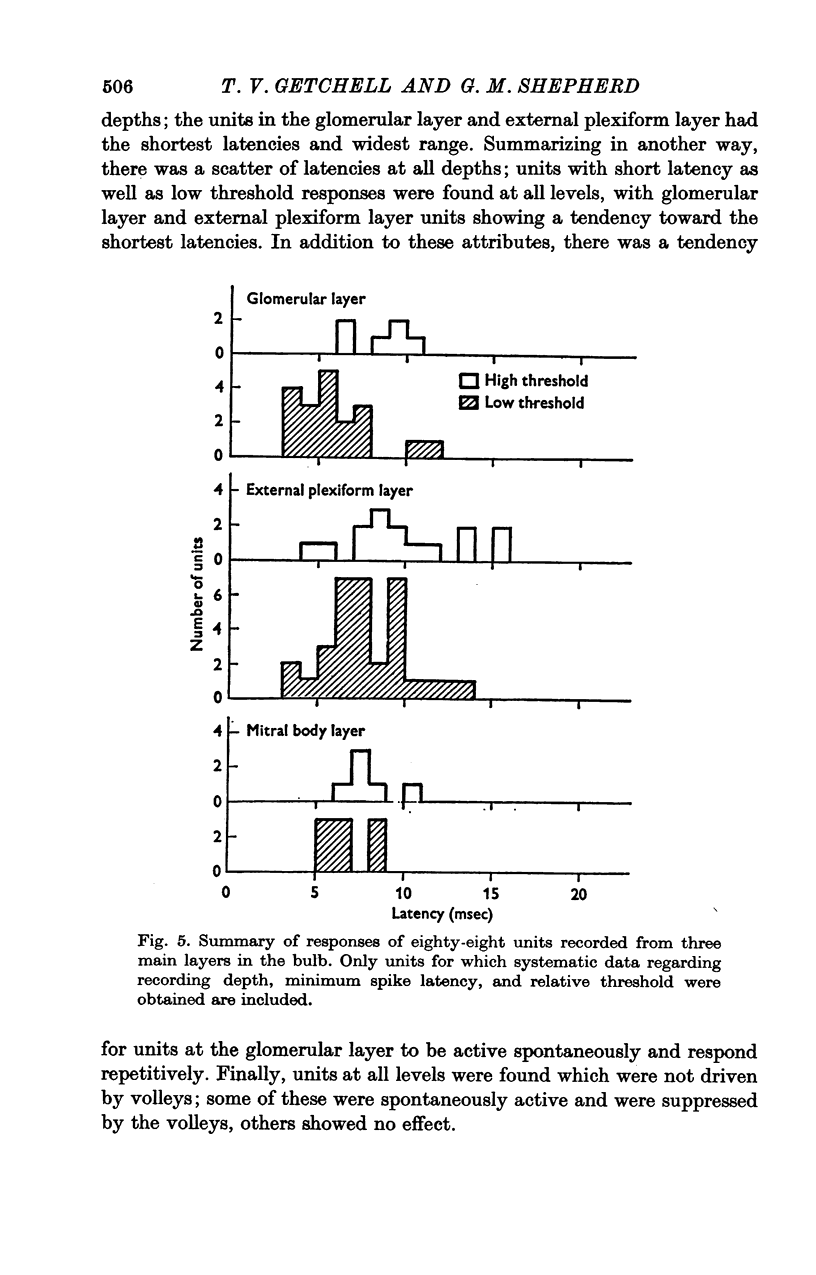
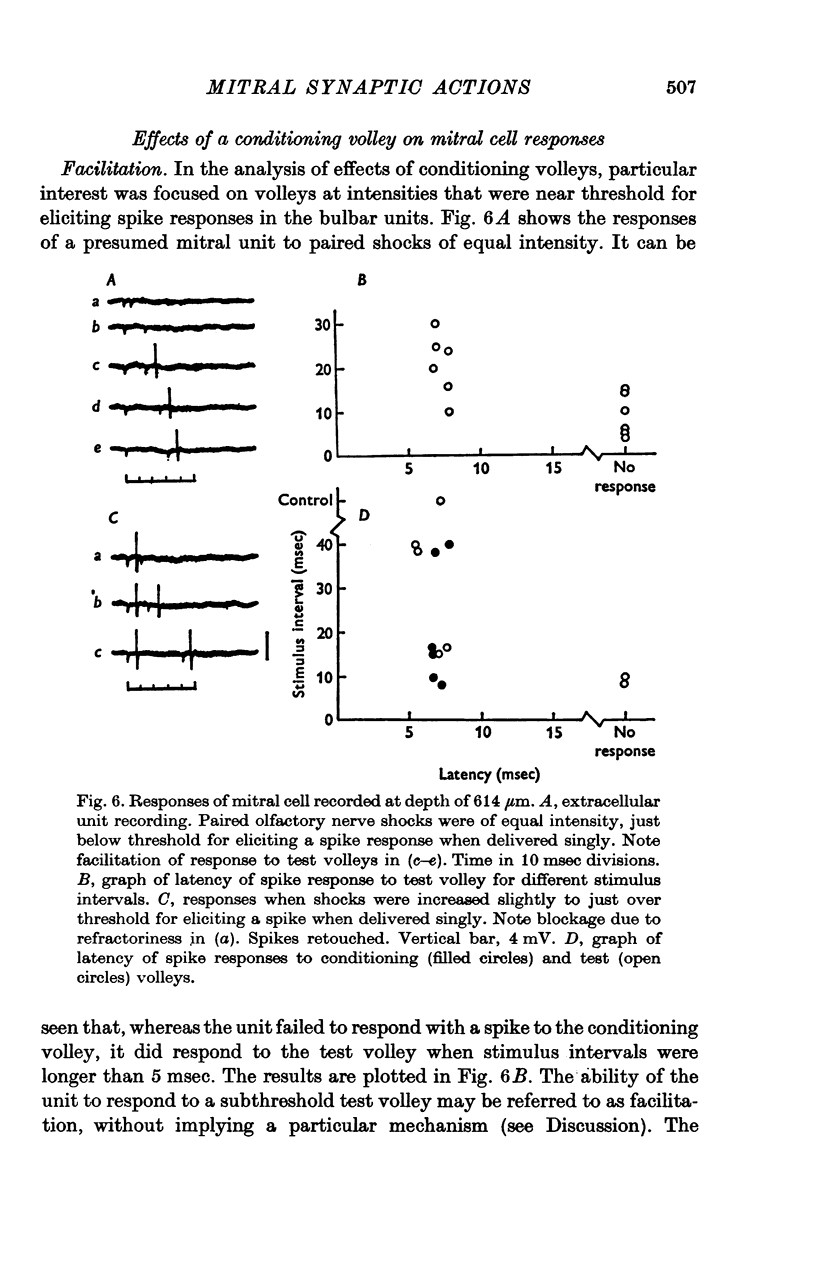
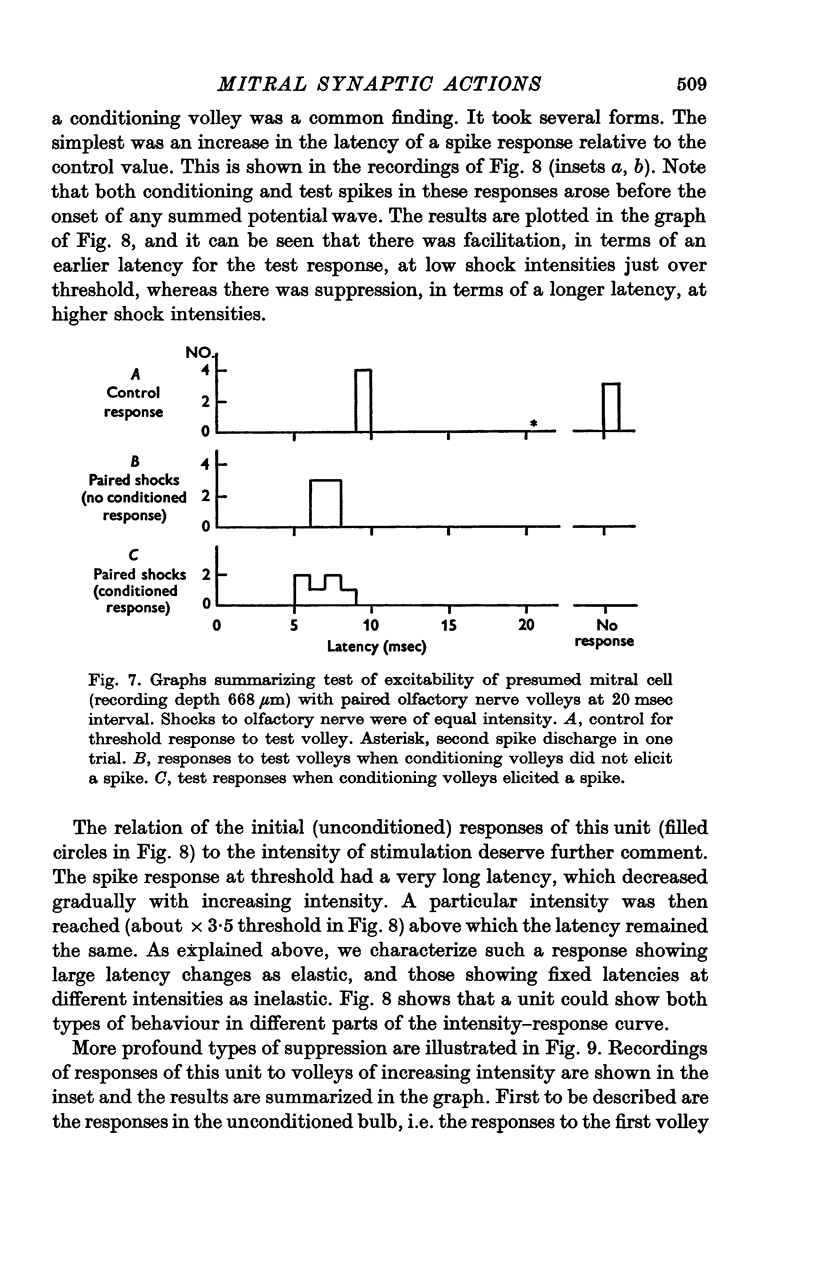
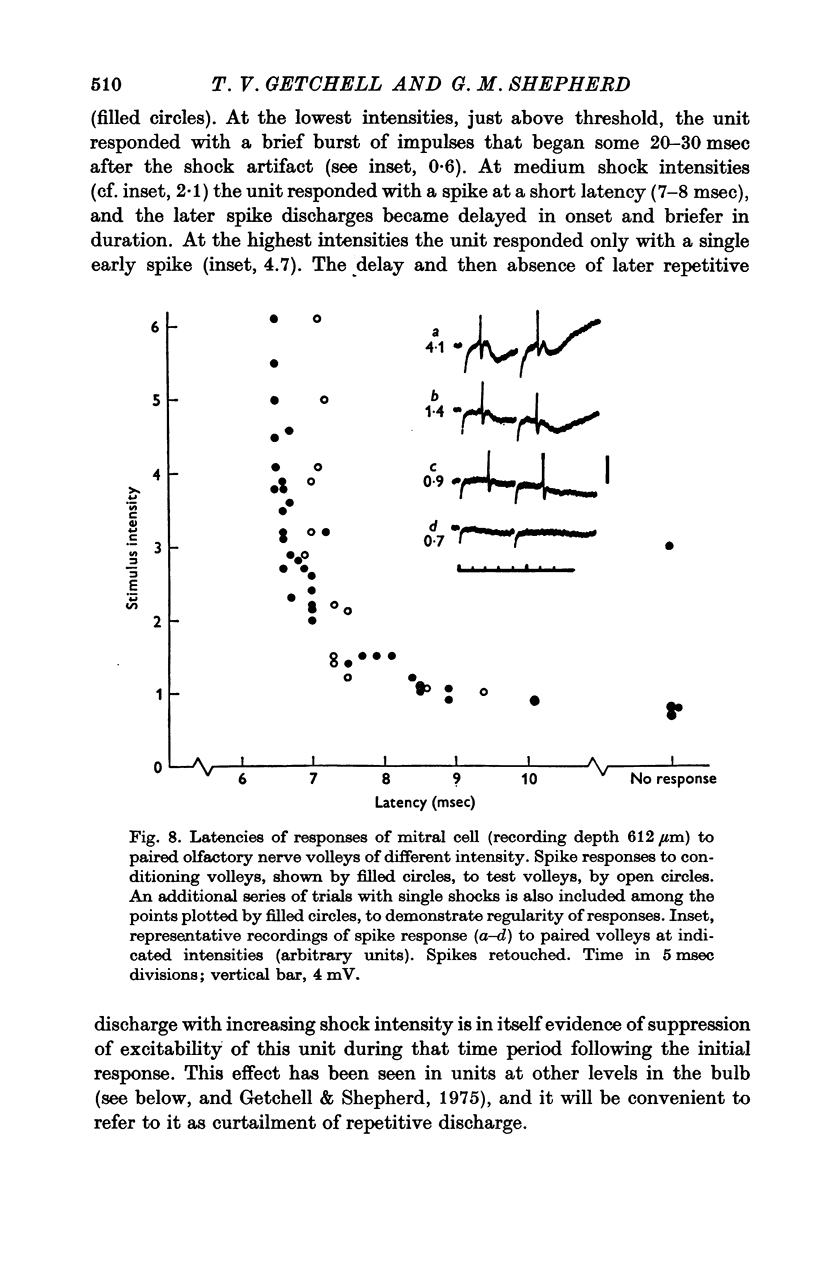
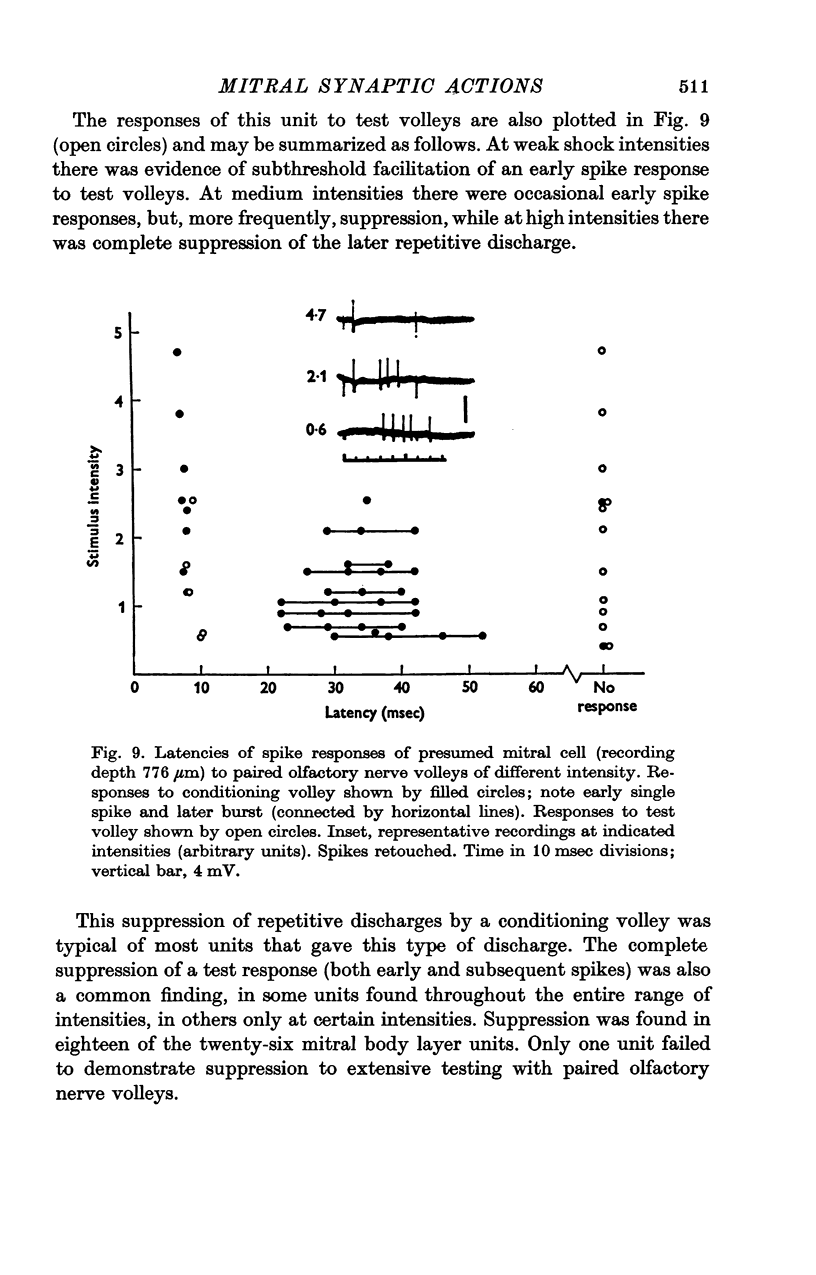
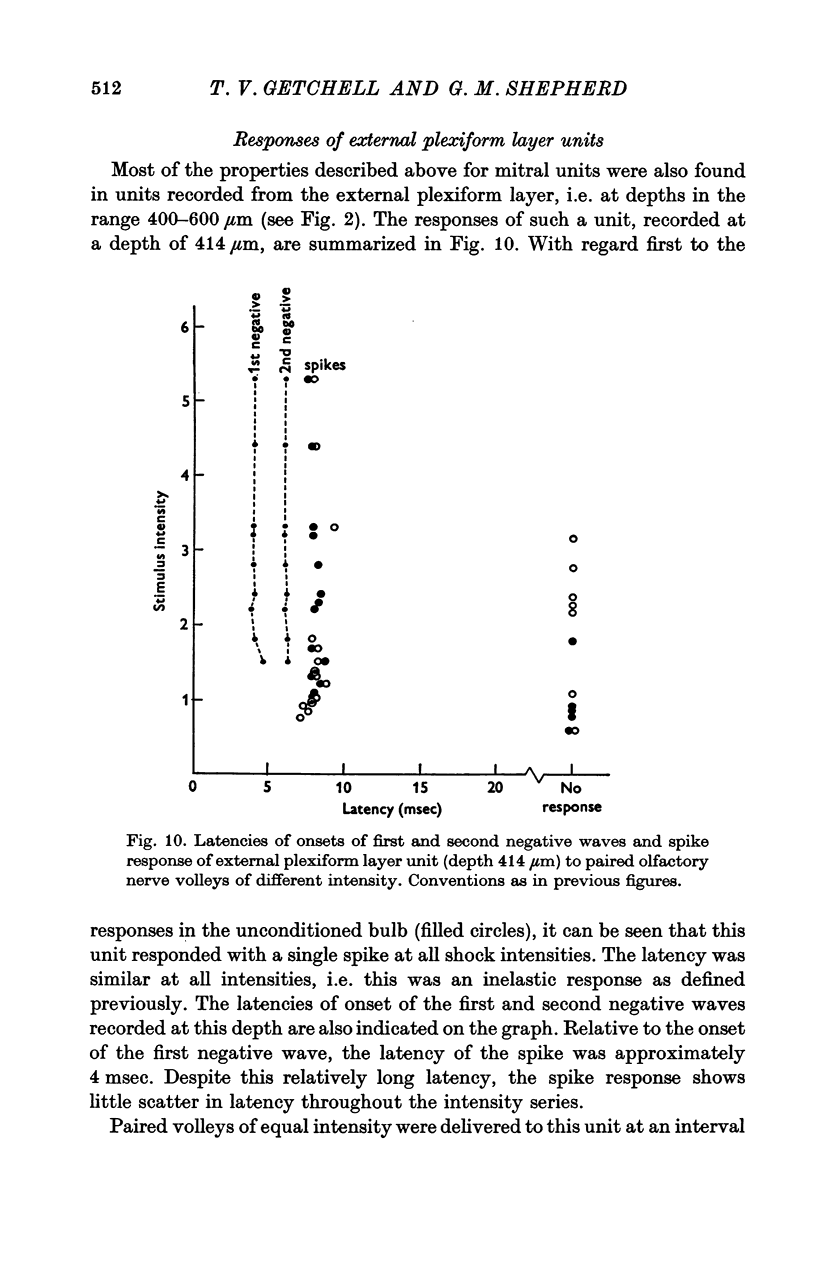
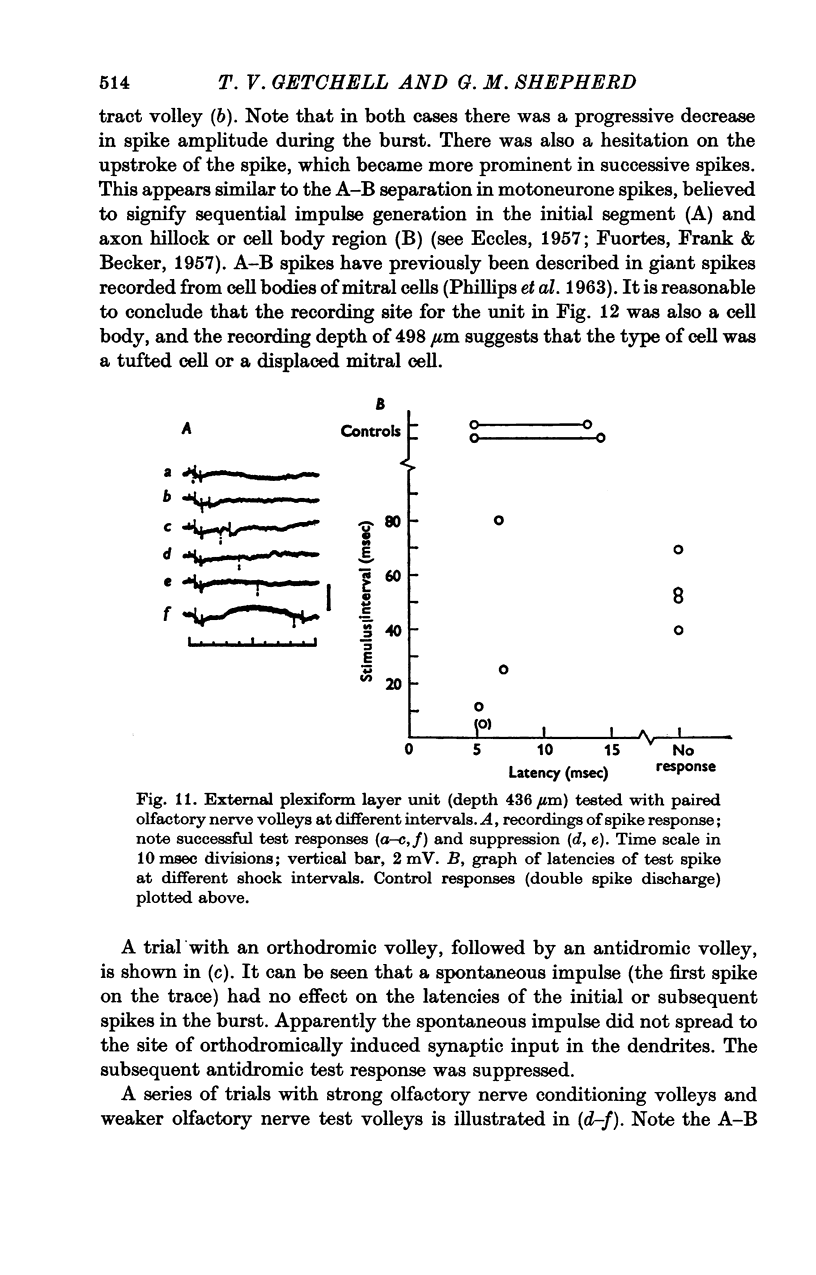
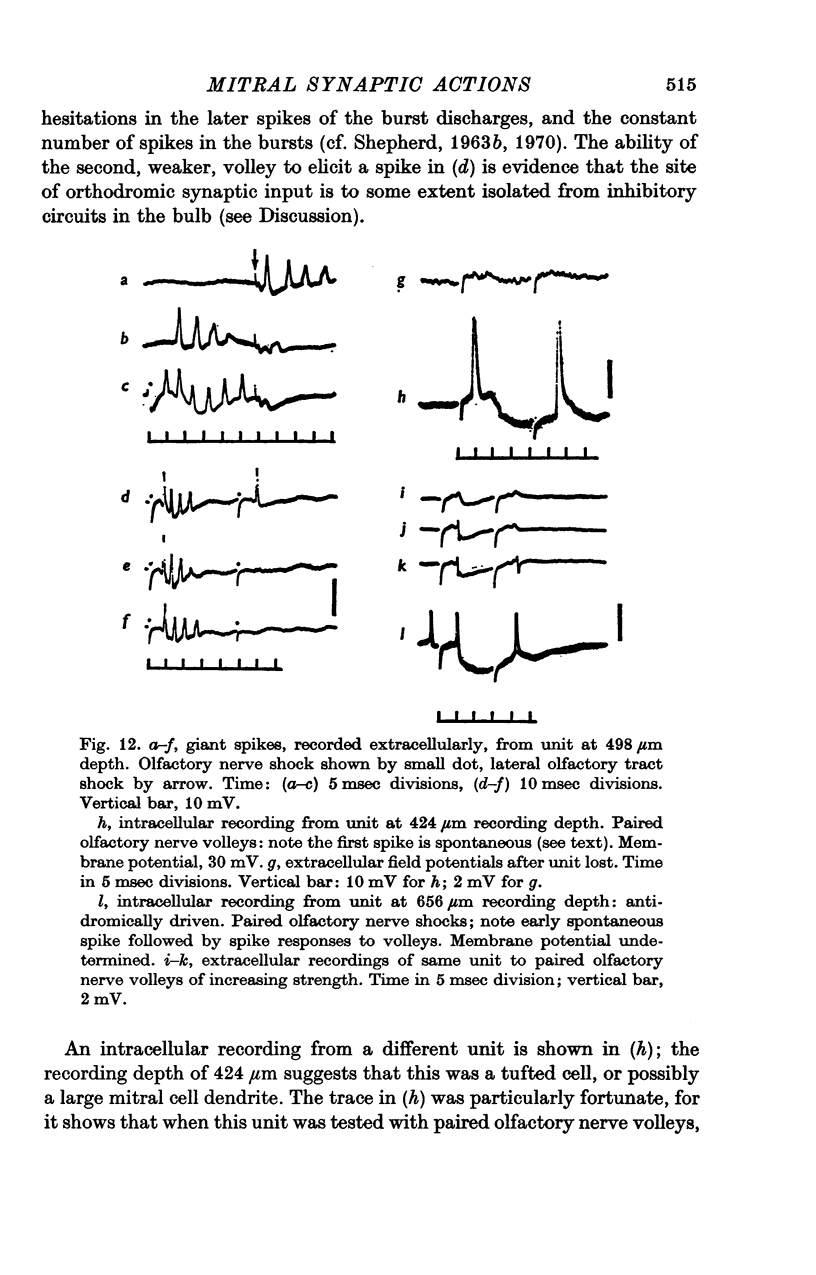
Abstract
1. A unitary study has been carried out of mitral and tufted cell responses to olfactory nerve volleys in the olfactory bulb of rabbits lightly anaesthetized with urethane-chloralose. 2. With volleys of different strengths, some mitral cells responded with a spike whose latency decreased considerably as the strength increased (elastic response); other cells responded at an invariant latency (inelastic response). The former may reflect diffuse olfactory nerve inputs to the dendritic tufts in the olfactory glomeruli, while tha latter may reflect input from discrete bundles of fibres. 3. The shortest spike latencies are consistent with monosynaptic excitation by the olfactory nerves; longer latencies may be due to longer pathways through the nerves, or polysynaptic pathways within the glomerular layer. 4. Facilitation, in terms of lower threshold and shorter spike latency, was found when testing with paired volleys of weak intensity at relatively short intervals (less than 40 msec). Suppression, in terms of raised threshold, longer latency and briefer repetitive discharges, was found at intervals up to several hundred msec. The facilitation and suppression are consistent with the hypothesis of synaptic excitation and inhibition, respectively, mediated through interneurones in the olfactory bulb. 5. Presumed tufted cells were similar in response properties to identified mitral cells. 6. Intracellular recordings revealed long-lasting hyperpolarization and in some cases, an initial depolarization leading to spike initiation, in response to an olfactory nerve volley.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK L. G., COOMBS J. S., ECCLES J. C. The nature of the monosynaptic excitatory and inhibitory processes in the spinal cord. Proc R Soc Lond B Biol Sci. 1952 Oct 16;140(899):170–176. [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. H., Bliss T. V., Keating M. J. The synaptic organization of optic afferents in the amphibian tectum. Proc R Soc Lond B Biol Sci. 1974 Nov 19;187(1089):421–447. doi: 10.1098/rspb.1974.0086. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Average transmission distance from mitral-tufted to granule cells in olfactory bulb. Electroencephalogr Clin Neurophysiol. 1974 Jun;36(6):609–618. doi: 10.1016/0013-4694(74)90227-2. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Relation of glomerular neuronal activity to glomerular transmission attenuation. Brain Res. 1974 Jan 4;65(1):91–107. doi: 10.1016/0006-8993(74)90338-2. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Spatial divergence and temporal dispersion in primary olfactory nerve of cat. J Neurophysiol. 1972 Nov;35(6):733–744. doi: 10.1152/jn.1972.35.6.733. [DOI] [PubMed] [Google Scholar]
- Freeman W. J. Topographic organization of primary olfactory nerve in cat and rabbit as shown by evoked potentials. Electroencephalogr Clin Neurophysiol. 1974 Jan;36(1):33–45. doi: 10.1016/0013-4694(74)90134-5. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V., Shepherd G. M. Short-axon cells in the olfactory bulb: dendrodendritic synaptic interactions. J Physiol. 1975 Oct;251(2):523–548. doi: 10.1113/jphysiol.1975.sp011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land L. J., Eager R. P., Shepherd G. M. Olfactory nerve projections to the olfactory bulb in rabbit: demonstration by means of a simplified ammoniacal silver degeneration method. Brain Res. 1970 Oct 13;23(2):250–254. doi: 10.1016/0006-8993(70)90044-2. [DOI] [PubMed] [Google Scholar]
- Land L. J. Localized projection of olfactory nerves to rabbit olfactory bulb. Brain Res. 1973 Dec 7;63:153–166. doi: 10.1016/0006-8993(73)90084-x. [DOI] [PubMed] [Google Scholar]
- Land L. J., Shepherd G. M. Autoradiographic analysis of olfactory receptor projections in the rabbit. Brain Res. 1974 Apr 26;70(3):506–510. doi: 10.1016/0006-8993(74)90259-5. [DOI] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Identification of tufted cells in the olfactory bulb. Nature. 1970 Aug 8;227(5258):623–625. doi: 10.1038/227623a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Inhibitory mechanisms in the rabbit olfactory bulb: dendrodendritic mechanisms. Brain Res. 1969 Jun;14(1):157–172. doi: 10.1016/0006-8993(69)90037-7. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Olfactory nerves and their excitatory action in the olfactory bulb. Exp Brain Res. 1972;14(2):185–197. doi: 10.1007/BF00234798. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. The effects of anaesthetics on synaptic excitation and inhibition in the olfactory bulb. J Physiol. 1972 Jun;223(3):803–814. doi: 10.1113/jphysiol.1972.sp009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS C. G., POWELL T. P., SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO STIMULATION OF THE LATERAL OLFACTORY TRACT IN THE RABBIT. J Physiol. 1963 Aug;168:65–88. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuron types of the glomerular layer of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):305–345. doi: 10.1242/jcs.9.2.305. [DOI] [PubMed] [Google Scholar]
- Pinching A. J., Powell T. P. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971 Sep;9(2):347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Rall W., Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J. 1973 Jul;13(7):648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M. Theoretical reconstruction of field potentials and dendrodendritic synaptic interactions in olfactory bulb. J Neurophysiol. 1968 Nov;31(6):884–915. doi: 10.1152/jn.1968.31.6.884. [DOI] [PubMed] [Google Scholar]
- SHEPHERD G. M. NEURONAL SYSTEMS CONTROLLING MITRAL CELL EXCITABILITY. J Physiol. 1963 Aug;168:101–117. doi: 10.1113/jphysiol.1963.sp007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPHERD G. M. RESPONSES OF MITRAL CELLS TO OLFACTORY NERVE VOLLEYS IN THE RABBIT. J Physiol. 1963 Aug;168:89–100. doi: 10.1113/jphysiol.1963.sp007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M. Physiological evidence for dendrodendritic synaptic interactions in the rabbit's olfactory glomerulus. Brain Res. 1971 Sep 10;32(1):212–217. doi: 10.1016/0006-8993(71)90168-5. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972 Oct;52(4):864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- Thomas R. C., Wilson V. J. Precise localization of Renshaw cells with a new marking technique. Nature. 1965 Apr 10;206(980):211–213. doi: 10.1038/206211b0. [DOI] [PubMed] [Google Scholar]
- White E. L. Synaptic organization in the olfactory glomerulus of the mouse. Brain Res. 1972 Feb 11;37(1):69–80. doi: 10.1016/0006-8993(72)90346-0. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO C., YAMAMOTO T., IWAMA K. The inhibitory systems in the olfactory bulb studied by intracellular recording. J Neurophysiol. 1963 May;26:403–415. doi: 10.1152/jn.1963.26.3.403. [DOI] [PubMed] [Google Scholar]


