Studies of periplasmic binding protein-dependent transporters date to the 1960s when it was realized that the transport of certain solutes was inhibited following an osmotic shock that released the contents of the periplasm (8, 80). The ATP-binding cassette (ABC) superfamily (52) was defined in 1986 when homology was detected between a binding protein-dependent transporter and a multidrug efflux pump cloned from human cancer cells (21, 42). These proteins, also known as traffic ATPases (4), number 80 in the Escherichia coli genome (13, 23) and 48 in the human genome where many have been linked to human disease (29). In addition to the periplasmic binding protein-dependent transporters that mediate uptake, bacterial cells also contain ABC transporters lacking a binding protein that mediate efflux of compounds such as lipopolysaccharides (15, 56, 95, 133), capsular polysaccharides (87, 90), antimicrobial agents (7, 98, 126), and toxins (35, 47, 57). A few ABC proteins also lack a transmembrane region and use the same architecture to perform alternative functions such as DNA repair (34).
A typical ABC transporter has four domains or subunits, two of which are hydrophobic and are predicted to span the membrane multiple times in an alpha-helical conformation and two of which bind nucleotide and are exposed to the cytoplasm. This domain architecture is clearly established in the recent structure of MsbA (20), an ABC transporter that mediates the export of lipid and lipid A in E. coli (32, 133). MsbA is a homodimer, and the intracellular loops between transmembrane-spanning helices of each monomer unit constitute a novel intracellular alpha-helical domain that physically separates the nucleotide-binding domain from the transmembrane domain. The nucleotide-binding domains and subunits share considerable sequence homology across the entire family (45) and assume a similar three-dimensional fold that consists of a core nucleotide-binding subdomain that is common to other ATPases and an alpha-helical subdomain that is specific to ABC proteins (50). The nucleotide-binding subdomain contains the canonical Walker A and B motifs (128) that are involved in nucleotide binding, while the helical subdomain contains the ABC family signature or LSGGQ motif the function of which is still debatable (31, 48, 53).
This minireview summarizes recent work using vanadate to stabilize the transition state for ATP hydrolysis that has provided new insight into the mechanism of action of this family of proteins.
BINDING PROTEIN-DEPENDENT UPTAKE SYSTEMS
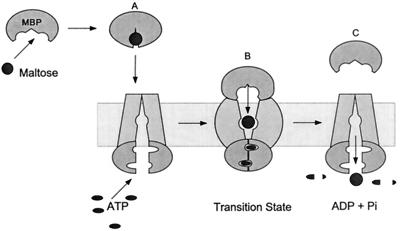
In the periplasmic binding protein-dependent transport systems, the soluble binding protein is the first component to interact with the substrate to be transported, acting as a high-affinity receptor for the substrate in the periplasm (93) (see Fig. 1). Interaction of the ligand-bound binding protein with the transporter stimulates the ATPase activity of the transporter and initiates transport (27, 88). A fuller understanding of how these binding proteins function in transport was realized following our recent observation in the maltose transport system that the periplasmic maltose binding protein (MBP) becomes tightly bound to the membrane transporter (MalFGK2, a complex of MalF, MalG, and two MalK proteins) in the presumed catalytic transition state for ATP hydrolysis (22). In the transition state conformation, the affinity of the binding protein for maltose is reduced (22) and the sugar is presumably transferred to the transporter and ultimately into the cell (Fig. 1).
FIG. 1.
Model for maltose transport. (A) MBP binds maltose, undergoing a change from an open conformation to a closed conformation, generating a high-affinity sugar-binding site. In the closed conformation, MBP binds MalFGK2 to initiate transport and hydrolysis. (B) In the transition state for ATP hydrolysis, MBP becomes tightly bound to MalFGK2, and internal sugar-binding sites are exposed to each other. This opening of MBP in the transition state reduces the affinity of MBP for maltose, facilitating the transfer of sugar to MalFGK2. (C) Maltose is transported, and MBP is released after reexposure of the membrane-binding site to the cytoplasm. MBP activates the ATPase activity of MalK by bringing the two MalK subunits into close proximity, completing the nucleotide-binding site(s) at the MalK-MalK interface with residues donated from the opposing subunit. Reprinted from reference 22.
EVIDENCE THAT VANADATE TRAPS THE TRANSITION STATE
The tight interaction between MBP and the maltose transporter was discovered during a study of the mechanism of inhibition of maltose transport by vanadate (22, 107). Vanadate is an analogue of inorganic phosphate that can mimic the gamma phosphate of ATP in the transition state for ATP hydrolysis (114). In the structure of the vanadate-inhibited myosin ATPase, Mg and ADP are present, and vanadate occupies the position of the gamma phosphate, having assumed the trigonal bipyrimidal conformation characteristic of the transition state (114). As was observed in myosin (41), vanadate inhibition results in the tight binding of ADP to MalFGK2, meaning that ADP does not dissociate following removal of free vanadate and free nucleotide from solution (107). Since ABC proteins normally do not bind nucleotide with high affinity (86, 124), the tight binding exhibited in the presence of vanadate likely reflects its ability to stabilize the transition state of the maltose transporter. Further evidence that vanadate traps the transition state in the maltose transport system comes from the observation that ATP hydrolysis is required for vanadate inhibition (107). This requirement is demonstrated first by the observation that both Mg and ATP are required for stable inhibition by vanadate and second by the fact that the wild-type transporter does not hydrolyze ATP in the absence of MBP (27), and neither stable inhibition of ATPase activity by vanadate nor trapping of nucleotide occurs in the absence of MBP (22, 107). Finally, even though ADP is trapped by vanadate, ADP will not substitute for ATP in formation of the inhibited species, indicating that vanadate trapping occurs after ATP has been hydrolyzed but before ADP is released (107). This result also suggests that there is an ADP-bound species formed following ATP hydrolysis and Pi release that has a higher free energy than the species formed when ADP binds to the transporter (Fig. 2) (107). In Fig. 3, we indicate that the free energy of ATP hydrolysis may be released in incremental steps that are tied to different conformational changes in the transporter (107). For example, for the mammalian P-glycoprotein, it has been suggested that Pi release may be coupled to transport (123). Structural comparisons of ATP-bound and ADP-bound ABC subunits from bacteria have revealed a conformational difference that may be important for nucleotide exchange in the active site (130).
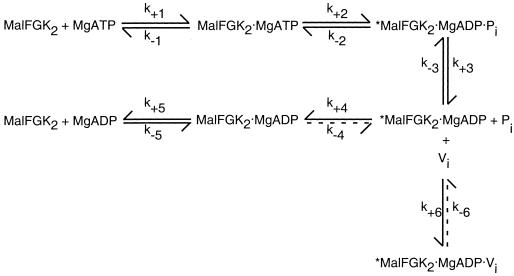
FIG. 2.
Scheme for ATP hydrolysis and vanadate inhibition. In this scheme for ATP hydrolysis by MalFGK2, ATP binding (step 1) and ATP hydrolysis (step 2) are followed by the ordered release of Pi (step 3) and then ADP (steps 4 and 5). Release of ADP is predicted to be a two-step process in which the transport complex undergoes a conformational change that is not readily reversible, from a high-energy species (∗) to a low-energy species (step 4) prior to ADP release (step 5). Vanadate will inhibit ATPase activity (step 6) only if it binds to the high-energy species formed immediately following ATP hydrolysis and Pi release (steps 1 to 3). Once formed, the vanadate-bound species is quite stable in the maltose system (107). Reprinted from reference 107 with permission of the publisher.
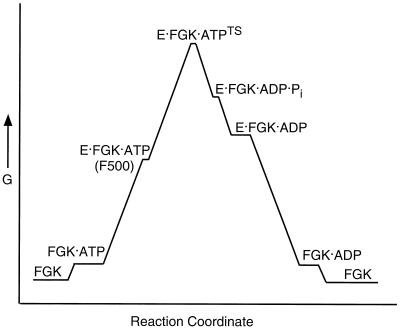
FIG. 3.
Free energy changes for MalFGK2 during ATP hydrolysis. For illustrative purposes, we have constructed this hypothetical energy diagram (G, Gibbs free energy) for MalFGK2 (FGK) during ATP hydrolysis. Binding of both ATP and MBP (E) to FGK will trigger ATP hydrolysis. E becomes more tightly bound to FGK as the complex approaches the transition state (EFGKTS), passing through an intermediate conformation that may resemble the binding protein-independent MalF500 mutant (63, 121). Following ATP hydrolysis, dissociation of Pi is likely to precede dissociation of ADP, allowing vanadate to bind and stabilize the complex in the transition state (TS) conformation. The ADP-bound intermediate may exist in both a high-energy and low-energy conformation, since ADP plus vanadate cannot trap the transition state.
IMPORTANCE OF CONFORMATIONAL CHANGE IN PERIPLASMIC BINDING PROTEINS
The finding that vanadate traps a complex of MBP and MalFGK2 underscores the central importance of the binding protein to the transport process; it is an integral part of the translocator, not just a peripheral component. Binding proteins undergo a ligand-induced conformational change that has been detected by many techniques, including fluorescence (67, 71, 135), nuclear magnetic resonance (17, 37, 46), X-ray scattering (82, 108), electron paramagnetic resonance (44), and cross-linking (18). The nature of this conformational change is evident from the structures of these proteins, which have been determined both in the presence and absence of ligand (70, 76, 77, 81, 85, 89, 97, 106, 116, 119, 134). These binding proteins have two globular domains attached by a flexible hinge, and in the ligand-bound structures, the ligand is buried deep within the cleft between the two domains. In the absence of ligand, the cleft between the domains is more open and exposed to solvent. The gain in entropy resulting from desolvation of the ligand in the binding cleft is likely to provide the driving force for domain closure (65).
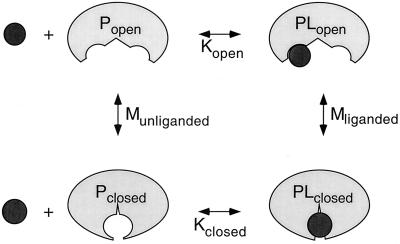
The influence of conformational change on ligand-binding affinity can be understood in terms of the thermodynamic cycle shown in Fig. 4. Since the conformational equilibrium of a binding protein strongly favors the open form (Popen) in the absence of ligand and the closed form (Pclosed) in the presence of ligand (65, 93) (Munliganded < Mliganded in Fig. 4), the equilibrium constant describing the binding of ligand to the closed form (Kclosed) must be greater than the equilibrium constant describing the binding of ligand to the open form (Kopen), meaning that the closed conformation has a higher affinity for ligand than the open conformation. Because the ligand is buried in the closed conformation, it is assumed that ligand binds and dissociates only when binding protein is in the open conformation (59, 71). Since this interconversion occurs rapidly in the presence of ligand, binding proteins display both high-affinity binding and high on and off rates (59, 71).
FIG. 4.
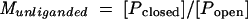 |
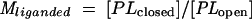 |
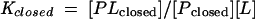 |
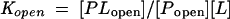 |
This conformational change also plays an important role in signal transduction as illustrated in the maltose transport system. Mutational studies have identified two regions on either side of the ligand-binding cleft in MBP that appear to interact with the transporter MalFGK2 (36, 49, 66, 120). Opening and closing the cleft will alter the positions of these regions relative to each other, providing a means of signaling to the transporter that maltose is present. Although both ligand-free and ligand-bound forms of the binding proteins interact with the transporters (5, 69, 109), ligand-bound binding protein more efficiently stimulates the ATPase activity of the transporter (5, 27), suggesting that the closed form of the binding protein binding initiates a transport event. The relatively high concentration of binding protein required to achieve one-half maximal transport activity (25 to 100 μM) (28, 64, 91) suggests that the binding protein and the transporter initially interact with low affinity, which progresses to a high-affinity interaction in the transition state.
Finally, this conformational change is likely to be an integral part of the translocation process itself. MBP displays a remarkable ability to retain ligand during gel filtration (38), yet maltose is absent in the vanadate-trapped complex (22). These data suggest that MBP is trapped in the low-affinity, or open, conformation by vanadate. If, as generally believed (27, 66, 93), binding proteins initiate interaction with the transporters when in the closed conformation, then a cycle of opening and closing coincides with a cycle of ATP hydrolysis (Fig. 1). The opening of the binding protein and the complementary changes in the transporter that generate the tight-binding interaction stabilize the transition state of ATP and promote catalysis (22). Opening of the binding protein in the transition state will also couple transport to ATP hydrolysis because the simultaneous decrease in the affinity of the binding protein for ligand and increase in the affinity of the binding protein for the transporter will trigger the release of ligand from the binding protein directly into the transporter and hence into the cell (Fig. 1).
CONFORMATIONAL CHANGE IN THE TRANSMEMBRANE DOMAINS
The transient nature of the tight-binding interaction between MBP and MalFGK2 (22) suggests that both proteins undergo conformational changes during the catalytic cycle. We have predicted the nature of the conformational change in the binding protein based on its structure and ligand-binding interactions. However, there is little foundation upon which to model conformational changes in the transmembrane region of this transporter. Mutant transporters that are independent of the binding protein still transport substrate specifically, albeit with lower affinity, indicating that there is a substrate-binding site in the membrane (111, 115, 121). Peter Mitchell has suggested that transporters may employ a central binding site that is alternately exposed to each side of the membrane (73, 74). This mobile barrier hypothesis has been incorporated into the model in Fig. 1. We suggest that in the ground state, before and after ATP hydrolysis, this low-affinity binding site is accessible only from the cytoplasm and in the transition state it is accessible only from the periplasmic surface (22). With binding protein tightly bound at the periplasmic surface of the transporter, accessibility to the membrane-binding site may be limited to substrate that is released by the binding protein in the transition state complex. If true, then substrate translocation may be complete before ADP dissociates from the transporter, since maltose is absent from the vanadate-trapped species (22). Since the binding protein is locked onto the transporter in the transition state, it is also conceivable that a channel that spans the membrane region opens, analogous to the maltoporin channel in the outer membrane (31, 33, 103). At least one ABC protein, the cystic fibrosis transmembrane regulator (CFTR), functions as a channel (10). Now that it is possible to trap the transporter in the transition state, these models can be tested. For example, cysteine-scanning mutagenesis can be used to determine the exposure of residues in presumed transmembrane regions to the aqueous environment (39, 129).
MECHANISM OF ACTIVATION OF ATPase ACTIVITY
Several nucleotide-binding subunits from bacterial uptake systems have been crystallized in the absence of their transmembrane partners (31, 50, 54, 130), providing valuable information about the nature of the nucleotide-binding site. Unlike most ATPases, bound nucleotide is highly exposed on the surface of the monomer, suggesting that residues from another subunit in the transport complex may complete the active sites (48, 53). Since the nucleotide-binding subunits can form dimers in the absence of the membrane components (55, 84), the active sites could be completed with residues from a second nucleotide-binding subunit. This suggestion, first introduced by Jones and George (53), was confirmed in the structure of Rad50cd, an ABC protein involved in DNA repair, where residues from the conserved family signature or LSGGQ motif of one ATPase subunit complete the active site in the second subunit (48). There is still uncertainty as to whether the Rad50 dimer is representative of a true ABC transporter, since several different dimer interfaces have been observed in different structures (31, 50, 130). In the structure of the MsbA dimer, which includes the transmembrane domains, the monomers contact each other only in the region presumed to span the outer leaflet of the membrane bilayer and the two nucleotide-binding domains are separated by 50 Å, yielding no information about how they might interact (20). Arguments in favor of the universality of the Rad50 dimer structure are, however, mounting. The high degree of conservation of the LSGGQ motif in ABC proteins argues that it will have the same function in all ABC proteins. The contacts between the LSGGQ motif and the nucleotide in Rad50 perfectly correct deficiencies in the monomer subunits that would be expected to prevent ATP hydrolysis (48, 130), and mutations in the LSGGQ motif inhibit ATPase activity even in the absence of the transmembrane subunits (104). Finally, the idea that the nucleotide-binding sites are shared between subunits explains the requirement for two nucleotide-binding domains in these systems (48). Even in those cases where the sequence of one nucleotide-binding site has diverged from the consensus sequence and is assumed to be nonfunctional (53), two domains or subunits are still required to generate a single functional site.
The ATPase activity of Rad50 appears to be controlled through the dimerization of the two catalytic domains and ATP promotes dimer formation (48). In Fig. 1, we proposed a similar mechanism for the activation of ATPase activity, with the degree of subunit association being controlled by the interaction of the binding protein with the transporter (63). Consistent with this hypothesis, we have detected a decrease in the solvent accessibility of the Walker A motif that is associated with activation of the ATPase activity in MalFGK2 (63). By comparing the structures of ATP-bound and ADP-bound ABC proteins, Yuan and colleagues discovered a possible switch mechanism that rotates the alpha-helical subdomain relative to the nucleotide-binding subdomain when a conserved glutamine residue interacts with the gamma phosphate of ATP (130). When the ATP- and ADP-bound species were fitted into the Rad50 dimer, the nucleotide-binding site was more exposed in the ADP-bound structure than the ATP-bound structure, leading the researchers to suggest that the switch may be important to exchange ADP for ATP (130). In our view, this switch could also be a component of the conformational change that brings the LSGGQ motif into the nucleotide-binding site and activates the ATPase activity. In the intact complex, this switch may be controlled by the interaction between the binding protein and the transporter, thereby preventing ATP hydrolysis in the absence of binding protein.
BINDING PROTEIN-INDEPENDENT MUTANTS STABILIZE INTERMEDIATES IN HYDROLYSIS CYCLE
Our model in Fig. 1 predicts that residues in the Walker A motif will become less accessible to solvent as a result of activation of the ATPase activity by MBP. Mutants capable of transporting maltose in the absence of MBP have gained the ability to hydrolyze ATP in the absence of MBP (27). A fluorophore attached to the Walker A motif is in fact less solvent accessible in the binding protein-independent MalF500 mutant than in the wild type (63), suggesting that the MalF500 mutant is already in an activated conformation. In contrast to the wild type, where vanadate trapping of the transition state results in a decrease in solvent accessibility of the Walker A motif, the solvent accessibility of the MalF500 mutant is unchanged by vanadate trapping (63). Similarly, the MalF500 mutant binds tightly to MBP both in the presence and absence of vanadate (63). Thus, it appears that the conformation of the MalF500 mutation resembles the transition state both in terms of the conformation of the nucleotide-binding site and the increased affinity for MBP. MBP can inhibit the activity of the binding protein-independent transporters (49, 120, 121), and it has been suggested that the increased affinity between MBP and the binding protein independent transporters leads to inhibition through the formation of nonproductive complexes (28, 110). In the case of the MalF500 mutant, the failure of MBP to dissociate and rebind maltose may explain why it inhibits transport activity. In the energy diagram in Fig. 3, we indicate that the MalF500 mutant may resemble an intermediate that lies between the ground state and the transition state in the reaction pathway of the wild type. We further suggest that this intermediate exists only transiently, as we have been unable to stabilize an intermediate resembling the MalF500 transporter by adding MBP to the wild-type transporter. At this point in the pathway, MBP would be tightly bound to the transporter and could be in either an open or closed conformation. It has been suggested that MBP need not close completely to trigger a productive transport interaction with another of the binding protein-independent mutants (43).
INTERACTION BETWEEN NUCLEOTIDE- BINDING SITES
ABC transporters have two nucleotide-binding domains or subunits and hence two distinct nucleotide-binding sites, both of which can bind ATP (3). ATP is hydrolyzed with positive cooperativity by the periplasmic binding protein-dependent transporters, suggesting that the two sites interact (24, 61). Mutation or modification of a single nucleotide-binding site usually inhibits the function of both sites in both prokaryotic and eukaryotic transporters, providing further evidence that the two sites interact (2, 9, 26, 62). A possible exception is reported for the bacterial histidine transporter where mutation of a conserved histidine that eliminates activity if present in both subunits (112) reduces activity by only 50% if present in just one of the two nucleotide-binding subunits (83). This result was interpreted to mean that one intact nucleotide-binding site is sufficient to mediate transport (83); however, the effect of only one mutation was studied in this report and the researchers state that the apparent difference between the histidine transporter and other ABC transporters may be a function of the particular mutant studied (83). Based on vanadate-trapping experiments in the mammalian P-glycoprotein, it is suggested that the two nucleotide-binding sites alternate in ATP hydrolysis (123, 124). Both sites can hydrolyze ATP, since ADP can be found in either site when vanadate is used to trap the transition state for ATP hydrolysis (123); however, trapping of one site in the transition state prevents the second site from turning over (124). In the maltose transport system, vanadate inhibition was also associated with a one-to-one stoichiometry of binding of ADP to the transporter, indicating that the binding protein-dependent transporters may function by a similar mechanism (22, 107).
The question of whether both nucleotide-binding sites perform the same function in transport has not been fully addressed. No stoichiometric measurements have been accurate enough to determine unequivocally whether one or two ATP molecules are hydrolyzed for each substrate molecule transported (12, 25, 61, 72). However, comparison of the growth yields of bacteria under anaerobic conditions for different sugars indicates that only one ATP is expended per maltose or maltodextrin transported (78). In the bacterial transport systems, asymmetries between the nucleotide-binding subunits have been detected both in the intact complex (58a) and in the isolated subunits (31); however, these differences may be a manifestation of alternating catalytic sites rather than of functional differences between the two nucleotide-binding sites. In several of the mammalian ABC proteins including CFTR (1, 19, 51), sulfonylurea receptor protein 1 (68, 122), and multidrug resistance protein 1 (79), nucleotide interacts differently with the two nucleotide-binding sites, whereas in P-glycoprotein, the nucleotide binding sites appear to behave identically, leading to the suggestion in the alternating catalytic sites model for P-glycoprotein (105) that substrate is transported each time ATP is hydrolyzed. However, this hypothesis has recently been challenged (99).
EFFLUX OF PROTEINS, CARBOHYDRATES, AND ANTIBIOTICS
ABC transporters also mediate the efflux of several different classes of compounds from the bacterial cell. Despite the fact that some transporters mediate uptake and some efflux, the fundamental mechanism by which ATP hydrolysis is coupled to transport may be the same. Excellent progress has been made in several different model systems, as discussed in recent reviews (57, 92, 113). Of special interest is the recent work on the ABC multidrug transporter LmrA from Lactococcus lactis (127). This protein, like many of the efflux proteins in bacteria (100) including MsbA (20) has the transmembrane domain fused to the ATP-binding cassette domain and has been shown to function as a homodimer (127). LmrA exports a variety of cytotoxic compounds including vinblastine, a substrate of the human P-glycoprotein (126, 127). Like P-glycoprotein, it may extrude amphiphilic substrates from the inner leaflet of the lipid bilayer (14), and evidence of allosteric interactions between at least two substrate-binding sites has been reported (126). Vinblastine binds to LmrA with positive cooperativity; binding to the first site (Kd = 150 nM) initiates a second higher-affinity binding event (Kd = 30 nM). LmrA is inhibited by vanadate, and only one vinblastine molecule binds to the vanadate-trapped intermediate, at a low-affinity site (Kd = 160 nM) that is exposed to the extracellular surface (127). Based on this and other data, the researchers suggest that LmrA has two drug-binding sites each of which can convert from a high-affinity inward-facing site to a low-affinity outward-facing site. Transport is likened to a two-piston engine in which hydrolysis at one nucleotide-binding site resets one low-affinity binding site to a high-affinity site as the second high-affinity site is converted to a low-affinity site. Subsequent hydrolysis at the second nucleotide-binding site would return the protein to the original conformation and result in the transport of two drug molecules at the expense of two ATP molecules. In the vanadate-trapped intermediate, the high-affinity site is temporarily occluded as it switches from inside to outside and the low-affinity site is still accessible from the outside of the cell (127).
The parallels between drug efflux and solute uptake are striking. Evidence for both low-and high-affinity substrate-binding sites is present in both systems (121, 127), and trapping of the transition state results in the ablation of high-affinity binding (22, 94, 127). The models proposed for binding protein-dependent uptake (Fig. 1) (22) and efflux (127) differ in two significant ways. First, in the maltose transport system, the high- and low-affinity sites are clearly distinct, a high-affinity site is seen in the crystal structure of MBP (116), and a low-affinity site(s) is present in MalFGK2, as evidenced by the substrate specificity of a binding protein-independent mutant (121). As discussed, ablation of high-affinity binding is likely achieved through opening of the substrate-binding cleft in MBP after which the substrate is transferred to the low-affinity site in the membrane (Fig. 1). In the two-cylinder model for LmrA, there is no transfer of substrate from one site to another, rather, substrate is translocated while bound to a single site whose affinity and orientation changes. Second, in the maltose transport system, only one path across the membrane is illustrated (Fig. 1); in LmrA, two distinct paths are proposed (127). An alternate model for drug efflux, more similar to the model for maltose transport, has been proposed for the mammalian P-glycoprotein in which drug initially binds to one site near the cytoplasmic surface and is transferred to a second (release) site when the affinity of the first site is reduced as a result of ATP hydrolysis (30, 94, 125). In the structure of MsbA, considered to be a homolog of the multidrug efflux pumps, the region of the protein predicted to be in the inner membrane leaflet contains a cluster of positively charged residues, while the region contacting the outer leaflet is largely hydrophobic (20). It has recently been proposed that hydrolysis of ATP may promote conformational changes in the transmembrane region that generate unfavorable interactions between the lipid substrate and the charged cluster, causing the lipid molecule to flip from the inner leaflet to the outer leaflet (20). However, more structural information will be required to determine exactly how lipids interact with this site and whether there are two separate pathways for translocation across the membrane.
The secretion of some protein toxins from gram-negative organisms requires an ABC transporter and two accessory proteins, one from the membrane fusion protein family and one from a family that includes the outer membrane protein TolC (6, 11, 16). The structure of TolC has been elucidated (58); it forms a channel that has the potential to span both the outer membrane and the periplasmic space, explaining how toxin secretion from the cytoplasm bypasses the periplasm. These three proteins can be detected as a complex if the substrate is present, even in the absence of ATP hydrolysis (60, 118). By analogy to the systems discussed here, ATP hydrolysis should result in release of either all or some portion of the toxin protein into a channel that spans both membranes. It is theoretically possible that trapping of the transition state of these exporters with vanadate may capture the open-channel conformation of this export apparatus. TolC is predicted to undergo a conformational change that open and closes the channel at its periplasmic orifice (58).
SIMILAR MECHANISMS FUNCTION IN DIVERSE SYSTEMS
Just as the ATPase activity of the maltose transporter is stimulated by MBP, G proteins have a very low intrinsic GTPase activity that is stimulated either by a GAP (GTPase-activating protein) or a RGS protein (regulator of G-protein signaling) (40, 117). Aluminum fluoride, like vanadate, can mimic the transition state of the gamma phosphate of a nucleotide triphosphate during hydrolysis and stabilizes these normally transient interactions between the G proteins and their activators (75). The three-dimensional structures of the aluminum fluoride-stabilized complexes reveal that RGS proteins promote catalysis by repositioning residues in the active site and that an arginine side chain from the GAP proteins is inserted into the active site of the G proteins to help stabilize the transition state for hydrolysis (101, 117). Similar to RGS or GAP, MBP stimulates the ATPase activity of MalFGK2 by stabilizing the transition state and is tightly bound to MalFGK2 in the presence of a transition state analogue (22).
Mechanistically, the maltose system may bear an even closer resemblance to the nitrogenase system, a multiprotein enzyme that catalyzes biological nitrogen fixation in an ATP-dependent fashion (102). During fixation, electrons are transferred from the metal cluster of the Fe-containing protein to the FeMo-containing protein in an ATP-dependent fashion. As in the maltose system and in the G proteins, a transient interaction between these two proteins promotes nucleotide hydrolysis and aluminum fluoride will trap an inhibited complex containing nucleotide and both proteins. Examination of the crystal structure of this presumed transition state complex reveals that, during ATP hydrolysis, the electron-donating FeS cluster of the Fe-containing protein is positioned close to the electron-receiving metal center of the MoFe-containing protein to facilitate the transfer reaction. Additional structural changes surrounding the nucleotide-binding sites that stabilize the transition state (102) are visible. In contrast to the G proteins, where the primary purpose of the hydrolysis event is to alter the signaling state of the molecule (GTP- versus GDP-bound states) (96), a key physiological event for nitrogenase (electron transfer) occurs during hydrolysis, as we have predicted for the maltose transport system.
The recent finding that the nucleotide-binding component of the arsenic transporter ArsA (131, 132) has the same fold as the Fe-containing protein of nitrogenase strengthens the connection between nitrogenase and the ABC transporters. The ArsA protein binds arsenic with high affinity, and it is tempting to predict that, in the transition state, loss of high-affinity binding will promote the transfer of the metal from ArsA to the membrane-spanning subunit ArsB.
CONCLUDING REMARKS
Work with the inhibitor vanadate has already provided a wealth of information about the mechanism of action of ABC proteins and is likely to continue to do so. These experiments have provided a framework for understanding the mechanism by which transport is coupled to ATP hydrolysis and the opportunity to hypothesize further on what may occur in other bacterial transport systems. This minireview has only briefly touched upon the large body of work devoted to the characterization of vanadate inhibition in eukaryotic ABC proteins, in particular those such as CFTR and sulfonylurea receptor that may function differently from a classical transporter (1, 79, 122). Recent structural information on the ATP-binding components has provided a wealth of detail for investigating the mechanistic aspects of ATP hydrolysis by this relatively new class of transporters. One day soon, the structures of the ground state and transition state of a complete ABC transporter can be compared to gain an understanding of the nature of the translocation pathway across the membrane.
Acknowledgments
Work in our laboratory is supported in part by grants GM49261 from NIH and Q-1391 from the Robert A. Welch Foundation.
I thank Jue Chen, Fred Gimble, and members of the Davidson lab for reading the manuscript.
REFERENCES
- 1.Aleksandrov, L., A. Mengos, X. Chang, A. Aleksandrov, and J. R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 276:12918-12923. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shawi, M. K., and A. E. Senior. 1993. Characterization of the adenosine triphosphatase activity of Chinese hamster P-glycoprotein. J. Biol. Chem. 268:4197-4206. [PubMed] [Google Scholar]
- 3.Al-Shawi, M. K., I. L. Urbatsch, and A. E. Senior. 1994. Covalent inhibitors of P-glycoprotein ATPase activity. J. Biol. Chem. 269:8986-8992. [PubMed] [Google Scholar]
- 4.Ames, G. F., C. S. Mimura, and V. Shyamala. 1990. Bacterial periplasmic permeases belong to a family of transport proteins operating from Escherichia coli to humans: traffic ATPases. FEMS Microbiol. Rev. 6:429-446. [DOI] [PubMed] [Google Scholar]
- 5.Ames, G. F. L., C. E. Liu, A. K. Joshi, and K. Nikaido. 1996. Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J. Biol. Chem. 271:14264-14270. [DOI] [PubMed] [Google Scholar]
- 6.Andersen, C., C. Hughes, and V. Koronakis. 2001. Protein export and drug efflux through bacterial channel-tunnels. Curr. Opin. Cell Biol. 13:412-416. [DOI] [PubMed] [Google Scholar]
- 7.Andrade, A. C., J. G. Van Nistelrooy, R. B. Peery, P. L. Skatrud, and M. A. De Waard. 2000. The role of ABC transporters from Aspergillus nidulans in protection against cytotoxic agents and in antibiotic production. Mol. Gen. Genet. 263:966-977. [DOI] [PubMed] [Google Scholar]
- 8.Anraku, Y. 1967. The reduction and restoration of galactose transport in osmotically shocked cells of Escherichia coli. J. Biol. Chem. 242:793-800. [PubMed] [Google Scholar]
- 9.Azzaria, M., E. Schurr, and P. Gros. 1989. Discrete mutations introduced in the predicted nucleotide-binding sites of the mdr1 gene abolish its ability to confer multidrug resistance. Mol. Cell. Biol. 9:5289-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bear, C. E., C. H. Li, N. Kartner, R. J. Bridges, T. J. Jensen, M. Ramjeesingh, and J. R. Riordan. 1992. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 68:809-818. [DOI] [PubMed] [Google Scholar]
- 11.Binet, R., S. Letoffe, J. M. Ghigo, P. Delepelaire, and C. Wandersman. 1997. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene 192:7-11. [DOI] [PubMed] [Google Scholar]
- 12.Bishop, L., R. Agbayani, Jr., S. V. Ambudkar, P. C. Maloney, and G. F.-L. Ames. 1989. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc. Natl. Acad. Sci. USA 86:6953-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 14.Bolhuis, H., H. W. van Veen, D. Molenaar, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance in Lactococcus lactis: evidence for ATP-dependent drug extrusion from the inner leaflet of the cytoplasmic membrane. EMBO J. 15:4239-4245. [PMC free article] [PubMed] [Google Scholar]
- 15.Bronner, D., B. R. Clarke, and C. Whitfield. 1994. Identification of an ATP-binding cassette transport system required for translocation of lipopolysaccharide O-antigen side-chains across the cytoplasmic membrane of Klebsiella pneumoniae serotype O1. Mol. Microbiol. 14:505-519. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan, S. K. 2001. Type 1 secretion and multidrug efflux: transport through the TolC channel-tunnel. Trends Biochem. Sci. 26:3-6. [DOI] [PubMed] [Google Scholar]
- 17.Buckel, S. D., P. F. Cottam, V. Simplaceanu, and C. Ho. 1989. Formation of intermolecular and intramolecular hydrogen bonds in histidine-binding protein J of Salmonella typhimurium upon binding L-histidine. A proton nuclear magnetic resonance study. J. Mol. Biol. 208:477-489. [DOI] [PubMed] [Google Scholar]
- 18.Careaga, C. L., J. Sutherland, J. Sabeti, and J. J. Falke. 1995. Large amplitude twisting motions of an interdomain hinge: a disulfide trapping study of the galactose-glucose binding protein. Biochemistry 34:3048-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson, M. R., S. M. Travis, and M. J. Welsh. 1995. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J. Biol. Chem. 270:1711-1717. [DOI] [PubMed] [Google Scholar]
- 20.Chang, G., and C. B. Roth. 2001. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293:1793-1800. [DOI] [PubMed] [Google Scholar]
- 21.Chen, C.-J., J. E. Chin, K. Ueda, D. P. Clark, I. Pastan, M. M. Gottesman, and I. G. Roninson. 1986. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381-389. [DOI] [PubMed] [Google Scholar]
- 22.Chen, J., S. Sharma, F. A. Quiocho, and A. L. Davidson. 2001. Trapping the transition state of an ATP-binding-cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98:1525-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dassa, E., M. Hofnung, I. T. Paulsen, and M. H. Saier, Jr. 1999. The Escherichia coli ABC transporters: an update. Mol. Microbiol. 32:887-889. [DOI] [PubMed] [Google Scholar]
- 24.Davidson, A. L., S. S. Laghaeian, and D. E. Mannering. 1996. The maltose transport system of Escherichia coli displays positive cooperativity in ATP hydrolysis. J. Biol. Chem. 271:4858-4863. [PubMed] [Google Scholar]
- 25.Davidson, A. L., and H. Nikaido. 1990. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J. Biol. Chem. 265:4254-4260. [PubMed] [Google Scholar]
- 26.Davidson, A. L., and S. Sharma. 1997. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J. Bacteriol. 179:5458-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davidson, A. L., H. A. Shuman, and H. Nikaido. 1992. Mechanism of maltose transport in Escherichia coli: transmembrane signalling by periplasmic binding proteins. Proc. Natl. Acad. Sci. USA 89:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dean, D. A., L. I. Hor, H. A. Shuman, and H. Nikaido. 1992. Interaction between maltose-binding protein and the membrane-associated maltose transporter complex in Escherichia coli. Mol. Microbiol. 6:2033-2040. [DOI] [PubMed] [Google Scholar]
- 29.Dean, M., Y. Hamon, and G. Chimini. 2001. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42:1007-1017. [PubMed] [Google Scholar]
- 30.Dey, S., M. Ramachandra, I. Pastan, M. M. Gottesman, and S. V. Ambudkar. 1997. Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc. Natl. Acad. Sci. USA 94:10594-10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diederichs, K., J. Diez, G. Greller, C. Muller, J. Breed, C. Schnell, C. Vonrhein, W. Boos, and W. Welte. 2000. Crystal structure of MalK, the ATPase subunit of the trehalose/maltose ABC transporter of the archaeon Thermococcus litoralis. EMBO J. 19:5951-5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doerrler, W. T., M. C. Reedy, and C. R. Raetz. 2001. An Escherichia coli mutant defective in lipid export. J. Biol. Chem. 276:11461-11464. [DOI] [PubMed] [Google Scholar]
- 33.Doige, C. A., and G. F.-L. Ames. 1993. ATP-dependent transport systems in bacteria and humans: relevance of cystic fibrosis and multidrug resistance. Annu. Rev. Microbiol. 47:291-319. [DOI] [PubMed] [Google Scholar]
- 34.Doolittle, R. F., M. S. Johnson, I. Husain, B. Van Houten, D. C. Thomas, and A. Sancar. 1986. Domainal evolution of a prokaryotic DNA repair protein and its relationship to active-transport proteins. Nature 323:451-453. [DOI] [PubMed] [Google Scholar]
- 35.Duong, F., A. Lazdunski, and M. Murgier. 1996. Protein secretion by heterologous bacterial ABC-transporters: the C-terminus secretion signal of the secreted protein confers high recognition specificity. Mol. Microbiol. 21:459-470. [DOI] [PubMed] [Google Scholar]
- 36.Duplay, P., S. Szmelcman, H. Bedouelle, and M. Hofnung. 1987. Silent and functional changes in the periplasmic maltose binding protein of Escherichia coli K12. I. Transport of maltose. J. Mol. Biol. 194:663-673. [DOI] [PubMed] [Google Scholar]
- 37.Evenas, J., V. Tugarinov, N. R. Skrynnikov, N. K. Goto, R. Muhandiram, and L. E. Kay. 2001. Ligand-induced structural changes to maltodextrin-binding protein as studied by solution NMR spectroscopy. J. Mol. Biol. 309:961-974. [DOI] [PubMed] [Google Scholar]
- 38.Ferenci, T., and U. Klotz. 1978. Affinity chromatographic isolation of the periplasmic maltose binding-protein of Escherichia coli. FEBS Lett. 94:213-217. [DOI] [PubMed] [Google Scholar]
- 39.Frillingos, S., M. Sahin-Toth, J. Wu, and H. R. Kaback. 1998. Cys-scanning mutagenesis: a novel approach to structure function relationships in polytopic membrane proteins. FASEB J. 12:1281-1299. [DOI] [PubMed] [Google Scholar]
- 40.Gideon, P., J. John, M. Frech, A. Lautwein, R. Clark, J. E. Scheffler, and A. Wittinghofer. 1992. Mutational and kinetic analyses of the GTPase-activating protein (GAP)-p21 interaction: the C-terminal domain of GAP is not sufficient for full activity. Mol. Cell. Biol. 12:2050-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodno, C. C. 1982. Myosin active-site trapping with vanadate ion. Methods Enzymol. 85:116-123. [DOI] [PubMed] [Google Scholar]
- 42.Gros, P., J. Croop, and D. Housman. 1986. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell 47:371-380. [DOI] [PubMed] [Google Scholar]
- 43.Hall, J. A., A. K. Ganesan, J. Chen, and H. Nikaido. 1997. Two modes of ligand binding in maltose-binding protein of Escherichia coli. Functional significance in active transport. J. Biol. Chem. 272:17615-17622. [DOI] [PubMed] [Google Scholar]
- 44.Hall, J. A., T. E. Thorgeirsson, J. Liu, Y. K. Shin, and H. Nikaido. 1997. Two modes of ligand binding in maltose-binding protein of Escherichia coli. Electron paramagnetic resonance study of ligand-induced global conformational changes by site-directed spin labeling. J. Biol. Chem. 272:17610-17614. [DOI] [PubMed] [Google Scholar]
- 45.Higgins, C. F. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 46.Ho, C., Y. Giza, S. Takahashi, K. E. Ugen, P. F. Cottam, and S. R. Dowd. 1980. A proton nuclear magnetic resonance investigation of histidine-binding protein J of Salmonella typhimurium: a model for transport of L-histidine across cytoplasmic membrane. J. Supramol. Struct. 13:131-145. [DOI] [PubMed] [Google Scholar]
- 47.Holland, I. B., and M. A. Blight. 1999. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J. Mol. Biol. 293:381-399. [DOI] [PubMed] [Google Scholar]
- 48.Hopfner, K. P., A. Karcher, D. S. Shin, L. Craig, L. M. Arthur, J. P. Carney, and J. A. Tainer. 2000. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101:789-800. [DOI] [PubMed] [Google Scholar]
- 49.Hor, L. I., and H. A. Shuman. 1993. Genetic analysis of periplasmic binding protein dependent transport in Escherichia coli. Each lobe of maltose-binding protein interacts with a different subunit of the MalFGK2 membrane transport complex. J. Mol. Biol. 233:659-670. [DOI] [PubMed] [Google Scholar]
- 50.Hung, L. W., I. X. Wang, K. Nikaido, P. Q. Liu, G. F. Ames, and S. H. Kim. 1998. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396:703-707. [DOI] [PubMed] [Google Scholar]
- 51.Hwang, T. C., G. Nagel, A. C. Nairn, and D. C. Gadsby. 1994. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc. Natl. Acad. Sci. USA 91:4698-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyde, S. C., P. Emsley, M. J. Hartshorn, M. M. Mimmack, U. Gileadi, S. R. Pearch, M. P. Gallagher, D. R. Gill, R. E. Hubbard, and C. F. Higgins. 1990. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362-365. [DOI] [PubMed] [Google Scholar]
- 53.Jones, P. M., and A. M. George. 1999. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol. Lett. 179:187-202. [DOI] [PubMed] [Google Scholar]
- 54.Karpowich, N., O. Martsinkevich, L. Millen, Y. Yuan, P. L. Dai, K. MacVey, P. J. Thomas, and J. F. Hunt. 2001. Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure 9:571-586. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy, K. A., and B. Traxler. 1999. MalK forms a dimer independent of its assembly into the MalFGK2 ATP-binding cassette transporter of Escherichia coli. J. Biol. Chem. 274:6259-6264. [DOI] [PubMed] [Google Scholar]
- 56.Kido, N., V. I. Torgov, T. Sugiyama, K. Uchiya, H. Sugihara, T. Komatsu, N. Kato, and K. Jann. 1995. Expression of the O9 polysaccharide of Escherichia coli: sequencing of the E. coli O9 rfb gene cluster, characterization of mannosyl transferases, and evidence for an ATP-binding cassette transport system. J. Bacteriol. 177:2178-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koronakis, V., and C. Hughes. 1993. Bacterial signal peptide-independent protein export: HlyB-directed secretion of hemolysin. Semin. Cell Biol. 4:7-15. [DOI] [PubMed] [Google Scholar]
- 58.Koronakis, V., A. Sharff, E. Koronakis, B. Luisi, and C. Hughes. 2000. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature 405:914-919. [DOI] [PubMed] [Google Scholar]
- 58a.Kreimer, D. I., K. P. Chai, and G. F.-L. Ames. 2000. Nonequivalence of the nucleotide-binding subunits of an ABC transporter, the histidine permease, and conformational changes in the membrane complex. Biochemistry 39:14183-14195. [DOI] [PubMed]
- 59.Ledvina, P. S., A. L. Tsai, Z. Wang, E. Koehl, and F. A. Quiocho. 1998. Dominant role of local dipolar interactions in phosphate binding to a receptor cleft with an electronegative charge surface: equilibrium, kinetic, and crystallographic studies. Protein Sci. 7:2550-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Letoffe, S., P. Delepelaire, and C. Wandersman. 1996. Protein secretion in gram-negative bacteria: assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding. EMBO J. 15:5804-5811. [PMC free article] [PubMed] [Google Scholar]
- 61.Liu, C. E., P. Q. Liu, and G. F. L. Ames. 1997. Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter). J. Biol. Chem. 272:21883-21891. [DOI] [PubMed] [Google Scholar]
- 62.Loo, T. W., and D. M. Clarke. 1996. Rapid purification of human P-glycoprotein mutants expressed transiently in HEK 293 cells by nickel-chelate chromatography and characterization of their drug-stimulated ATPase activities. J. Biol. Chem. 270:21449-21452. [DOI] [PubMed] [Google Scholar]
- 63.Mannering, D. E., S. Sharma, and A. L. Davidson. 2001. Demonstration of conformational changes associated with activation of the maltose transport complex. J. Biol. Chem. 376:12362-12368. [DOI] [PubMed] [Google Scholar]
- 64.Manson, M. D., W. Boos, P. J. Bassford, Jr., and B. A. Rasmussen. 1985. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J. Biol. Chem. 260:9727-9733. [PubMed] [Google Scholar]
- 65.Mao, B., M. R. Pear, J. A. McCammon, and F. A. Quiocho. 1982. Hinge-bending in L-arabinose-binding protein. The “Venus's-flytrap” model. J. Biol. Chem. 257:1131-1133. [PubMed] [Google Scholar]
- 66.Martineau, P., W. Saurin, M. Hofnung, J. C. Spurlino, and F. A. Quiocho. 1990. Progress in the identification of interaction sites on the periplasmic maltose binding protein from E. coli. Biochimie 72:397-402. [DOI] [PubMed] [Google Scholar]
- 67.Martineau, P., S. Szmelcman, J. C. Spurlino, F. A. Quiocho, and M. Hofnung. 1990. Genetic approach to the role of tryptophan residues in the activities and fluorescence of a bacterial periplasmic maltose-binding protein. J. Mol. Biol. 214:337-352. [DOI] [PubMed] [Google Scholar]
- 68.Matsuo, M., N. Kioka, T. Amachi, and K. Ueda. 1999. ATP binding properties of the nucleotide-binding folds of SUR1. J. Biol. Chem. 274:37479-37482. [DOI] [PubMed] [Google Scholar]
- 69.Merino, G., W. Boos, H. A. Shuman, and E. Bohl. 1995. The inhibition of maltose transport by the unliganded form of the maltose-binding protein of Escherichia coli: experimental findings and mathematical treatment. J. Theor. Biol. 177:171-179. [DOI] [PubMed] [Google Scholar]
- 70.Milburn, M. V., G. G. Prive, D. L. Milligan, W. G. Scott, J. Yeh, J. Jancarik, D. E. Koshland, Jr., and S. H. Kim. 1991. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science 254:1342-1347. [DOI] [PubMed] [Google Scholar]
- 71.Miller, D. M., J. S. Olson, J. W. Pflugrath, and F. A. Quiocho. 1983. Rates of ligand binding to periplasmic proteins involved in bacterial transport and chemotaxis. J. Biol. Chem. 258:13665-13672. [PubMed] [Google Scholar]
- 72.Mimmack, J. L., M. P. Gallagher, M. P. Hyde, S. R. Pearce, I. R. Booth, and C. F. Higgins. 1989. Energy-coupling to periplasmic binding protein-dependent transport systems: stoichiometry of ATP hydrolysis during transport. Proc. Natl. Acad. Sci. USA 86:8257-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mitchell, P. 1957. A general theory of membrane transport from studies of bacteria. Nature 180:134-136. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell, P. 1990. Osmochemistry of solute translocation. Res. Microbiol. 141:286-289. [DOI] [PubMed] [Google Scholar]
- 75.Mittal, R., M. R. Ahmadian, R. S. Goody, and A. Wittinghofer. 1996. Formation of a transition-state analog of the Ras GTPase reaction by Ras-GDP, tetrafluoroaluminate, and GTPase-activating proteins. Science 273:115-117. [DOI] [PubMed] [Google Scholar]
- 76.Mowbray, S. L., and L. B. Cole. 1992. 1.7 A X-ray structure of the periplasmic ribose receptor from Escherichia coli. J. Mol. Biol. 225:155-175. [DOI] [PubMed] [Google Scholar]
- 77.Mowbray, S. L., and G. A. Petsko. 1983. The x-ray structure of the periplasmic galactose binding protein from Salmonella typhimurium at 3.0-A resolution. J. Biol. Chem. 258:7991-7997. [DOI] [PubMed] [Google Scholar]
- 78.Muir, M., L. Williams, and T. Ferenci. 1985. Influence of transport energization on the growth yield of Escherichia coli. J. Bacteriol. 163:1237-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagata, K., M. Nishitani, M. Matsuo, N. Kioka, T. Amachi, and K. Ueda. 2000. Nonequivalent nucleotide trapping in the two nucleotide binding folds of the human multidrug resistance protein MRP1. J. Biol. Chem. 275:17626-17630. [DOI] [PubMed] [Google Scholar]
- 80.Neu, H. C., and L. A. Heppel. 1965. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J. Biol. Chem. 240:3685-3692. [PubMed] [Google Scholar]
- 81.Newcomer, M. E., G. L. Gilliland, and F. A. Quiocho. 1981. l-Arabinose-binding protein-sugar complex at 2.4 A resolution. Stereochemistry and evidence for a structural change. J. Biol. Chem. 256:13213-13217. [PubMed] [Google Scholar]
- 82.Newcomer, M. E., B. A. Lewis, and F. A. Quiocho. 1981. The radius of gyration of L-arabinose-binding protein decreases upon binding of ligand. J. Biol. Chem. 256:13218-13222. [PubMed] [Google Scholar]
- 83.Nikaido, K., and G. F. Ames. 1999. One intact ATP-binding subunit is sufficient to support ATP hydrolysis and translocation in an ABC transporter, the histidine permease. J. Biol. Chem. 274:26727-26735. [DOI] [PubMed] [Google Scholar]
- 84.Nikaido, K., P. Q. Liu, and G. F. Ames. 1997. Purification and characterization of HisP, the ATP-binding subunit of a traffic ATPase (ABC transporter), the histidine permease of Salmonella typhimurium. Solubility, dimerization, and ATPase activity. J. Biol. Chem. 272:27745-27752. [DOI] [PubMed] [Google Scholar]
- 85.Oh, B. H., J. Pandit, C. H. Kang, K. Nikaido, S. Gokcen, G. F. Ames, and S. H. Kim. 1993. Three-dimensional structures of the periplasmic lysine/arginine/ornithine-binding protein with and without a ligand. J. Biol. Chem. 268:11348-11355. [PubMed] [Google Scholar]
- 86.Panagiotidis, C. H., M. Reyes, A. Sievertsen, W. Boos, and H. A. Shuman. 1993. Characterization of the structural requirements for assembly and nucleotide binding of an ATP-binding cassette transporter. The maltose transport system of Escherichia coli. J. Biol. Chem. 268:23685-23696. [PubMed] [Google Scholar]
- 87.Pavelka, M. S., Jr., S. F. Hayes, and R. P. Silver. 1994. Characterization of KpsT, the ATP-binding component of the ABC-transporter involved with the export of capsular polysialic acid in Escherichia coli K1. J. Biol. Chem. 269:20149-20158. [PubMed] [Google Scholar]
- 88.Petronilli, V., and G. F.-L. Ames. 1991. Binding protein-independent histidine permease mutants. Uncoupling of ATP hydrolysis from transmembrane signaling. J. Biol. Chem. 266:16293-16296. [PubMed] [Google Scholar]
- 89.Pflugrath, J. W., and F. A. Quiocho. 1988. The 2 A resolution structure of the sulfate-binding protein involved in active transport in Salmonella typhimurium. J. Mol. Biol. 200:163-180. [DOI] [PubMed] [Google Scholar]
- 90.Pigeon, R. P., and R. P. Silver. 1994. Topological and mutational analysis of KpsM, the hydrophobic component of the ABC-transporter involved in the export of polysialic acid in Escherichia coli K1. Mol. Microbiol. 14:871-881. [DOI] [PubMed] [Google Scholar]
- 91.Prossnitz, E., A. Gee, and G. F.-L. Ames. 1989. Reconstitution of the histidine periplasmic transport system in membrane vesicles. Energy coupling and interaction between the binding protein and the membrane complex. J. Biol. Chem. 264:5006-5014. [PubMed] [Google Scholar]
- 92.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quiocho, F. A. 1990. Atomic structures of periplasmic binding proteins and the high-affinity active transport systems in bacteria. Philos. Trans. R. Soc. Lond. B 326:341-351. [DOI] [PubMed] [Google Scholar]
- 94.Ramachandra, M., S. V. Ambudkar, D. Chen, C. A. Hrycyna, S. Dey, M. M. Gottesman, and I. Pastan. 1998. Human P-glycoprotein exhibits reduced affinity for substrates during a catalytic transition state. Biochemistry 37:5010-5019. [DOI] [PubMed] [Google Scholar]
- 95.Rocchetta, H. L., and J. S. Lam. 1997. Identification and functional characterization of an ABC transport system involved in polysaccharide export of A-band lipopolysaccharide in Pseudomonas aeruginosa. J. Bacteriol. 179:4713-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ross, E. M., and T. M. Wilkie. 2000. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69:795-827. [DOI] [PubMed] [Google Scholar]
- 97.Sack, J. S., S. D. Trakhanov, I. H. Tsigannik, and F. A. Quiocho. 1989. Structure of the L-leucine-binding protein refined at 2.4 A resolution and comparison with the Leu/Ile/Val-binding protein structure. J. Mol. Biol. 206:193-207. [DOI] [PubMed] [Google Scholar]
- 98.Saier, M. H., Jr., I. T. Paulsen, M. K. Sliwinski, S. S. Pao, R. A. Skurray, and H. Nikaido. 1998. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 12:265-274. [DOI] [PubMed] [Google Scholar]
- 99.Sauna, Z. E., and S. V. Ambudkar. 2001. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. The two ATP hydrolysis events in a single catalytic cycle are kinetically similar but affect different functional outcomes. J. Biol. Chem. 276:11653-11661. [DOI] [PubMed] [Google Scholar]
- 100.Saurin, W., M. Hofnung, and E. Dassa. 1999. Getting in or out: early segregation between importers and exporters in the evolution of ATP-binding cassette (ABC) transporters. J. Mol. Evol. 48:22-41. [DOI] [PubMed] [Google Scholar]
- 101.Scheffzek, K., M. R. Ahmadian, W. Kabsch, L. Wiesmuller, A. Lautwein, F. Schmitz, and A. Wittinghofer. 1997. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277:333-338. [DOI] [PubMed] [Google Scholar]
- 102.Schindelin, H., C. Kisker, J. L. Schlessman, J. B. Howard, and D. C. Rees. 1997. Structure of ADP × AIF4(−)-stabilized nitrogenase complex and its implications for signal transduction. Nature 387:370-376. [DOI] [PubMed] [Google Scholar]
- 103.Schirmer, T., T. A. Keller, Y. F. Wang, and J. P. Rosenbusch. 1995. Structural basis for sugar translocation through maltoporin channels at 3.1 A resolution. Science 267:512-514. [DOI] [PubMed] [Google Scholar]
- 104.Schmees, G., A. Stein, S. Hunke, H. Landmesser, and E. Schneider. 1999. Functional consequences of mutations in the conserved "signature sequence' of the ATP-binding-cassette protein MalK. Eur. J. Biochem. 266:420-430. [DOI] [PubMed] [Google Scholar]
- 105.Senior, A. E., M. K. Al-Shawi, and I. L. Urbatsch. 1995. The catalytic cycle of P-glycoprotein. FEBS Lett. 377:285-289. [DOI] [PubMed] [Google Scholar]
- 106.Sharff, A. J., L. E. Rodseth, J. E. Spurlino, and F. A. Quiocho. 1992. Crystallographic evidence of a large ligand-induced hinge-twist motion between the two domains of the maltodextrin binding protein involved in active transport and chemotaxis. Biochemistry 31:10657-10663. [DOI] [PubMed] [Google Scholar]
- 107.Sharma, S., and A. L. Davidson. 2000. Vanadate-induced trapping of nucleotide by the purified maltose transport complex requires ATP hydrolysis. J. Bacteriol. 182:6570-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shilton, B. H., M. M. Flocco, M. Nilsson, and S. L. Mowbray. 1996. Conformational changes of three periplasmic receptors for bacterial chemotaxis and transport: the maltose-, glucose/galactose- and ribose-binding proteins. J. Mol. Biol. 264:350-363. [DOI] [PubMed] [Google Scholar]
- 109.Shilton, B. H., and S. L. Mowbray. 1995. Simple models for the analysis of binding protein-dependent transport systems. Protein Sci. 4:1346-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shilton, B. H., H. A. Shuman, and S. L. Mowbray. 1996. Crystal structures and solution conformations of a dominant-negative mutant of Escherichia coli maltose-binding protein. J. Mol. Biol. 264:364-376. [DOI] [PubMed] [Google Scholar]
- 111.Shuman, H. A. 1982. Active transport of maltose in Escherichia coli K-12: role of the periplasmic maltose binding protein and evidence for a substrate recognition site in the cytoplasmic membrane. J. Biol. Chem. 257:5455-5461. [PubMed] [Google Scholar]
- 112.Shyamala, V., V. Baichwal, E. Beall, and G. F.-L. Ames. 1991. Structure-function analysis of the histidine permease and comparison with cystic fibrosis mutations. J. Biol. Chem. 266:18714-18719. [PubMed] [Google Scholar]
- 113.Silver, R. P., K. Prior, C. Nsahlai, and L. F. Wright. 2001. ABC transporters and the export of capsular polysaccharides from gram-negative bacteria. Res. Microbiol. 152:357-364. [DOI] [PubMed] [Google Scholar]
- 114.Smith, C. A., and I. Rayment. 1996. X-ray structure of the magnesium(II).ADP.vanadate complex of the Dictyostelium discoideum myosin motor domain to 1.9 A resolution. Biochemistry 35:5404-5417. [DOI] [PubMed] [Google Scholar]
- 115.Speiser, D. M., and G. F.-L. Ames. 1991. Salmonella typhimurium histidine periplasmic permease mutations that allow transport in the absence of histidine-binding proteins. J. Bacteriol. 173:1444-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spurlino, J. C., G. Y. Lu, and F. A. Quiocho. 1991. The 2.3-A resolution structure of the maltose- or maltodextrin-binding protein, a primary receptor of bacterial active transport and chemotaxis. J. Biol. Chem. 266:5202-5219. [DOI] [PubMed] [Google Scholar]
- 117.Tesmer, J. J., D. M. Berman, A. G. Gilman, and S. R. Sprang. 1997. Structure of RGS4 bound to AlF4-activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell 89:251-261. [DOI] [PubMed] [Google Scholar]
- 118.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. 1998. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 17:6487-6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trakhanov, S., D. I. Kreimer, S. Parkin, G. F. Ames, and B. Rupp. 1998. Cadmium-induced crystallization of proteins. II. Crystallization of the Salmonella typhimurium histidine-binding protein in complex with L-histidine, L-arginine, or L-lysine. Protein Sci. 7:600-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Treptow, N. A., and H. A. Shuman. 1988. Allele-specific malE mutations that restore interactions between maltose-binding protein and the inner-membrane components of the maltose transport system. J. Mol. Biol. 202:809-822. [DOI] [PubMed] [Google Scholar]
- 121.Treptow, N. A., and H. A. Shuman. 1985. Genetic evidence for substrate and periplasmic-binding-protein recognition by the MalF and MalG proteins, cytoplasmic membrane components of the Escherichia coli maltose transport system. J. Bacteriol. 163:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ueda, K., N. Inagaki, and S. Seino. 1997. MgADP antagonism to Mg2+-independent ATP binding of the sulfonylurea receptor SUR1. J. Biol. Chem. 272:22983-22986. [DOI] [PubMed] [Google Scholar]
- 123.Urbatsch, I. L., B. Sankaran, S. Bhagat, and A. E. Senior. 1995. Both P-glycoprotein nucleotide-binding sites are catalytically active. J. Biol. Chem. 270:26956-26962. [DOI] [PubMed] [Google Scholar]
- 124.Urbatsch, I. L., B. Sankaran, J. Weber, and A. E. Senior. 1995. P-glycoprotein is stably inhibited by vanadate-induced trapping of nucleotide at a single catalytic site. J. Biol. Chem. 270:19383-19390. [DOI] [PubMed] [Google Scholar]
- 125.Urbatsch, I. L., and A. E. Senior. 1995. Effects of lipids on ATPase activity of purified Chinese hamster P-glycoprotein. Arch. Biochem. Biophys. 316:135-140. [DOI] [PubMed] [Google Scholar]
- 126.van Veen, H. W., R. Callaghan, L. Soceneantu, A. Sardini, W. N. Konings, and C. F. Higgins. 1998. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature 391:291-295. [DOI] [PubMed] [Google Scholar]
- 127.van Veen, H. W., A. Margolles, M. Muller, C. F. Higgins, and W. N. Konings. 2000. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 19:2503-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yan, R. T., and P. C. Maloney. 1995. Residues in the pathway through a membrane transporter. Proc. Natl. Acad. Sci. USA 92:5973-5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuan, Y. R., S. Blecker, O. Martsinkevich, L. Millen, P. J. Thomas, and J. F. Hunt. 2001. The crystal structure of the MJ0796 ATP-binding cassette: implications for the structural consequences of ATP hydrolysis in the active site of an ABC-transporter. J. Biol. Chem. 276:32313-32321. [DOI] [PubMed] [Google Scholar]
- 131.Zhou, T., S. Radaev, B. P. Rosen, and D. L. Gatti. 2001. Conformational changes in four regions of the Escherichia coli ArsA ATPase link ATP hydrolysis to ion translocation. J. Biol. Chem. 276:30414-30422. [DOI] [PubMed] [Google Scholar]
- 132.Zhou, T., S. Radaev, B. P. Rosen, and D. L. Gatti. 2000. Structure of the ArsA ATPase: the catalytic subunit of a heavy metal resistance pump. EMBO J. 19:4838-4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou, Z., K. A. White, A. Polissi, C. Georgopoulos, and C. R. Raetz. 1998. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 273:12466-12475. [DOI] [PubMed] [Google Scholar]
- 134.Zou, J. Y., M. M. Flocco, and S. L. Mowbray. 1993. The 1.7 A refined X-ray structure of the periplasmic glucose/galactose receptor from Salmonella typhimurium. J. Mol. Biol. 233:739-752. [DOI] [PubMed] [Google Scholar]
- 135.Zukin, R. S., P. R. Hartig, and D. E. Koshland, Jr. 1977. Use of a distant reporter group as evidence for a conformational change in a sensory protein. Proc. Natl. Acad. Sci. USA 74:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]