Abstract
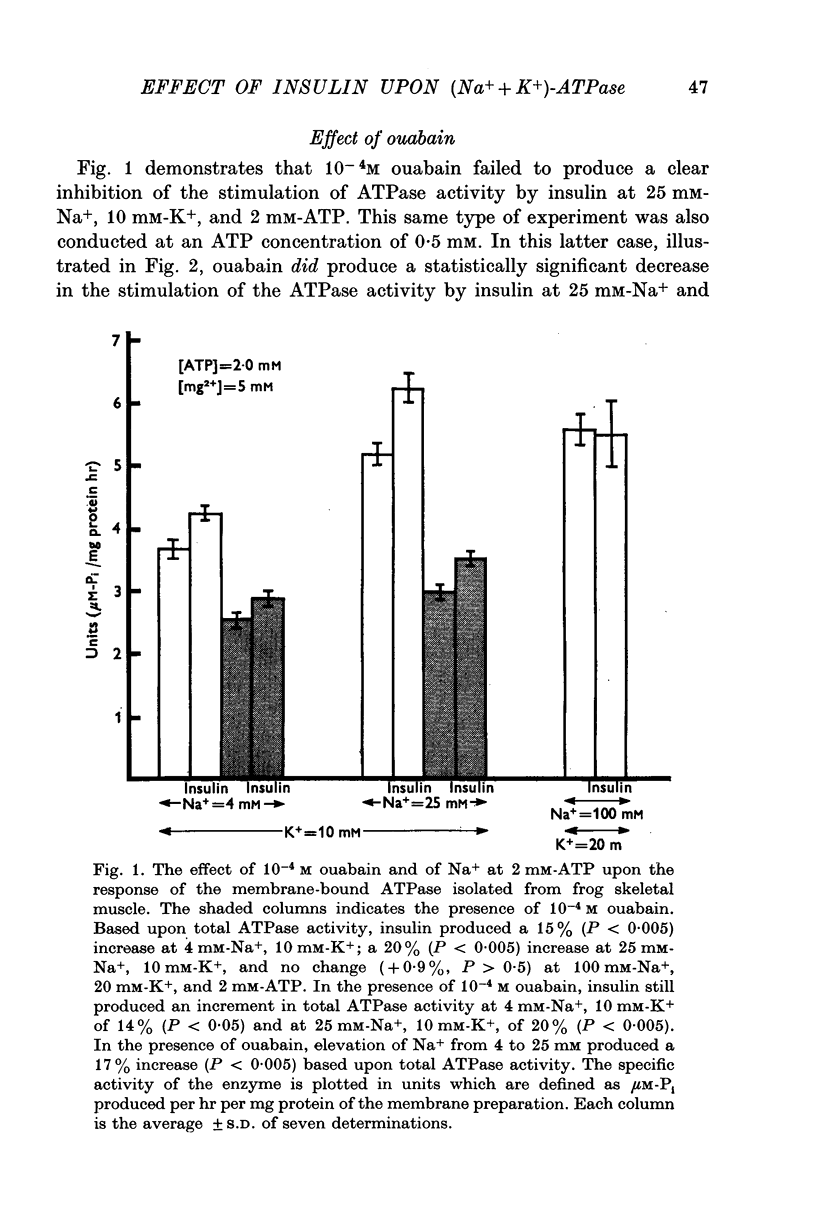
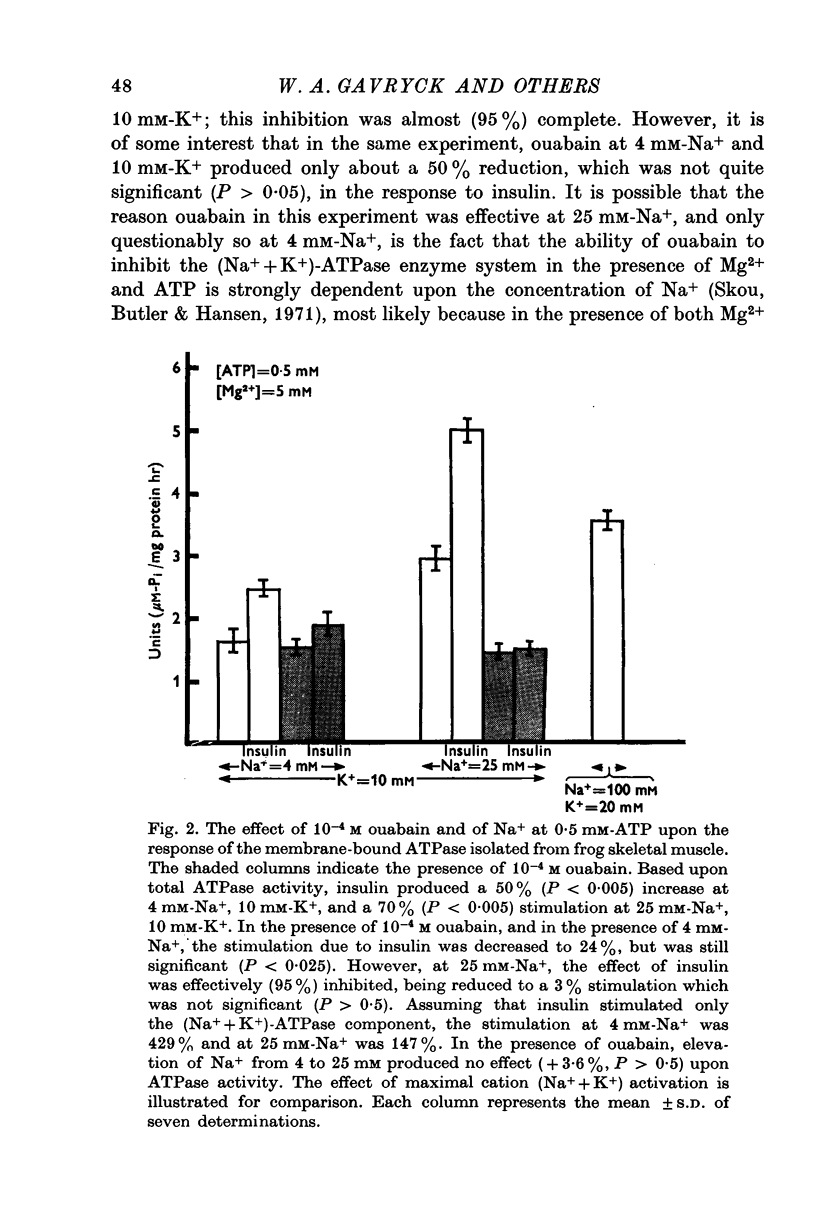
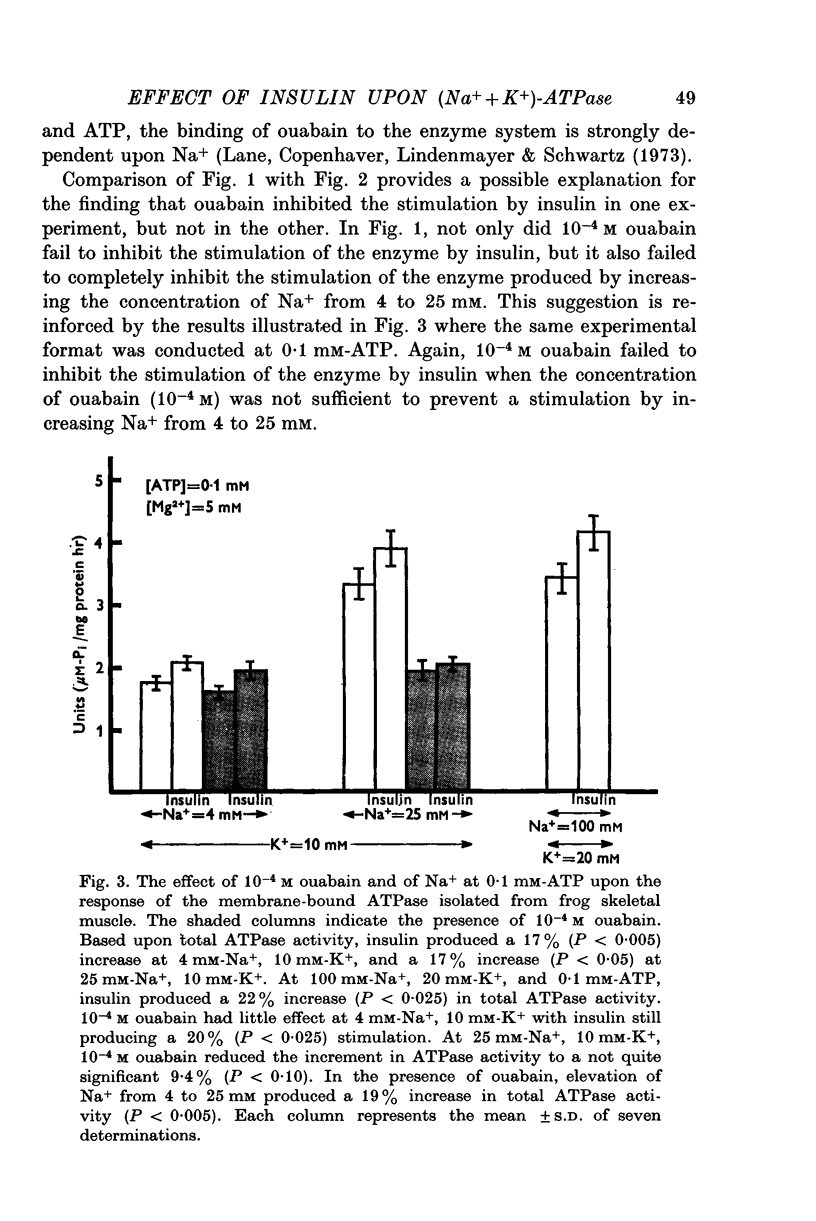
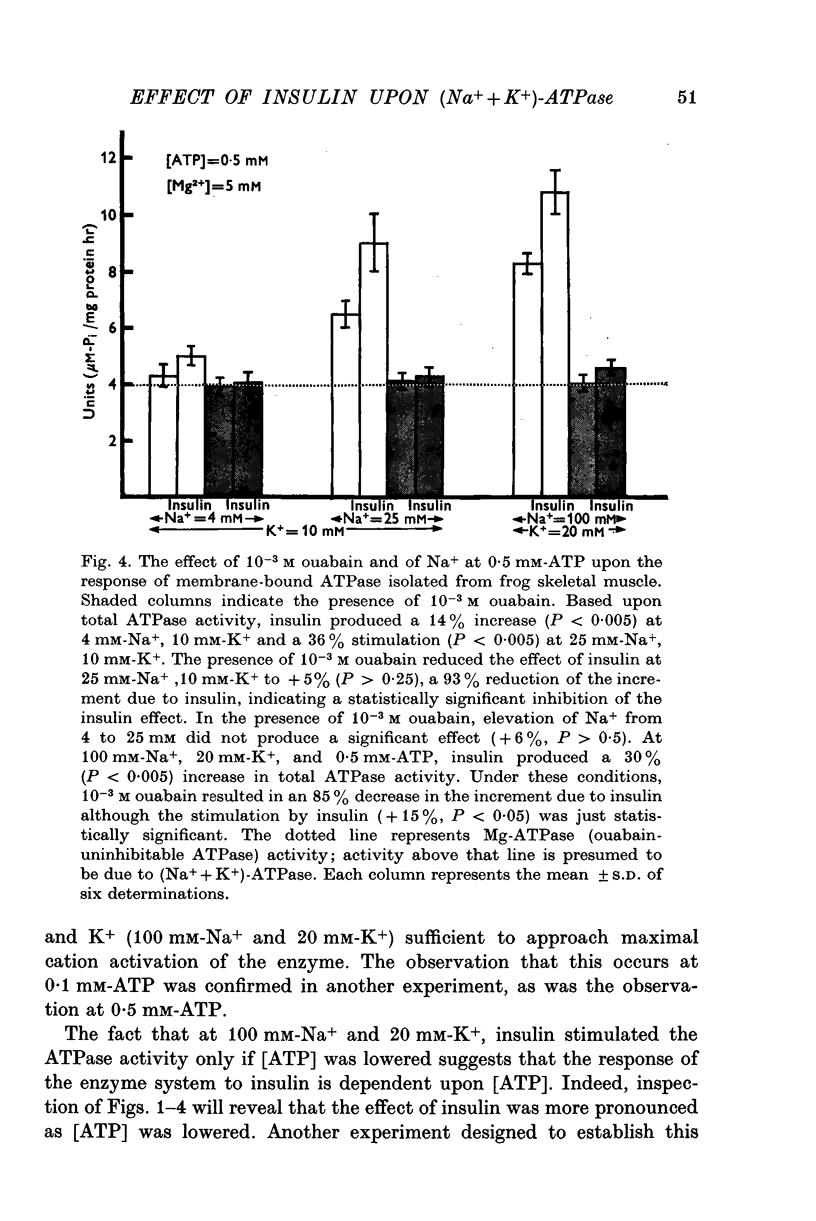
1. Insulin stimulates the activity of membrane-bound ATPase isolated from frog skeletal muscle and from rat brain. The increase in activity of the membrane-bound ATPase system isolated from frog ranged from 9-8 to 53% at concentrations of Na+ (25 mM), K+ (10 mM), and ATP (2 mM) similar to those in in vivo experiments conducted previously (Moore, 1973). The increased activity of the membrane-bound ATPase is, therefore, at least as great as the insulin-induced increase in Na efflux (10-38%) from intact cells (Moore, 1973). If the concentration of Na+ is lowered to 4 mM and that of ATP lowered to 0-5 mM albumin, and 10(6) M, the increase in ouabain-inhibitable ATPase activity can reach as high as 400%. 2. Ouabain, at a concentration (10(-3) M) sufficient to inhibit stimulation of the frog ATPase by increasing Na from 4 to 25 mM, completely blocked the stimulation of ATPase activity due to insulin. 3. At 2 mM-ATP, 100 mM-Na+, and 20 mM-K+, conditions which maximally activate the (Na+ + K+)-ATPase, insulin did not increase the ATPase, activity. Stimulation was consistently seen at 10 mM-K+, 0-5 mM-ATP, and either 4 mM or 25 mM-Na+. 4. The finding that insulin does not stimulate the ATPase activity in conditions in which the (Na+ + K+)-ATPase component is maximally activated and especially the fact that ouabain can reproducibly inhibit insulin stimulation of the membrane-bound ATPase activity strongly suggest that interaction of insulin with its receptor upon the plasma membrane somehow stimulates the (Na+ + K+)-ATPase system (ouabain sensitive; ATP phosphohydrolase, EC (3.6.1.3). These results are consistent with previous studies of the effect of insulin upon Na efflux from intact cells (Moore, 1973) and support the previous conclusion that the component of Na efflux stimulated by insulin is active. The evidence suggests that insulin probably does not affect Vmax of the (Na+ + K+)-ATPase system, but may increase the affinity of the enzyme system to one or more effectors, most likely Na+, ATP, and perhaps K+. 5. Oxidized glutathione (2-7 X 10(-6) M), 10(-6) M, 10(-7) M, and 10(-8) M cyclic AMP did not affect the ATPase activity 10(-6)Malbumin, and . 6. The results are consistent with the view that the Na pump, (Na+ + K+)-ATPase, is intimately involved with the physiological action of insulin and may be transducer between the binding of insulin to its receptor on the plasma membrane and the cellular actions of insulin.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONTING S. L., CANADY M. R. NA-K ACTIVATED ADENOSINE TRIPHOSPHATASE AND SODIUM TRANSPORT IN TOAD BLADDER. Am J Physiol. 1964 Nov;207:1005–1009. doi: 10.1152/ajplegacy.1964.207.5.1005. [DOI] [PubMed] [Google Scholar]
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Boegman R. J., Manery J. F., Pinteric L. The separation and partial purification of membrane-bound (Na + + K + )-dependent Mg 2+ -ATPase and (Na + +K + (Na + +K + )-independent Mg 2+ -ATPase from frog skeletal muscle. Biochim Biophys Acta. 1970 Jun 2;203(3):506–530. doi: 10.1016/0005-2736(70)90189-6. [DOI] [PubMed] [Google Scholar]
- Cortas N., Walser M. (Na + -K + )-activated ATPase in isolated mucosal cells of toad bladder. Biochim Biophys Acta. 1971 Oct 12;249(1):181–187. doi: 10.1016/0005-2736(71)90095-2. [DOI] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLISON R. J., WILSON W. P., WEISS E. B. Changes in cerebral potassium during insulin hypoglycemia. Proc Soc Exp Biol Med. 1958 May;98(1):128–129. doi: 10.3181/00379727-98-23962. [DOI] [PubMed] [Google Scholar]
- Fujita M., Nagano K., Mizuno N., Tashima Y., Nakao T., Nakao M. Comparison of some minor activities accompanying a preparation of sodium-plus-potassium ion-stimulated adenosine triphosphatase from pig brain. Biochem J. 1968 Jan;106(1):113–121. doi: 10.1042/bj1060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb P. S., Rodnight R. The role of bound potassium ions in the hydrolysis of low concentrations of adenosine triphosphate by preparations of membrane fragments from ox brain cerebral cortex. Biochem J. 1970 Nov;120(1):15–24. doi: 10.1042/bj1200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Erlij D. Insulin unmasks latent sodium pump sites in frog muscle. Nature. 1974 Sep 6;251(5470):57–58. doi: 10.1038/251057a0. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Wilson E. E., Good R. A., Coffey R. G. Direct action of insulin on plasma membrane ATPase activity in human lymphocytes. Nat New Biol. 1972 Feb 9;235(58):174–177. doi: 10.1038/newbio235174a0. [DOI] [PubMed] [Google Scholar]
- Horn R. S., Walaas O., Walaas E. The influence of sodium, potassium and lithium on the response of glycogen synthetase I to insulin and epinephrine in the isolated rat diaphragm. Biochim Biophys Acta. 1973 Jul 28;313(2):296–309. doi: 10.1016/0304-4165(73)90029-9. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The stimulation of adipocyte plasma membrane magnesium ion-stimulated adenosine triphosphatase by insulin and concanavalin A. J Biol Chem. 1974 Aug 25;249(16):5195–5199. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane L. K., Copenhaver J. H., Jr, Lindenmayer G. E., Schwartz A. Purification and characterization of and (3H)ouabain binding to the transport adenosine triphosphatase from outer medulla of canine kidney. J Biol Chem. 1973 Oct 25;248(20):7197–7200. [PubMed] [Google Scholar]
- Letarte J., Renold A. E. Ionic effects on glucose transport and metabolism by isolated mouse fat cells incubated with or without insulin. 3. Effects of replacement of Na+. Biochim Biophys Acta. 1969 Jul 15;183(2):366–374. doi: 10.1016/0005-2736(69)90093-5. [DOI] [PubMed] [Google Scholar]
- Lindenmayer G. E., Schwartz A. Nature of the transport adenosine triphosphatase digitalis complex. IV. Evidence that sodium-potassium competition modulates the rate of ouabain interaction iwth (Na + +K + ) adenosine triphosphatase during enzyme catalysis. J Biol Chem. 1973 Feb 25;248(4):1291–1300. [PubMed] [Google Scholar]
- Lostroh A. J., Krahl M. E. Insulin action. Accumulation in vitro of Mg 2 + and K + in rat uterus: ion pump activity. Biochim Biophys Acta. 1973 Jan 2;291(1):260–268. doi: 10.1016/0005-2736(73)90417-3. [DOI] [PubMed] [Google Scholar]
- Moore R. D. Effect of insulin upon the sodium pump in frog skeletal muscle. J Physiol. 1973 Jul;232(1):23–45. doi: 10.1113/jphysiol.1973.sp010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- Nizet A., Lefebvre P., Crabbé J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 1971;323(1):11–20. doi: 10.1007/BF00586561. [DOI] [PubMed] [Google Scholar]
- Post R. L., Hegyvary C., Kume S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J Biol Chem. 1972 Oct 25;247(20):6530–6540. [PubMed] [Google Scholar]
- Rogus E., Price T., Zierler K. L. Sodium plus potassium-activated, ouabain-inhibited adenosine triphosphatase from a fraction of rat skeletal muscle, and lack of insulin effect on it. J Gen Physiol. 1969 Aug;54(2):188–202. doi: 10.1085/jgp.54.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOU J. C. Preparation from mammallian brain and kidney of the enzyme system involved in active transport of Na ions and K ions. Biochim Biophys Acta. 1962 Apr 9;58:314–325. doi: 10.1016/0006-3002(62)91015-6. [DOI] [PubMed] [Google Scholar]
- Saudek C. D., Boulter P. R., Knopp R. H., Arky R. A. Sodium retention accompanying insulin treatment of diabetes mellitus. Diabetes. 1974 Mar;23(3):240–246. doi: 10.2337/diab.23.3.240. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Butler K. W., Hansen O. The effect of magnesium, ATP, P i , and sodium on the inhibition of the (Na + + K + )-activated enzyme system by g-strophanthin. Biochim Biophys Acta. 1971 Aug 13;241(2):443–461. doi: 10.1016/0005-2736(71)90044-7. [DOI] [PubMed] [Google Scholar]
- Swanson P. D., Bradford H. F., McIlwain H. Stimulation and solubilization of the sodium ion-activated adenosine triphosphatase of cerebral microsomes by surface-active agents, especially polyoxyethylene ethers: actions of phospholipases and a neuraminidase. Biochem J. 1964 Aug;92(2):235–247. doi: 10.1042/bj0920235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Walaas O., Walaas E., Wick A. N. The stimulatory effect by insulin on the incorporation of 32P radioactive inorganic phosphate into intracellular inorganic phosphate, adenine nucleotides and guanine nucleotides of the intact isolated rat diaphragm. Diabetologia. 1969 Apr;5(2):79–87. doi: 10.1007/BF01212001. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Hyperpolarization of muscle by insulin in a glucose-free environment. Am J Physiol. 1959 Sep;197:524–526. doi: 10.1152/ajplegacy.1959.197.3.524. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. EFFECT OF VERY SMALL CONCENTRATIONS OF INSULIN ON FOREARM METABOLISM. PERSISTENCE OF ITS ACTION ON POTASSIUM AND FREE FATTY ACIDS WITHOUT ITS EFFECT ON GLUCOSE. J Clin Invest. 1964 May;43:950–962. doi: 10.1172/JCI104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler K. L., Rogus E., Hazlewood C. F. Effect of insulin on potassium flux and water and electrolyte content of muscles from normal and from hypophysectomized rats. J Gen Physiol. 1966 Jan;49(3):433–456. doi: 10.1085/jgp.49.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


