Abstract
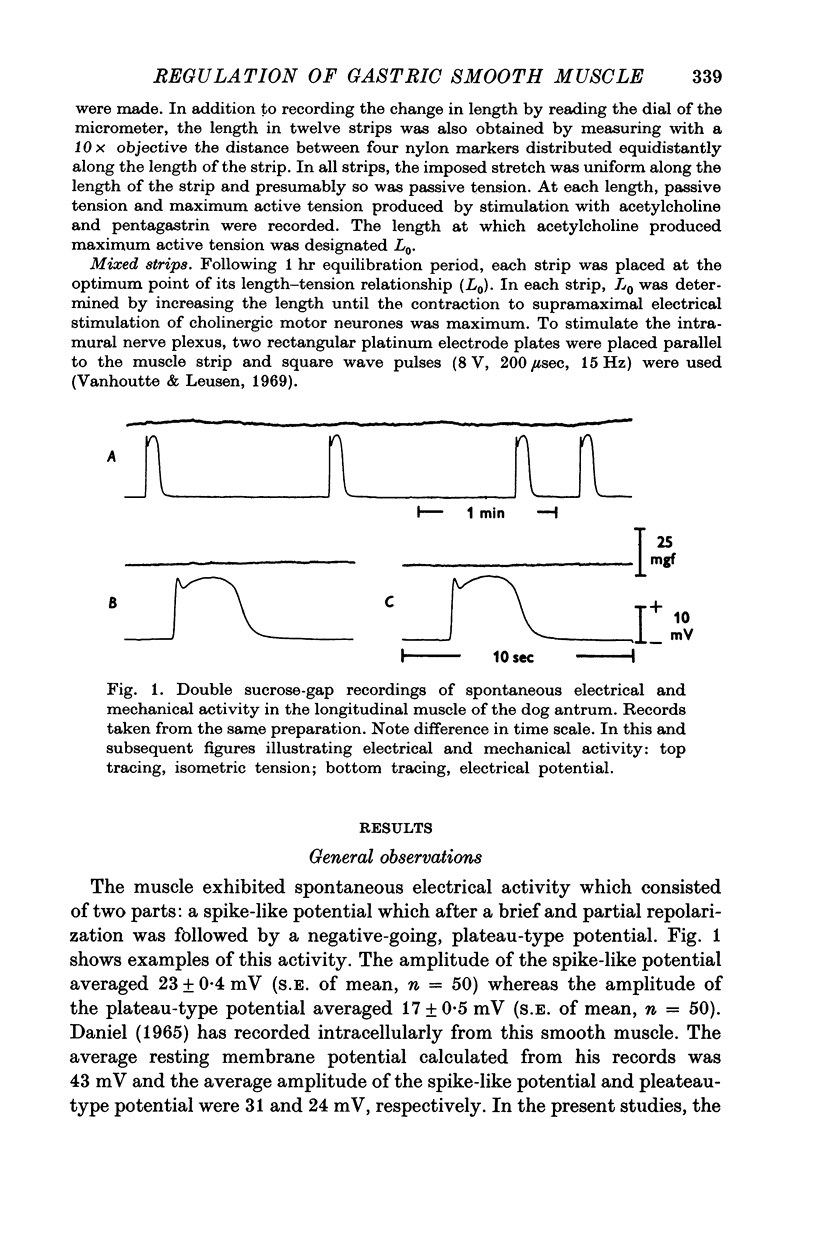
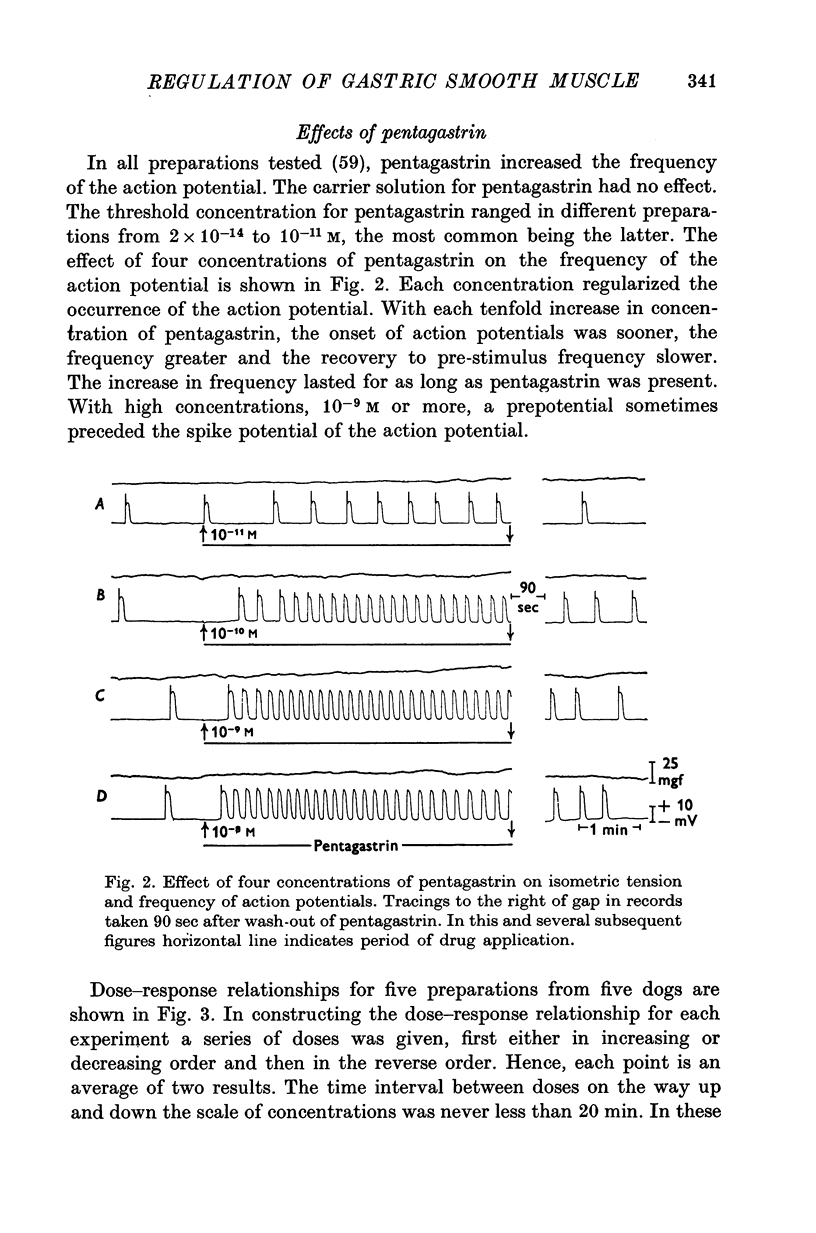
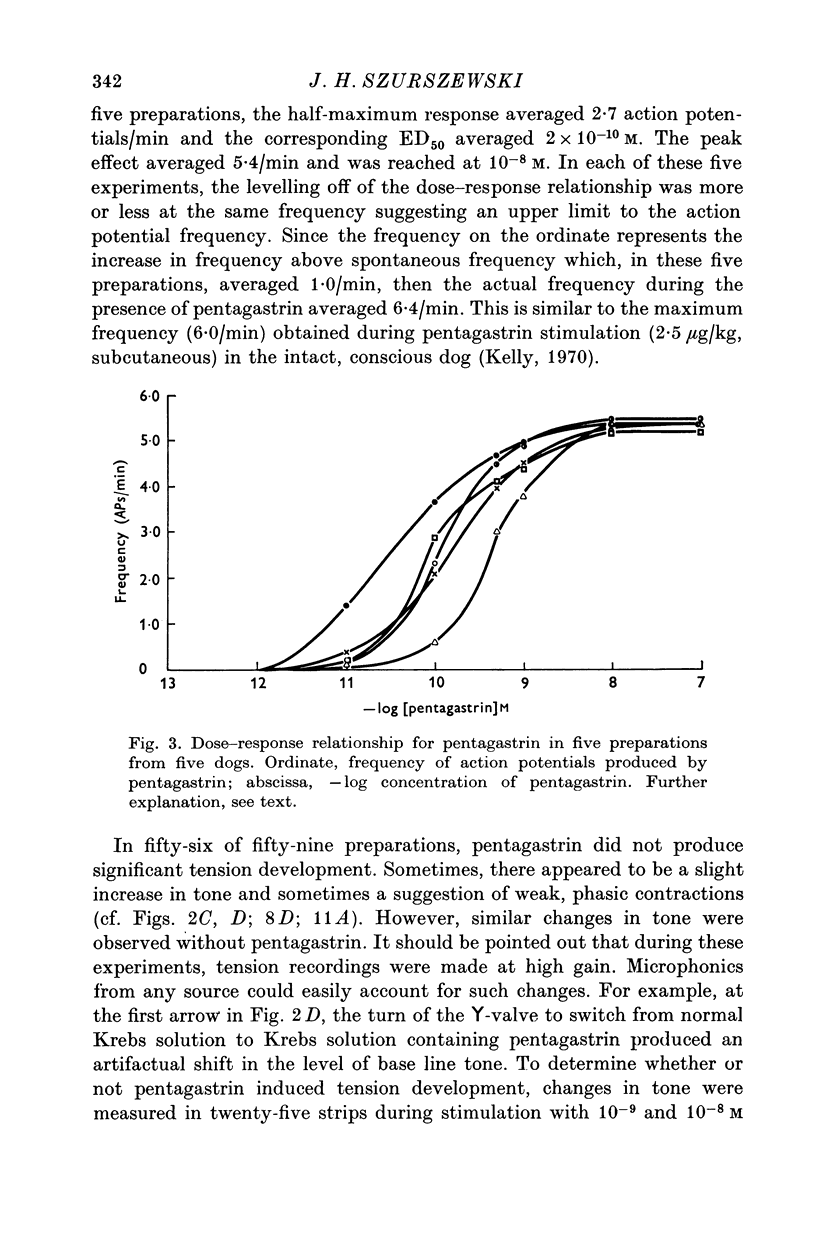
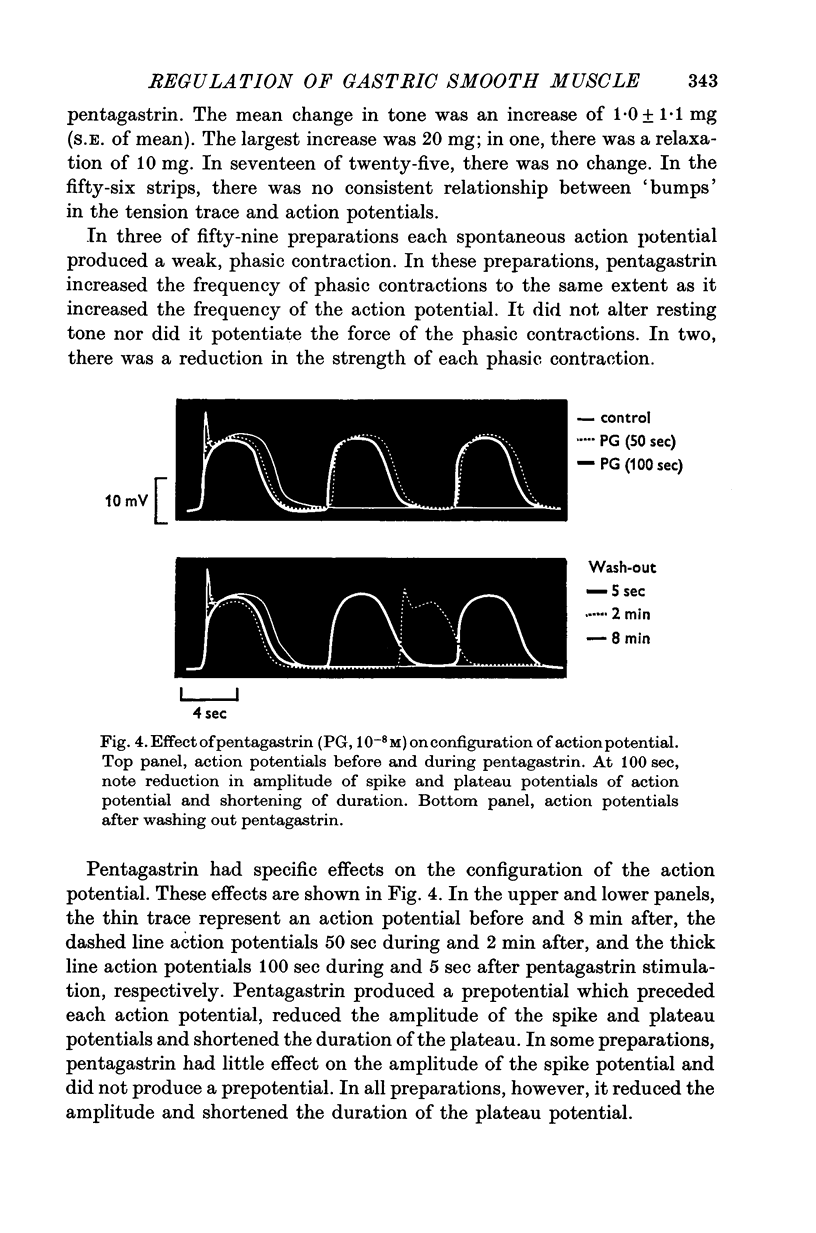
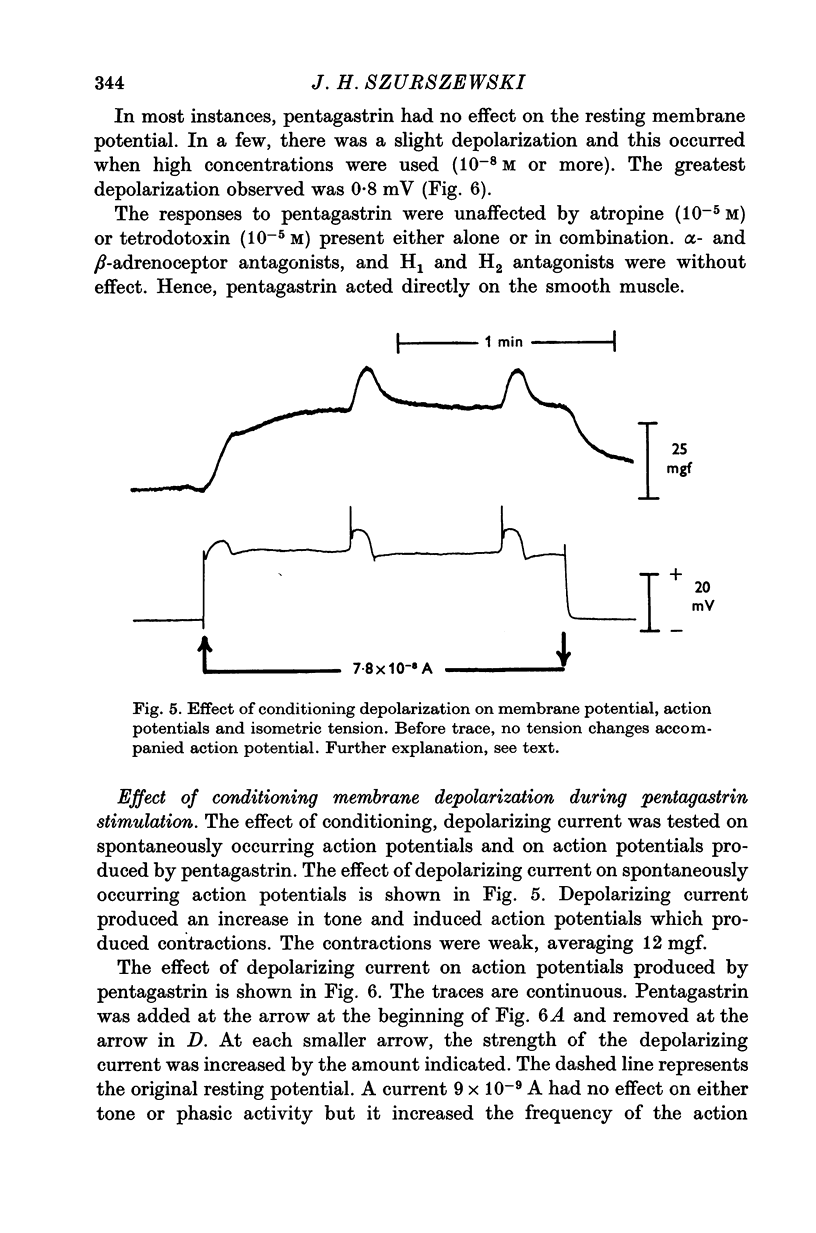
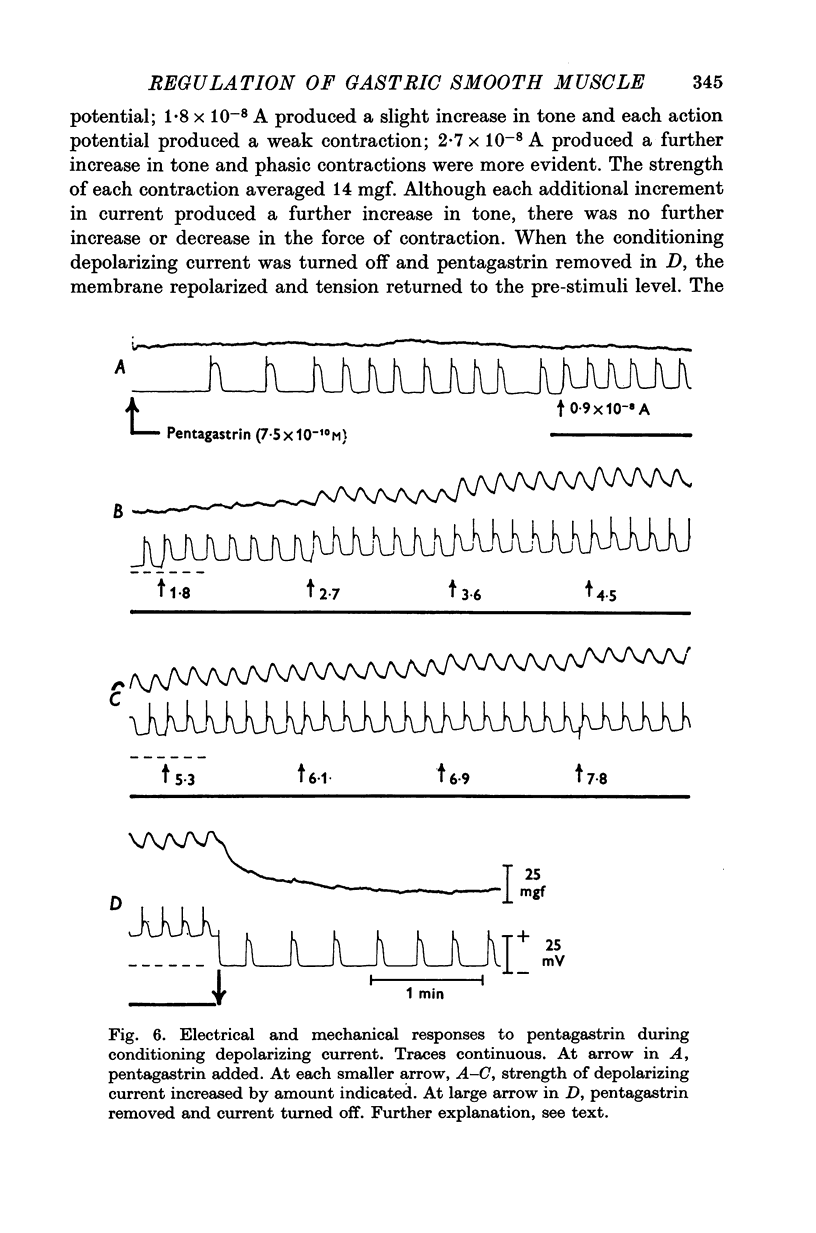
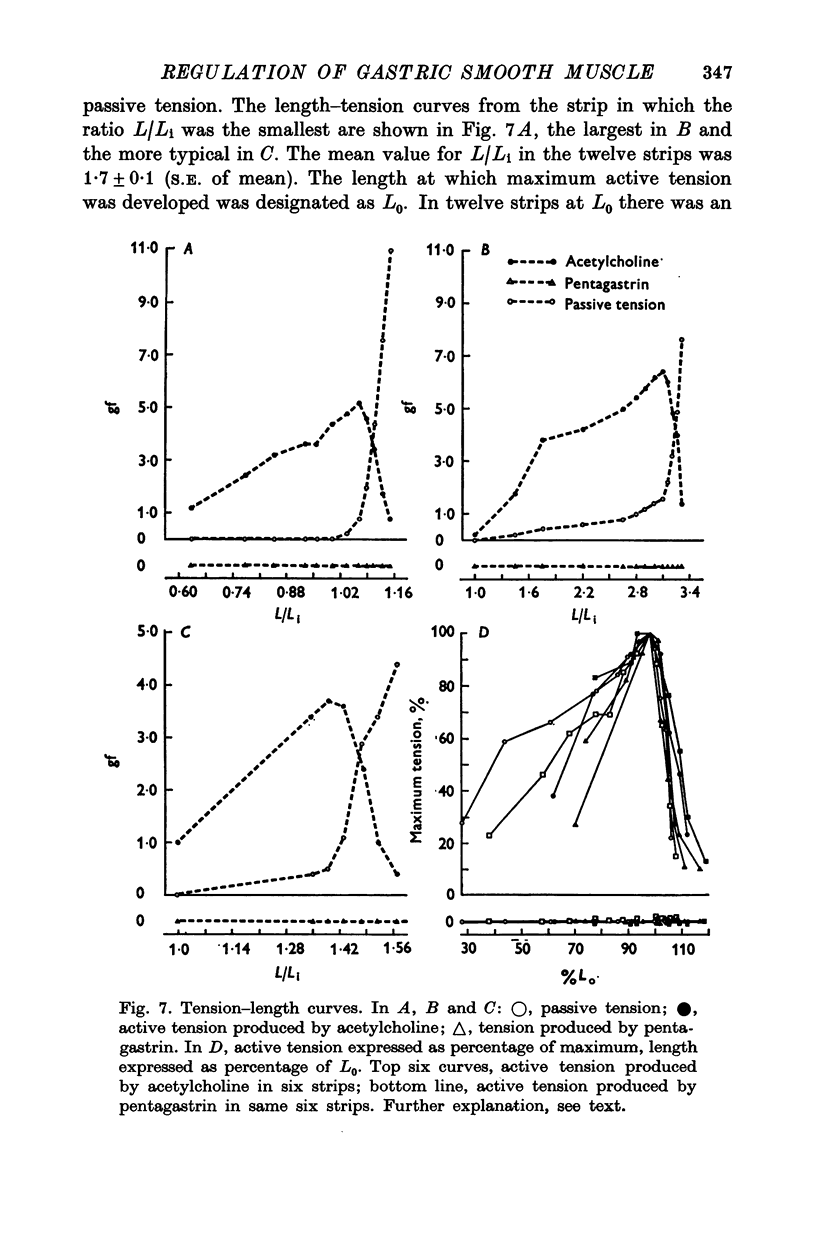
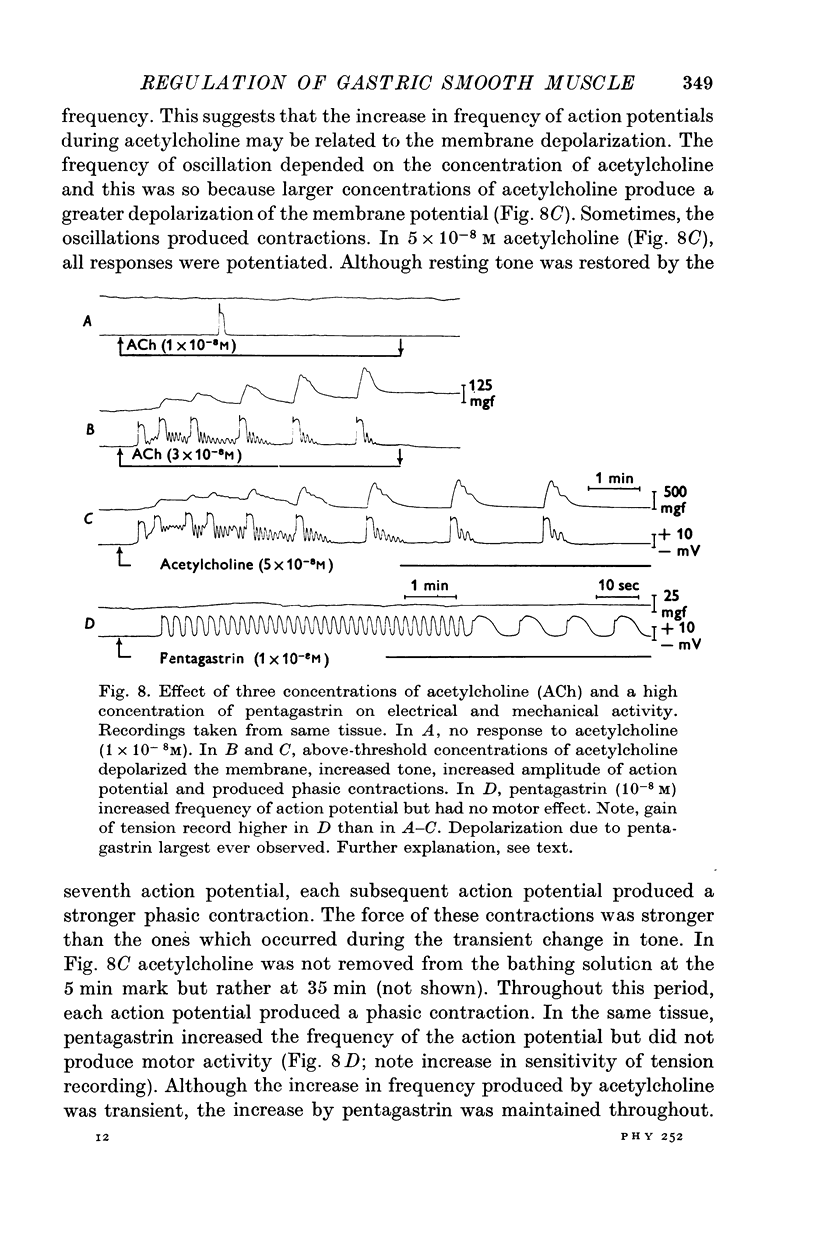
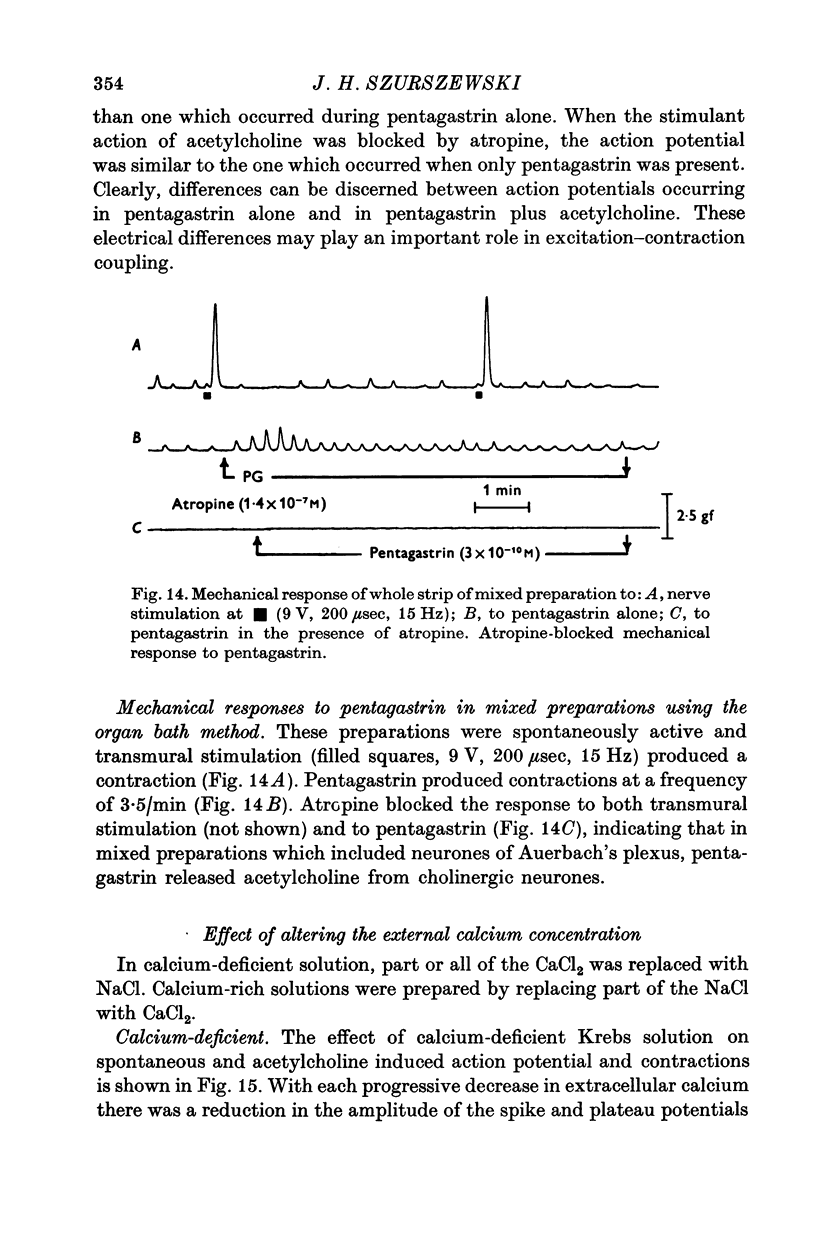
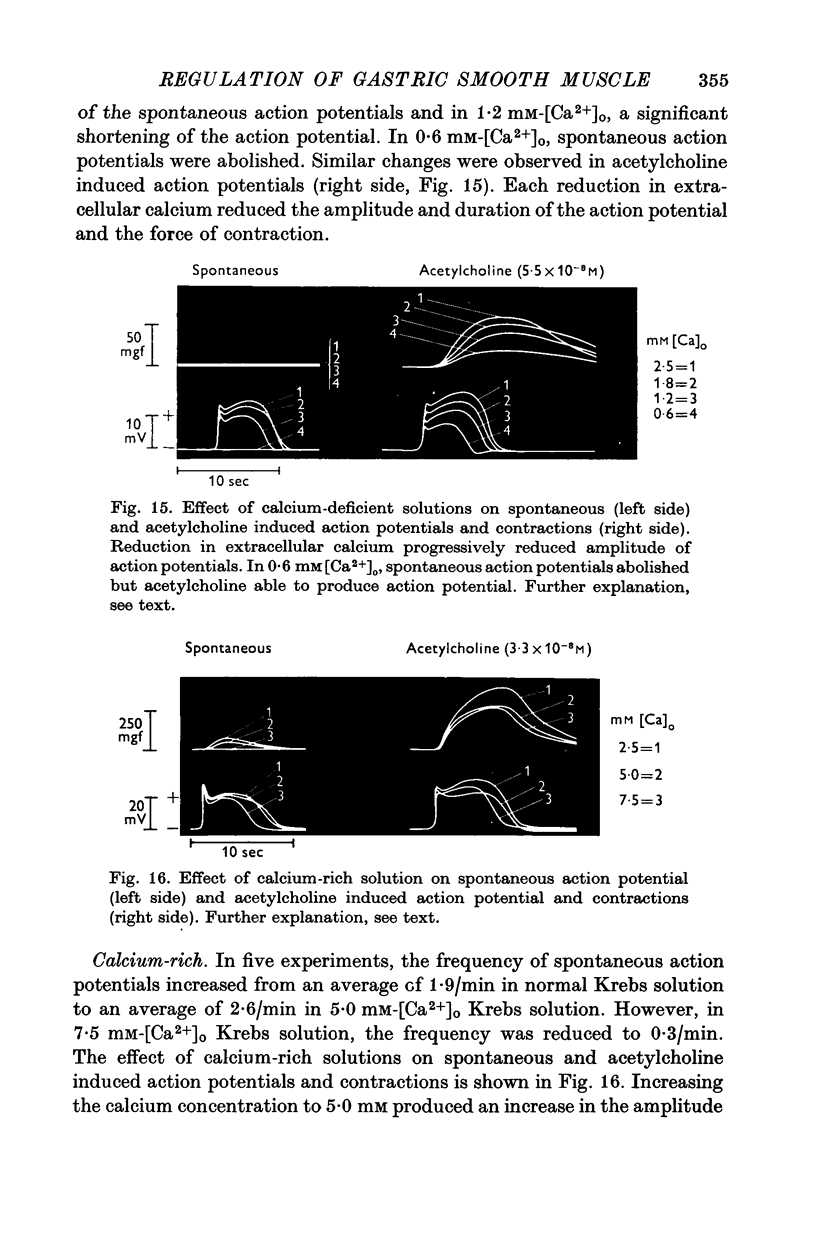
1. Electrical and mechanical activities of the longtitudinal muscle of the dog antrum were recorded with the double sucrose-gap technique. 2. The muscle exhibited spontaneous action potentials which consisted of a spike-like potential which, after a brief and partial repolarization, was followed by a negative-going, plateau-type potential. In 97% of the preparations, no tension changes were produced by spontaneous action potentials. 3. Tetrodotoxin, atropine, alpha- and beta-adrenoceptor antagonists, and H1 and H2 receptor blocking agents had no effect on the action potential. It was concluded that the action potential was myogenic in origin. 4. The mean frequency of the action potential at 37+/- 0.5 degrees C was 1.0/min+/-0.06 (s.e. of mean, n=92) and the mean duration 7.1+/-0.2 sec (s.e. of mean, n=11). 5. Steady depolarizing current increased whereas hyperpolarizing current decreased the frequency of the action potential. 6. Length-tension relations were studied. In twelve strips, the average resting, passive, tension at LO was 570 mg. The active force of isometric contraction produced by acetylcholine increased with strip length up to a maximum, then decreased wtih further increased in length. There were no mechanical responses to pentagastrin. 7. Pentagastrin had two sites of action. On smooth muscle, it increased the frequency of the action potential in a dose dependent fashion. Threshold concentraions ranged from 2X10-14 to 10-11M. The ED50 was 2X10-10M. The maximum response, 5.4/min, was reached at 10-8M. Pentagastrin also released acetylcholine from intramural cholinergic nerves. 8. Pentagastrin reduced the amplitude and duration of the action potential.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERG A. K., AXELSSON J. SOME MECHANICAL ASPECTS OF AN INTESTINAL SMOOTH MUSCLE. Acta Physiol Scand. 1965 May-Jun;64:15–27. doi: 10.1111/j.1748-1716.1965.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Bennett A. Effect of gastrin on isolated smooth muscle preparation. Nature. 1965 Oct 9;208(5006):170–173. doi: 10.1038/208170a0. [DOI] [PubMed] [Google Scholar]
- Bennett A., Misiewicz J. J., Waller S. L. Analysis of the motor effects of gastrin and pentagastrin on the human alimentary tract in vitro. Gut. 1967 Oct;8(5):470–474. doi: 10.1136/gut.8.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Goldman D. E. Origin of axon membrane hyperpolarization under sucrose-gap. Biophys J. 2008 Dec 31;6(4):453–470. doi: 10.1016/S0006-3495(66)86669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A., GOODALL M. Excitability, length tension relation and kinetics of uterine muscle contraction in relation to hormonal status. J Physiol. 1954 Nov 29;126(2):384–395. doi: 10.1113/jphysiol.1954.sp005216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. J., Phillips S. F., Summerskill W. H. Comparison of effects of gastrin, cholecystokinin-pancreozymin, secretin, and glucagon on human stomach muscle in vitro. Gastroenterology. 1970 Oct;59(4):539–545. [PubMed] [Google Scholar]
- Daniel E. E. The electrical and contractile activity of the pyloric region in dogs and the effects of drugs. Gastroenterology. 1965 Oct;49(4):403–418. [PubMed] [Google Scholar]
- GREGORY R. A., TRACY H. J. THE CONSTITUTION AND PROPERTIES OF TWO GASTRINS EXTRACTED FROM HOG ANTRAL MUCOSA. Gut. 1964 Apr;5:103–114. [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Isenberg J. I., Grossman M. I. Effect of gastrin and SC 15396 on gastric motility in dogs. Gastroenterology. 1969 Mar;56(3):450–455. [PubMed] [Google Scholar]
- Jacoby H. I., Marshall C. H. Gastric motor-stimulating activity of gastrin tetrapeptide in dogs. Gastroenterology. 1969 Jan;56(1):80–87. [PubMed] [Google Scholar]
- Kao C. Y. Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena. Pharmacol Rev. 1966 Jun;18(2):997–1049. [PubMed] [Google Scholar]
- Kelly K. A. Effect of gastrin on gastric myo-electric activity. Am J Dig Dis. 1970 May;15(5):399–405. doi: 10.1007/BF02283864. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong N. K., Brown B. H., Whittaker G. E., Duthie H. L. Effects of gastrin I, secretin and cholecystokinin-pancreozymin on the electrical activity, motor activity, and acid output of the stomach in man. Scand J Gastroenterol. 1972;7(2):161–170. [PubMed] [Google Scholar]
- Misiewicz J. J., Holdstock D. J., Waller S. L. Motor responses of the human alimentary tract to near-maximal infusions of pentagastrin. Gut. 1967 Oct;8(5):463–469. doi: 10.1136/gut.8.5.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Isaza J., Curt J., Woodward E. R. The effect of pentagastrin on gastric motility following vagotomy. J Surg Res. 1970 Feb;10(2):73–80. doi: 10.1016/0022-4804(70)90013-2. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Isaza J., Woodward E. R. Effect of gastrin on gastrin motor activity. Gastroenterology. 1969 Dec;57(6):649–658. [PubMed] [Google Scholar]
- TRACY H. J., GREGORY R. A. PHYSIOLOGICAL PROPERTIES OF A SERIES OF SYNTHETIC PEPTIDES STRUCTURALLY RELATED TO GASTRIN I. Nature. 1964 Dec 5;204:935–938. doi: 10.1038/204935a0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P., Leusen I. The reactivity of isolated venous preparations to electrical stimulation. Pflugers Arch. 1969;306(4):341–353. doi: 10.1007/BF00589159. [DOI] [PubMed] [Google Scholar]
- Vizi S. E., Bertaccini G., Impicciatore M., Knoll J. Evidence that acetylcholine released by gastrin and related polypeptides contributes to their effect on gastrointestinal motility. Gastroenterology. 1973 Feb;64(2):268–277. [PubMed] [Google Scholar]


