Abstract
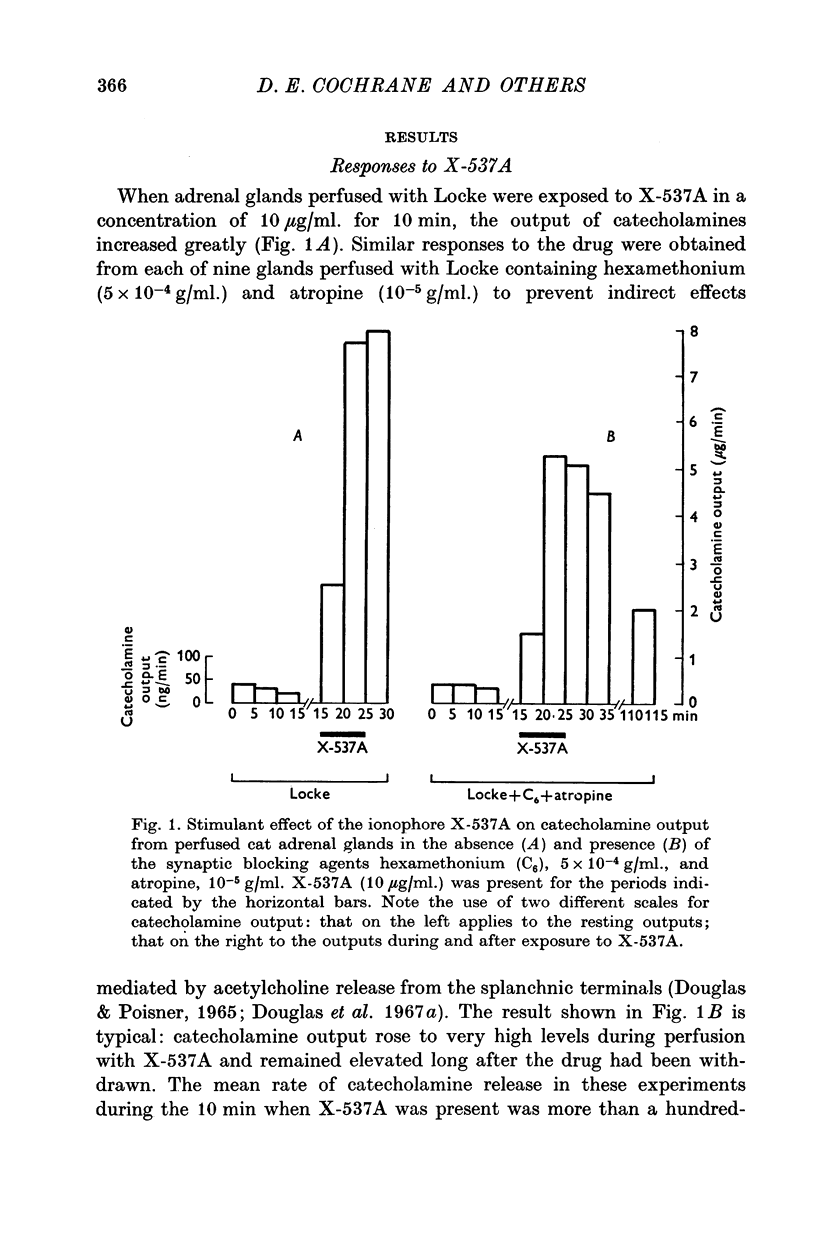
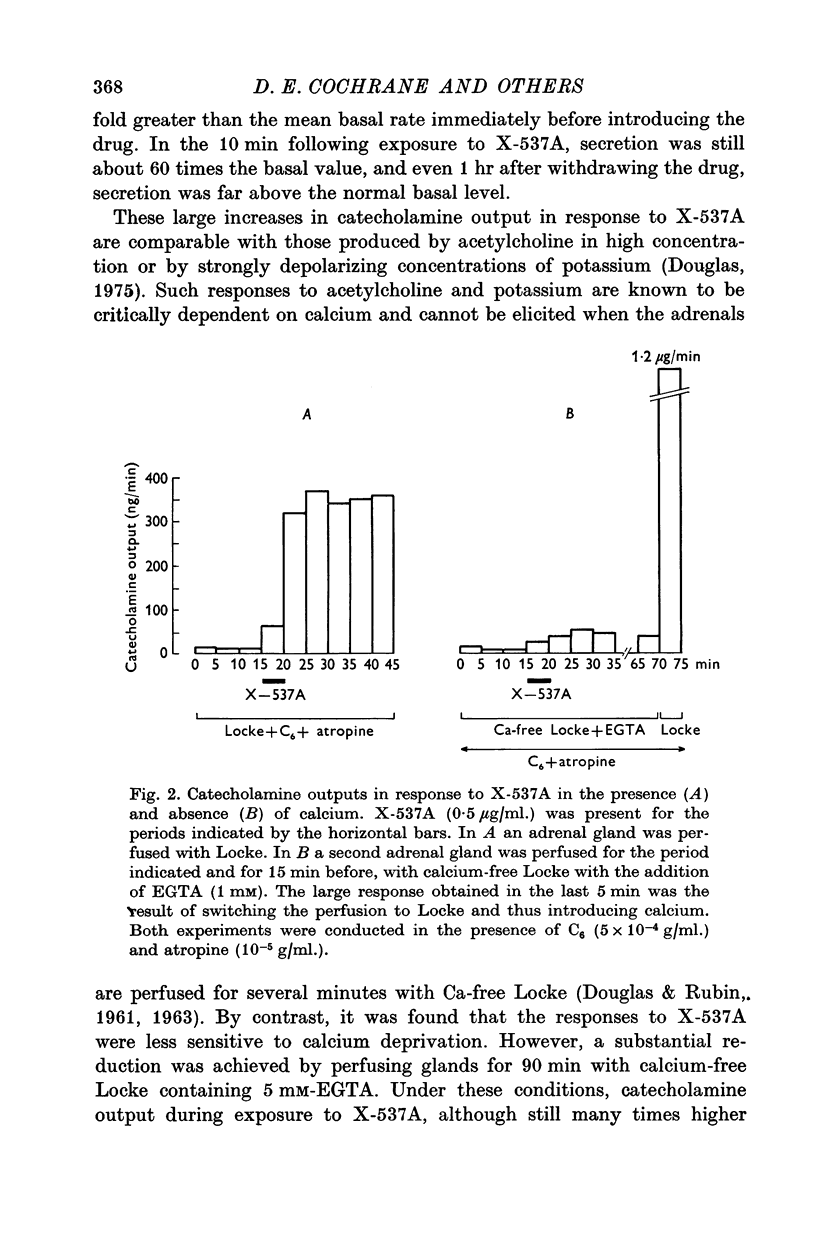
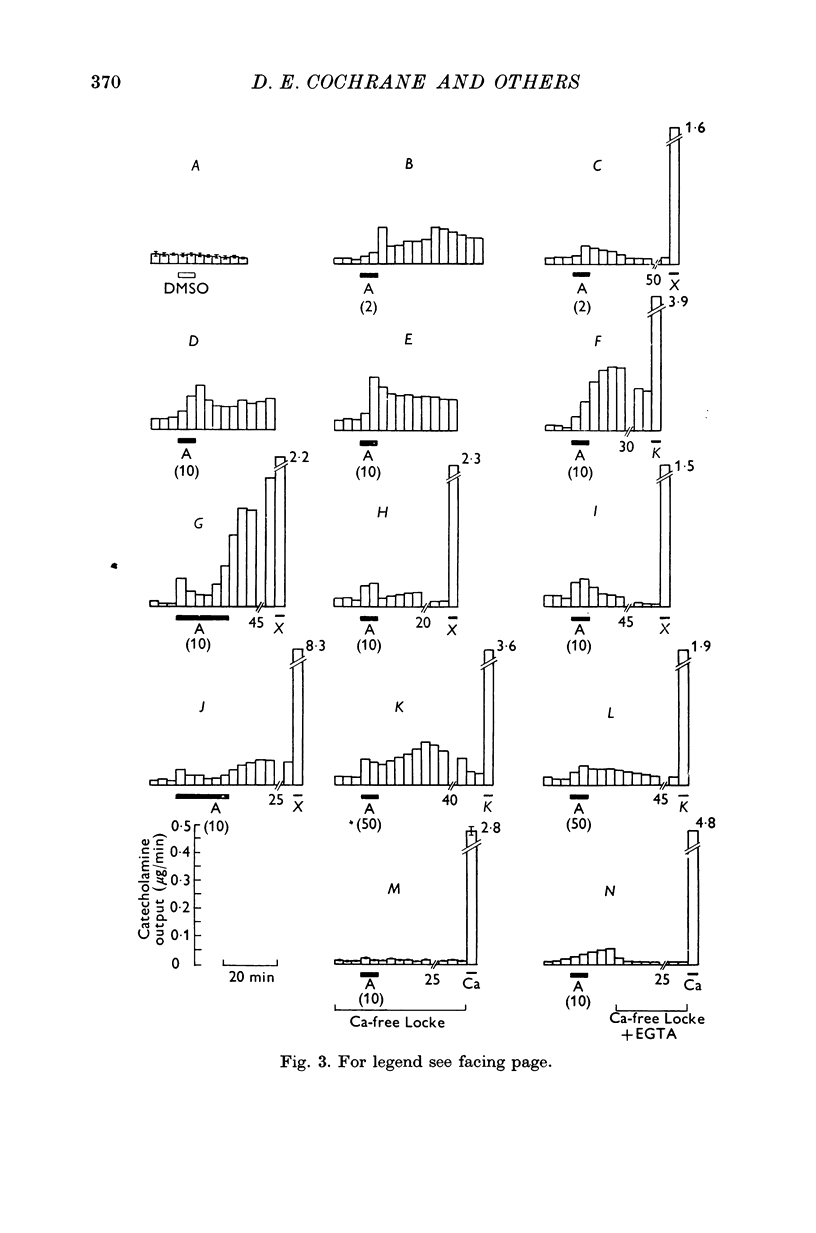
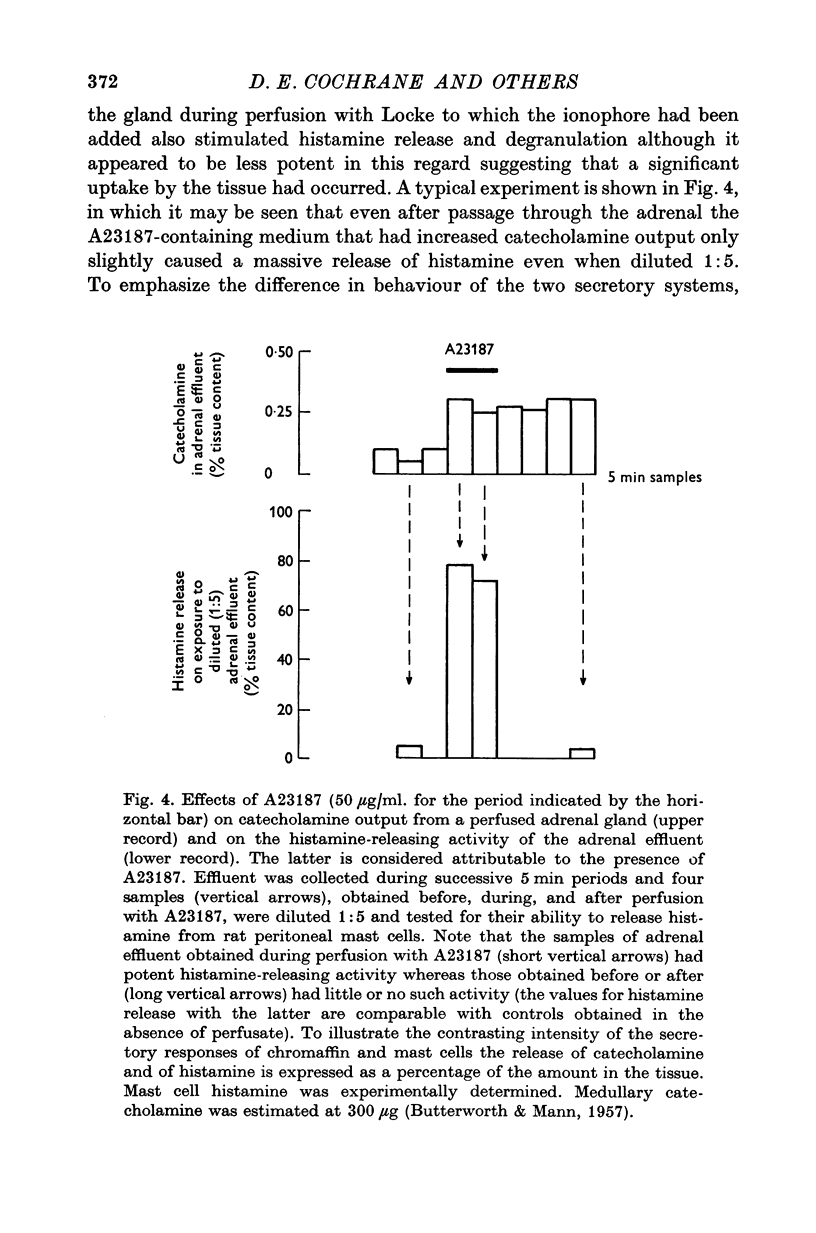
1. The ionophores X-537A and A23187, which are known to transfer calcuim across cell membranes, stimulated catecholamine release from perfused cat adrenal glands. 2. These stimulant effects persisted in the presence of hexamethonium and atropine and are therefore attributable to direct actions of the ionophores on the adrenal chromaffin cells. 3. Perfusion with calcium-free Locke abolished responses to A23187 and reduced those to X-537A. 4. Responses to X-537A were consistently large and comparable with those produced by 56 mM potassium. By contrast, responses to A23177, over the wide range of concentrations tested, were variable and much smaller. 5. That the two ionophores can stimulate through calcium-dependent mechanisms is considered fresh support for the calcium hypothesis of stimulus-secretion coupling. That they differ in effectiveness may mean that factors besides calcium are important. The greater potency of the less specific ionophore, X-537A, may be attributable to its ability to depolarize as well as carry calcuim, while the relatively small effects of A23187, a generally more effective ionophore for calcuim, may indicate that inward movement of calcium without a background of membrane perturbation such as may be produced by depolarization, is insufficient to elicit strong secretory responses.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreo C. S., Vallejos R. H. Uncoupling of photophosphorylation in spinach chloroplasts by the ionophorous antibiotic A23187. FEBS Lett. 1974 Sep 15;46(1):343–346. doi: 10.1016/0014-5793(74)80402-3. [DOI] [PubMed] [Google Scholar]
- BUTTERWORTH K. R., MANN M. A quantitative comparison of the sympathominetic amine content of the left and right adrenal glands of the cat. J Physiol. 1957 Apr 30;136(2):294–299. doi: 10.1113/jphysiol.1957.sp005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Chambers E. L., Pressman B. C., Rose B. The activation of sea urchin eggs by the divalent ionophores A23187 and X-537A. Biochem Biophys Res Commun. 1974 Sep 9;60(1):126–132. doi: 10.1016/0006-291x(74)90181-8. [DOI] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. How do neurones secrete peptides? Exocytosis and its consequences, including "synaptic vesicle" formation, in the hypothalamo-neurohypophyseal system. Prog Brain Res. 1973;39:21–39. doi: 10.1016/S0079-6123(08)64064-9. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S., Savion N., Heichal O., Selinger Z. Induction of enzyme secretion in rat pancreatic slices using the ionophore A-23187 and calcium. An experimental bypass of the hormone receptor pathway. J Biol Chem. 1974 Jun 25;249(12):3991–3993. [PubMed] [Google Scholar]
- Entman M. L., Gillette P. C., Wallick E. T., Pressman B. C., Schwartz A. A study of calcium binding and uptake by isolated cardiac sarcoplasmic reticulum: the use of a new ionophore (X537A). Biochem Biophys Res Commun. 1972 Aug 21;48(4):847–853. doi: 10.1016/0006-291x(72)90685-7. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Rao G. H. Effects of the lonophore A23187 on the blood platelets II. Influence on ultrastructure. Am J Pathol. 1974 Nov;77(2):151–166. [PMC free article] [PubMed] [Google Scholar]
- Gushchin I. S., Orlov S. M., Tsziu N. L. Membrannyi potentsial tuchnykh kletok i vysvobozhdenie iz nikh gistamina. Biull Eksp Biol Med. 1973 Nov;76(11):20–23. [PubMed] [Google Scholar]
- KREMZNER L. T., WILSON I. B. A procedure for the determination of histamine. Biochim Biophys Acta. 1961 Jun 24;50:365–367. doi: 10.1016/0006-3002(61)90339-0. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Douglas W. W. Electron microscope evidence of calcium-induced exocytosis in mast cells treated with 48-80 or the ionophores A-23187 and X-537A. J Cell Biol. 1974 Aug;62(2):519–526. doi: 10.1083/jcb.62.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Cochrane D. E., Douglas W. W. Exocytosis (secretory granule extrusion) induced by injection of calcium into mast cells. Can J Physiol Pharmacol. 1973 Dec;51(12):1001–1004. doi: 10.1139/y73-153. [DOI] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Calcium ionophore X-537A increases spontaneous and phasic quantal release of acetylcholine at frog neuromuscular junction. Nature. 1974 Aug 23;250(5468):658–660. doi: 10.1038/250658a0. [DOI] [PubMed] [Google Scholar]
- Levy J. V., Cohen J. A., Inesi G. Contractile effects of a calcium ionophore. Nature. 1973 Apr 13;242(5398):461–463. doi: 10.1038/242461a0. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Kun E. Mode of action of the antibiotic X-537A on mitochondrial glutamate oxidation. Biochem Biophys Res Commun. 1973 Feb 5;50(3):820–825. doi: 10.1016/0006-291x(73)91318-1. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Douglas W. W. Vasopressin release from the isolated neurohypophysis induced by a calcium ionophore, X-537A. Nature. 1974 May 31;249(456):479–481. doi: 10.1038/249479a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer D. R., Reed P. W., Lardy H. A. Ultraviolet and fluorescent spectral properties of the divalent cation ionophore A23187 and its metal ion complexes. Biochemistry. 1974 Sep 10;13(19):4007–4014. doi: 10.1021/bi00716a029. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Prince W. T., Rasmussen H., Berridge M. J. The role of calcium in fly salivary gland secretion analyzed with the ionophore A-23187. Biochim Biophys Acta. 1973 Nov 2;329(1):98–107. doi: 10.1016/0304-4165(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Rubin R. P. The role of energy metabolism in calcium-evoked secretion from the adrenal medulla. J Physiol. 1970 Jan;206(1):181–192. doi: 10.1113/jphysiol.1970.sp009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. T., Hansen E. L., Thorn N. A. Calcium and stimulus-secretion coupling in the neurohypophysis. III. Ca2+ ionophore (A-23187)-induced release of vasopressin from isolated rat neurophypophyses. Acta Endocrinol (Copenh) 1974 Nov;77(3):443–450. [PubMed] [Google Scholar]
- Scarpa A., Baldassare J., Inesi G. The effect of calcium ionophores on fragmented sarcoplasmic reticulum. J Gen Physiol. 1972 Dec;60(6):735–749. doi: 10.1085/jgp.60.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Inesi G. Ionophore mediated equilibration of calcium ion gradients in fragmented-sarcoplasmic reticulum. FEBS Lett. 1972 May 15;22(3):273–276. doi: 10.1016/0014-5793(72)80248-5. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lewis R. M., Hanley H. G., Munson R. G., Dial F. D., Ray M. V. Hemodynamic and biochemical effects of a new positive inotropic agent. Antibiotic ionophore RO 2-2985. Circ Res. 1974 Jan;34(1):102–111. doi: 10.1161/01.res.40.4.102. [DOI] [PubMed] [Google Scholar]
- Thoa N. B., Costa J. L., Moss J., Kopin I. J. Mechanism of release of norepinephrine from peripheral adrenergic neurones by the calcium ionophores X 537A and A 23187. Life Sci. 1974 May 1;14(9):1705–1719. doi: 10.1016/0024-3205(74)90272-0. [DOI] [PubMed] [Google Scholar]
- Williams J. A., Lee M. Pancreatic acinar cells: use of Ca++ ionophore to separate enzyme release from the earlier steps in stimulus-secretion coupling. Biochem Biophys Res Commun. 1974 Sep 23;60(2):542–548. doi: 10.1016/0006-291x(74)90274-5. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Wilkinson J. R., Hamill R. L., Horng J. S. Effects of antibiotic ionophore, A23187, on oxidative phosphorylation and calcium transport of liver mitochondria. Arch Biochem Biophys. 1973 Jun;156(2):578–585. doi: 10.1016/0003-9861(73)90308-1. [DOI] [PubMed] [Google Scholar]


