Abstract
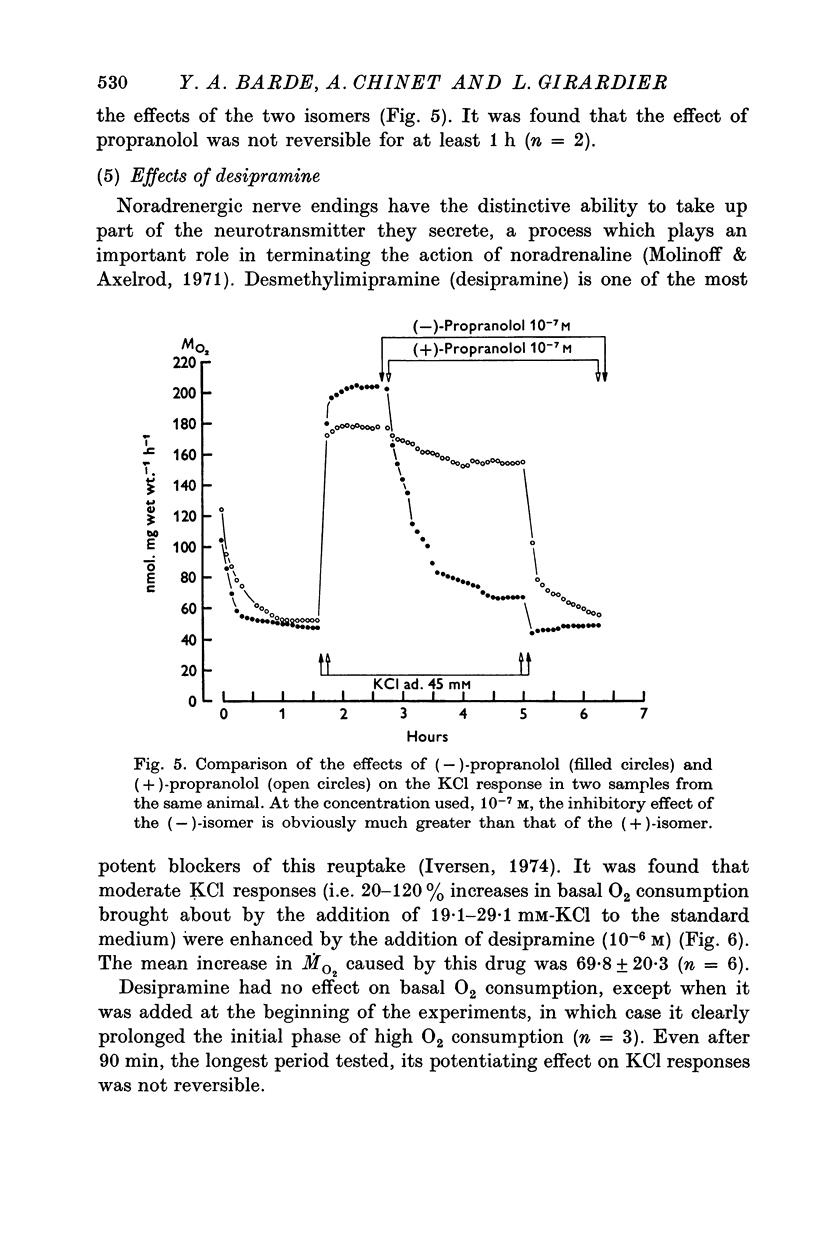
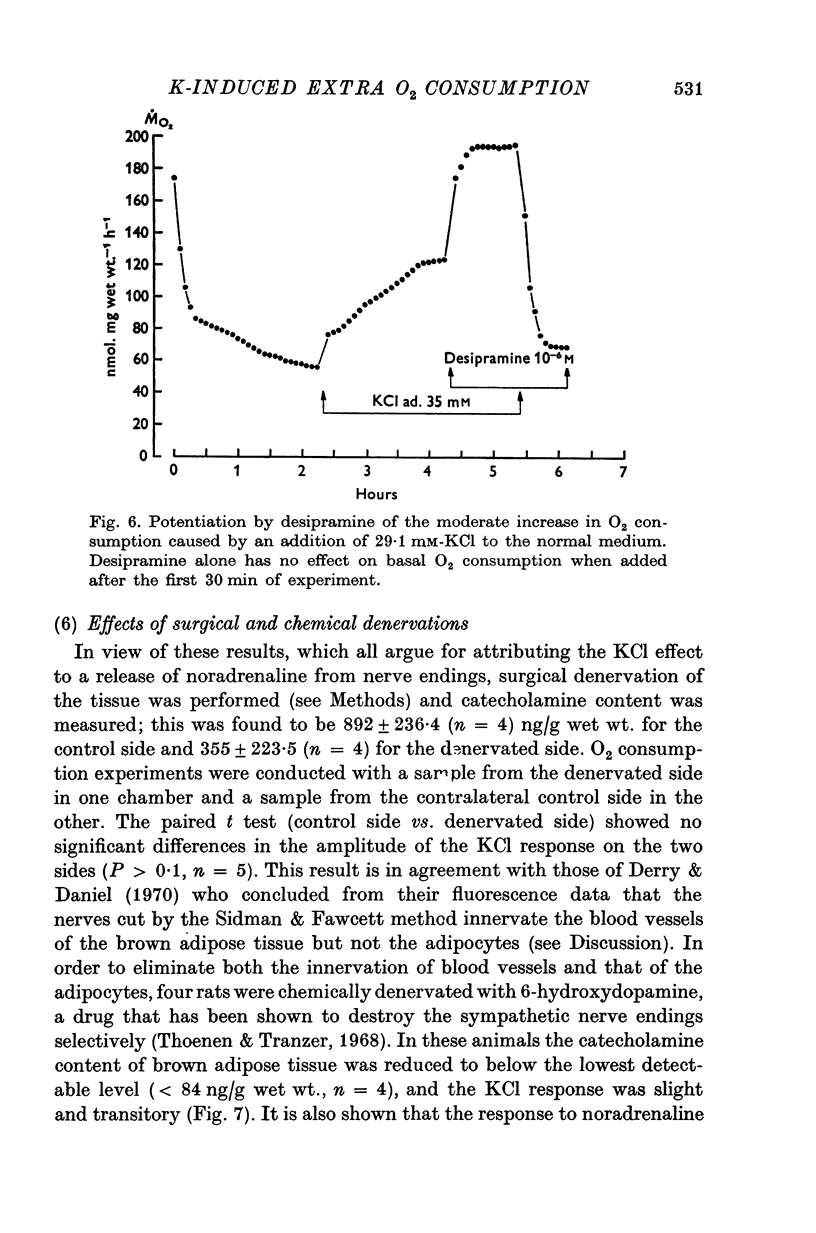
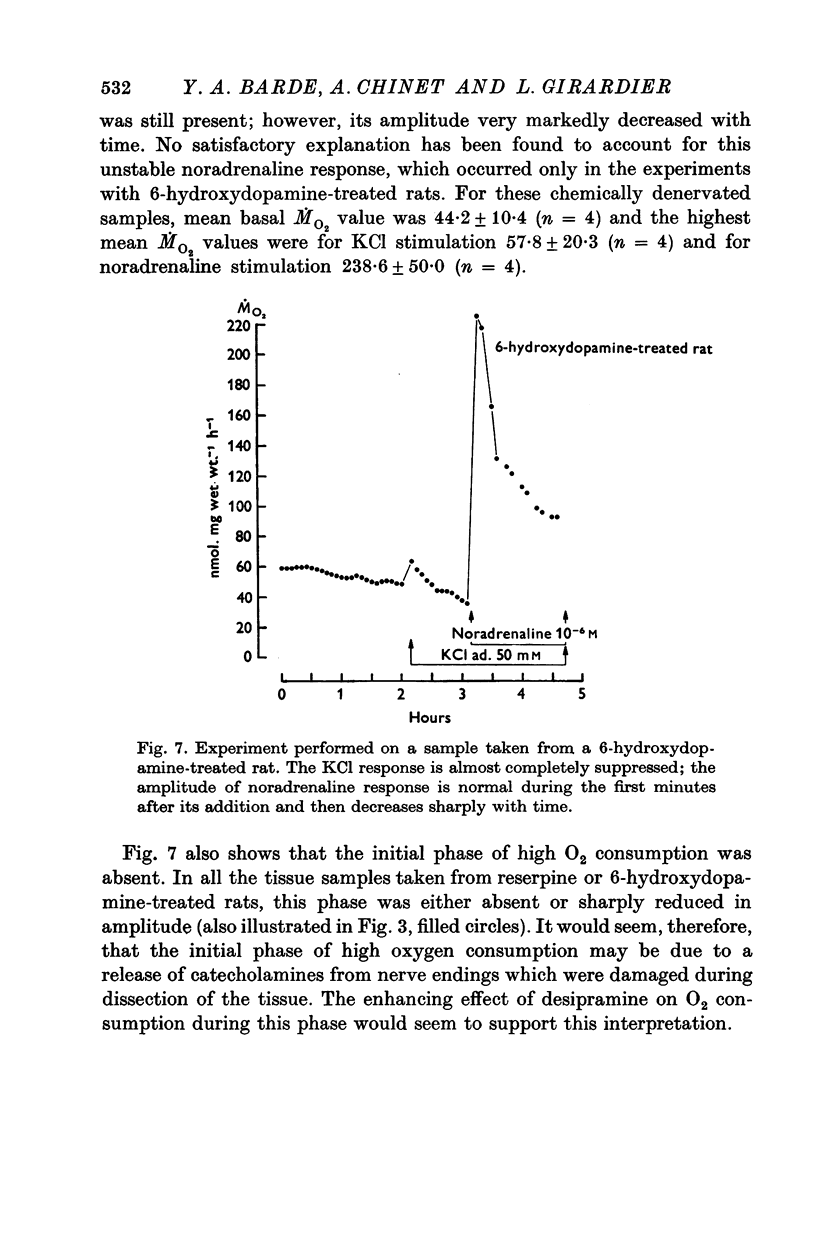
1. In brown adipose tissue, noradrenaline induces an increase in respiration and a depolarization of the cells. The effect of an increase in potassium concentration in a range known to depolarize the brown adipocytes was tested on the O2 consumption. 2. Isolated interscapular brown adipose tissue from the rat was incubated in chambers that allowed O2 consumption to be measured over prolonged periods. 3. 45-50 mM-KC1 were found to induce a more that fourfold increase in O2 consumption, which was stable, reversible and dependent upon the presence of calcium in the meduim. 4. When rats were pre-treated with reserpine or 6-hydroxydopamine the KC1-induced increase in O2 consumption was sharply reduced or entirely adsent. 5. The effect of KC1 was greatly inhibited by (-)-propranolol, but not by (+)-propranolol. 6. Moderate increases in O2 consumption induced by low concentrations of potassium were potentiated by desipramine, a drug which is known to block the uptake of catecholamines by adrenergic nerve endings. 7. Surgical denervation caused a decrease in the catecholamine content of the tissue, but had no effect on the KC1 response. 8. It is concluded that in brown adipose tissue, potassium stimulates O2 consumption by causing a release of noradrenaline from nerve endings. This implies that surgical denervation as it is commonly performed on this tissue does not denervate the brown adipocytes but probably only the blood vessels.
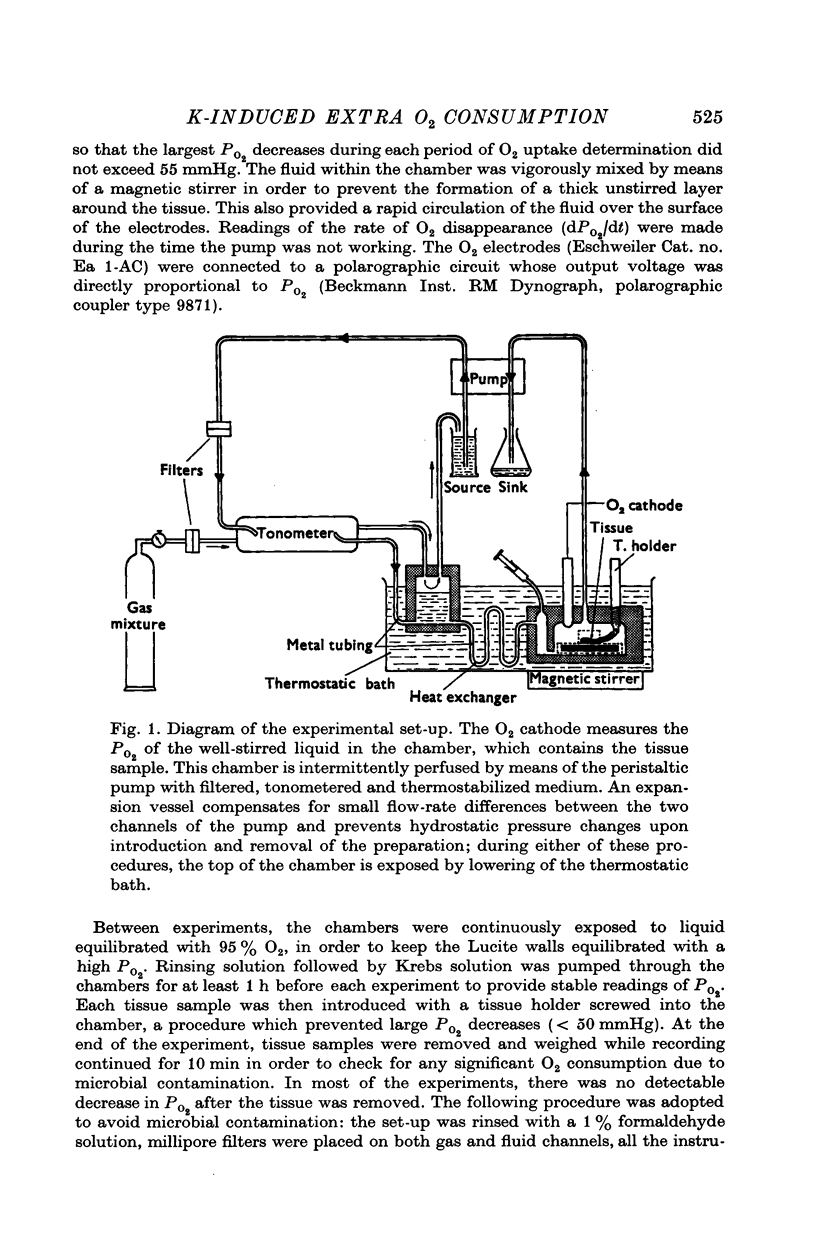
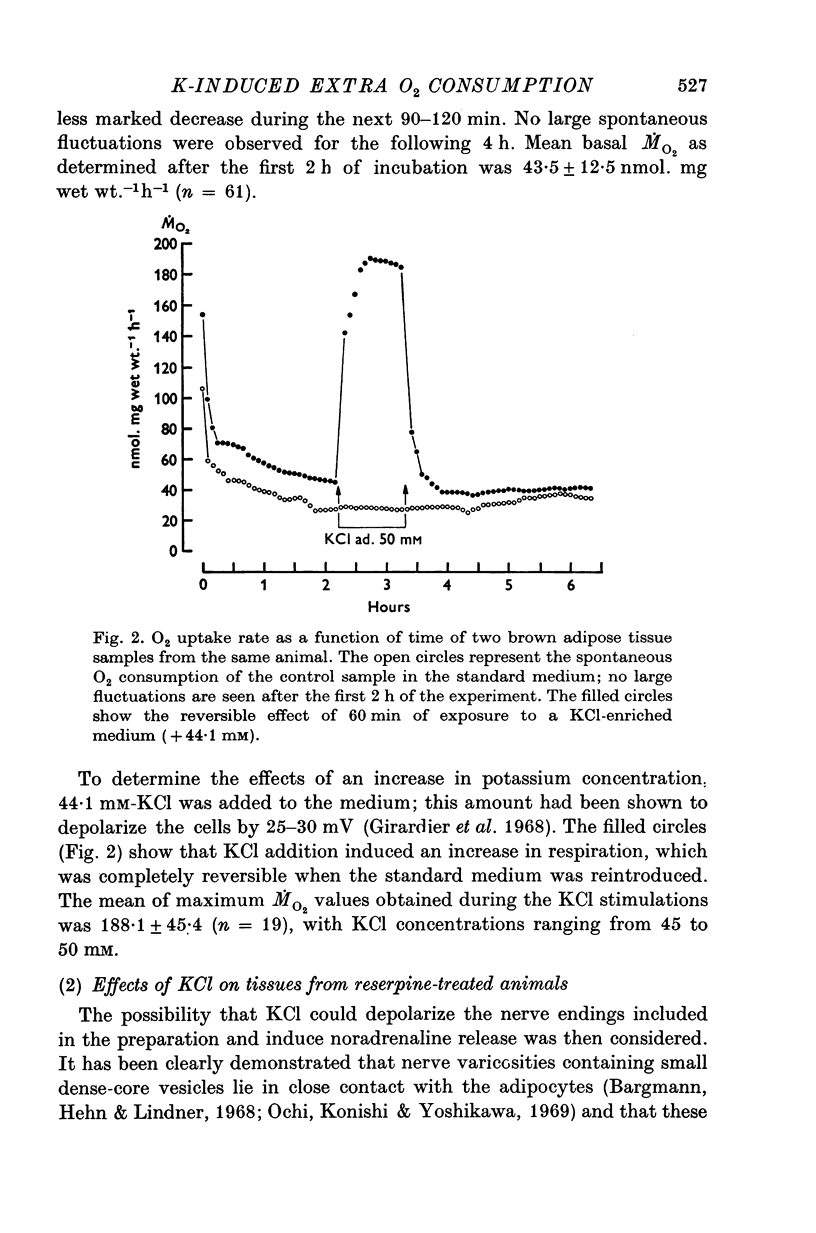
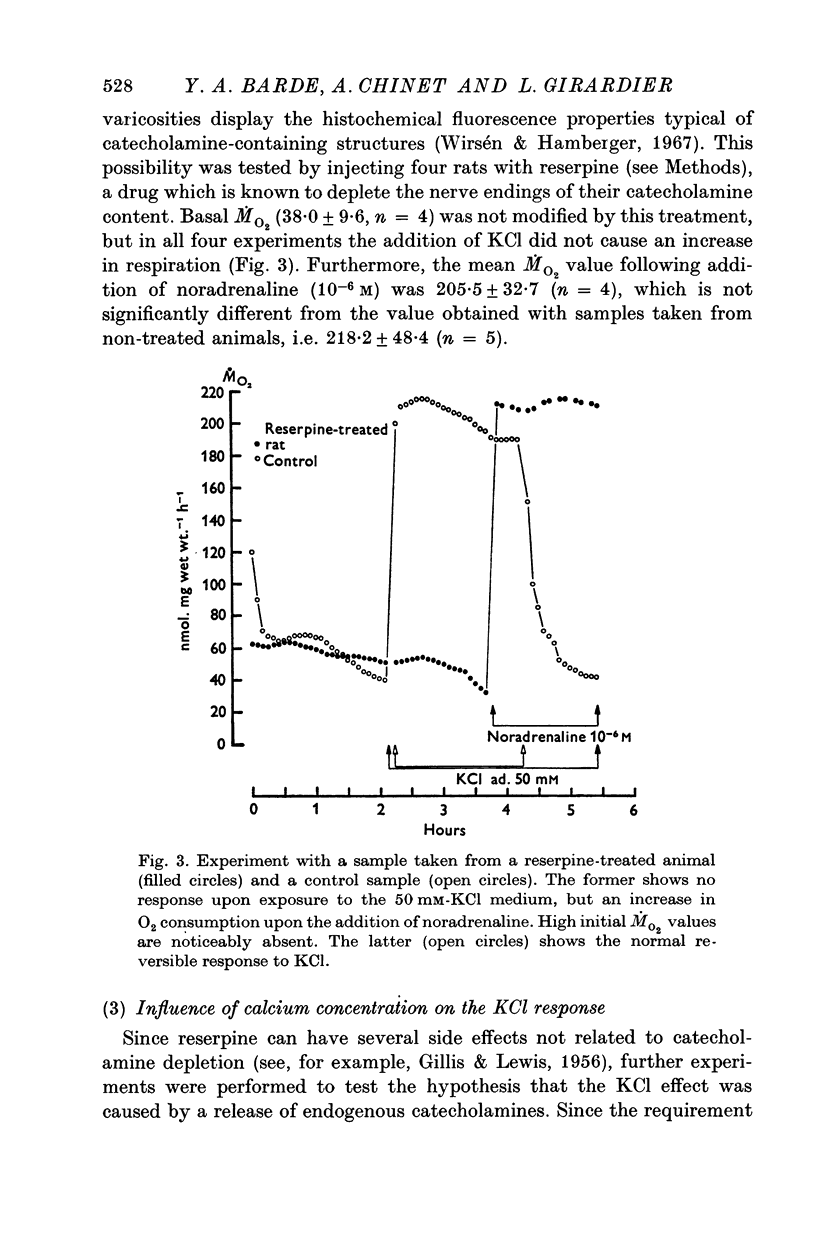
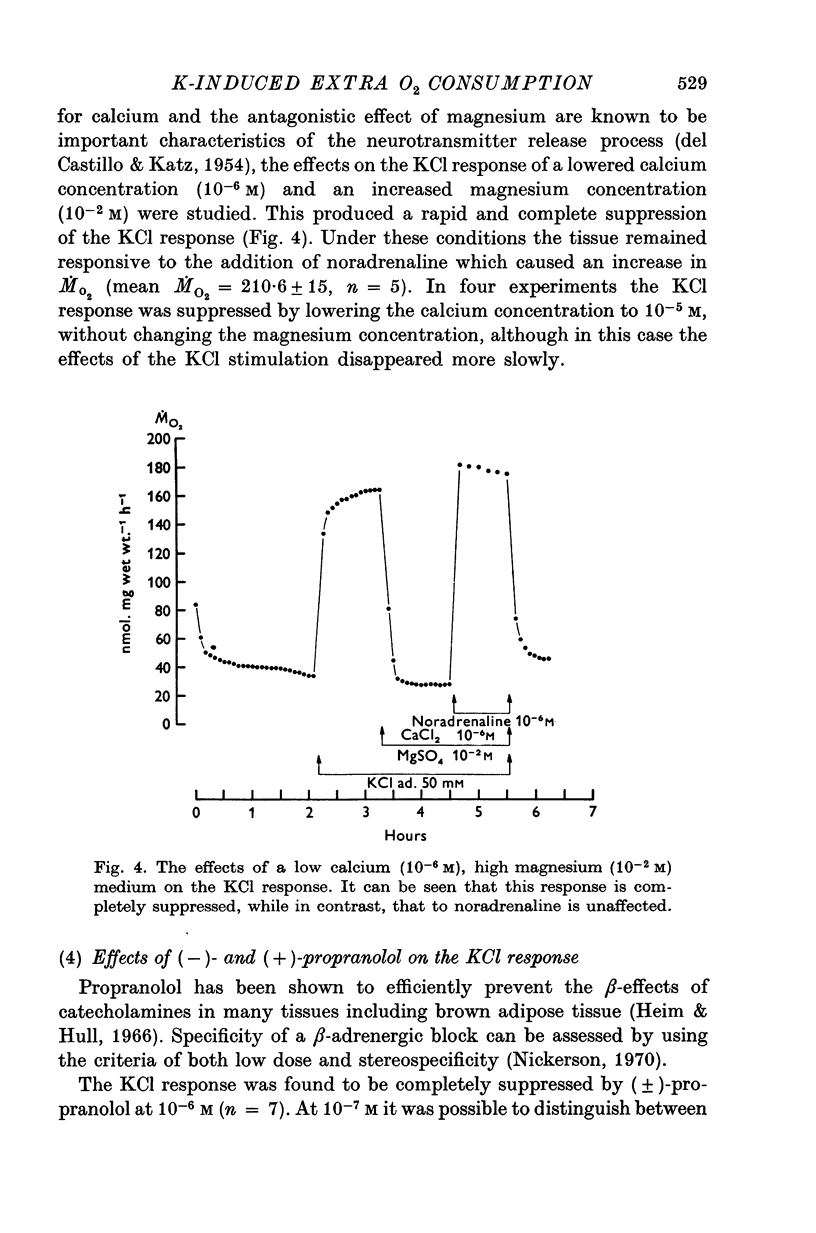
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argent B. E., Case R. M., Scratcherd T. Stimulation of amylase secretion from the perfused cat pancreas by potassium and other alkali metal ions. J Physiol. 1971 Aug;216(3):611–624. doi: 10.1113/jphysiol.1971.sp009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann W., von Hehn G., Lindner E. Uber die Zellen des braunen Fettgewebes und ihre Innervation. Z Zellforsch Mikrosk Anat. 1968;85(4):601–613. [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Glucose-induced electrical activity in pancreatic islet cells. J Physiol. 1970 Sep;210(2):255–264. doi: 10.1113/jphysiol.1970.sp009207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry D. M., Daniel H. Sympathetic nerve development in the brown adipose tissue of the rat. Can J Physiol Pharmacol. 1970 Mar;48(3):160–168. doi: 10.1139/y70-028. [DOI] [PubMed] [Google Scholar]
- GILLIS C. N., LEWIS J. J. A note on the pharmacology of reserpine. J Pharm Pharmacol. 1956 Aug;8(8):606–614. doi: 10.1111/j.2042-7158.1956.tb12192.x. [DOI] [PubMed] [Google Scholar]
- Girardier L., Seydoux J., Clausen T. Membrane potential of brown adipose tissue. A suggested mechanism for the regulation of thermogenesis. J Gen Physiol. 1968 Dec;52(6):925–940. doi: 10.1085/jgp.52.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Milner R. D. The role of sodium and potassium in insulin secretion from rabbit pancreas. J Physiol. 1968 Feb;194(3):725–743. doi: 10.1113/jphysiol.1968.sp008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim T., Hull D. The effect of propranalol on the calorigenic response in brown adipose tissue of new-born rabbits to catecholamines, glucagon, corticotrophin and cold exposure. J Physiol. 1966 Nov;187(2):271–283. doi: 10.1113/jphysiol.1966.sp008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L. Uptake mechanisms for neurotransmitter amines. Biochem Pharmacol. 1974 Jul 15;23(14):1927–1935. doi: 10.1016/0006-2952(74)90250-0. [DOI] [PubMed] [Google Scholar]
- Joel C. D. Effects of insulin and norepinephrine added in vitro on the metabolism of brown adipose tissue in the absence of added glucose. Lipids. 1970 Feb;5(2):224–230. doi: 10.1007/BF02532473. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- Ochi J., Konishi M., Yoshikawa H. Morphologischer Nachweis der sympathischen Innervation des braunen Fettgewebes gei der Ratte. Eine fluorescenz- und elektronenmikroskopische Studie. Z Anat Entwicklungsgesch. 1969;129(3):259–267. [PubMed] [Google Scholar]
- Orci L., Perrelet A., Ravazzola M., Malaisse-Lagae F., Renold A. E. A specialized membrane junction between nerve endings and B-cells in islets of Langerhans. Eur J Clin Invest. 1973 Dec;3(6):443–445. doi: 10.1111/j.1365-2362.1973.tb02212.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Cell communication, calcium ion, and cyclic adenosine monophosphate. Science. 1970 Oct 23;170(3956):404–412. doi: 10.1126/science.170.3956.404. [DOI] [PubMed] [Google Scholar]
- Schramm M. Amylase secretion in rat parotid slices by apparent activation of endogenous catecholamine. Biochim Biophys Acta. 1968 Oct 15;165(3):546–549. doi: 10.1016/0304-4165(68)90238-9. [DOI] [PubMed] [Google Scholar]
- Vieira F. L., Caplan S. R., Essig A. Energetics of sodium transport in frog skin. I. Oxygen consumption in the short-circuited state. J Gen Physiol. 1972 Jan;59(1):60–76. doi: 10.1085/jgp.59.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirsén C., Hamberger B. Catecholamines in brown fat. Nature. 1967 May 6;214(5088):625–626. doi: 10.1038/214625a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Hiroshige T. Calcium dependency of metabolic effects of excess potassium on brown adipose tissue. Jpn J Physiol. 1970 Oct 15;20(5):483–493. doi: 10.2170/jjphysiol.20.483. [DOI] [PubMed] [Google Scholar]


