Abstract
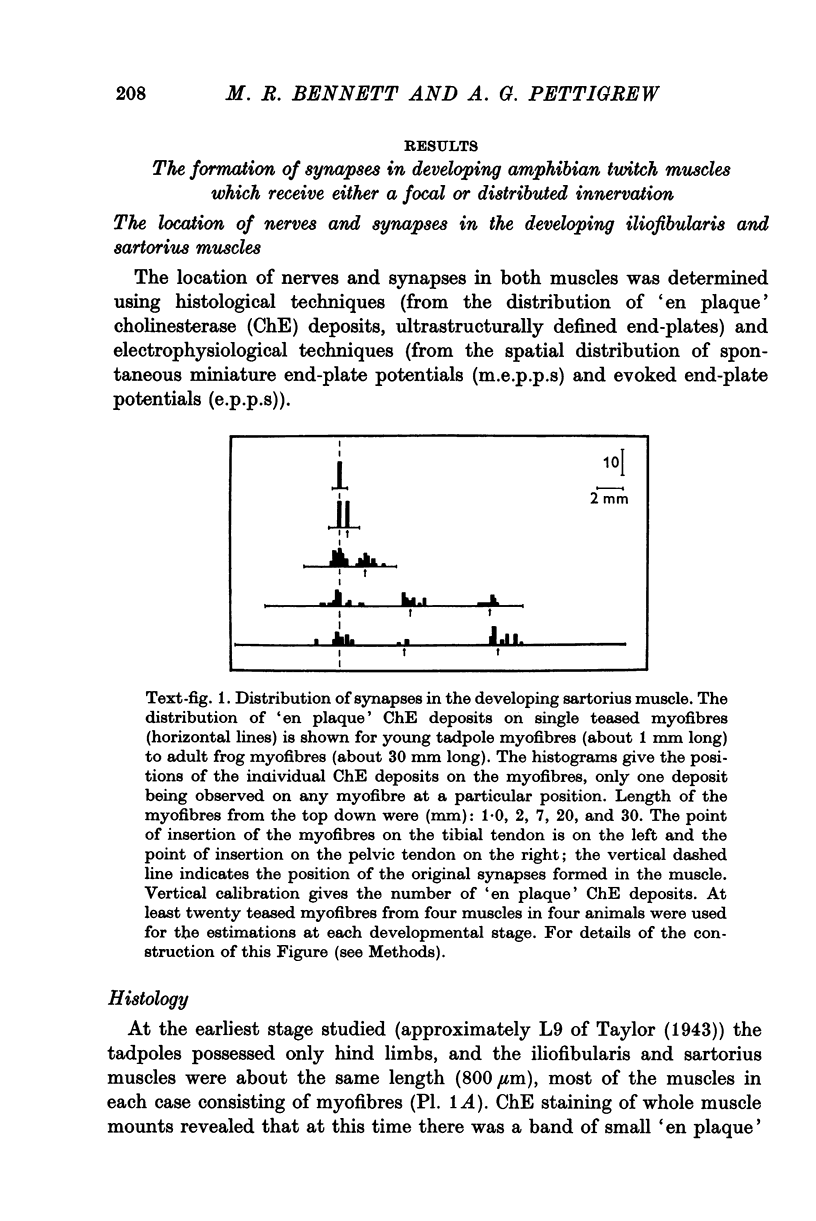
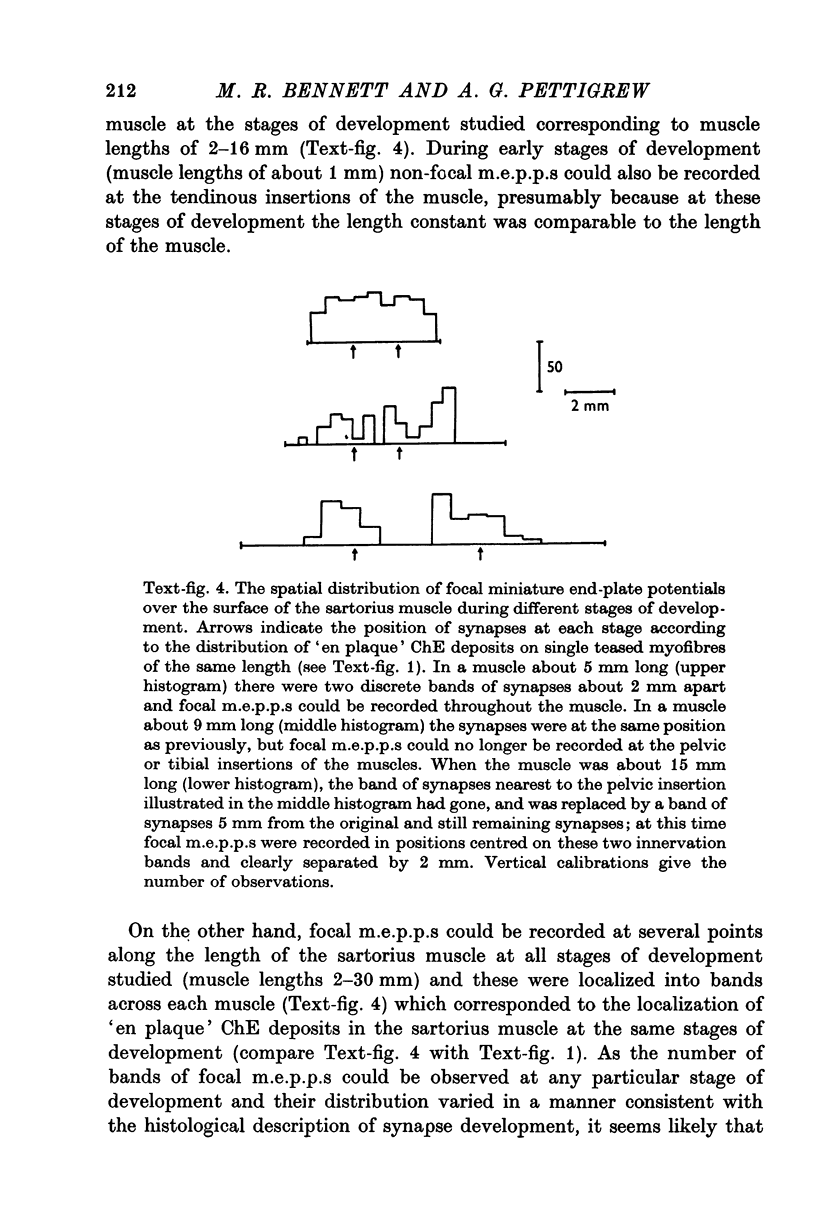
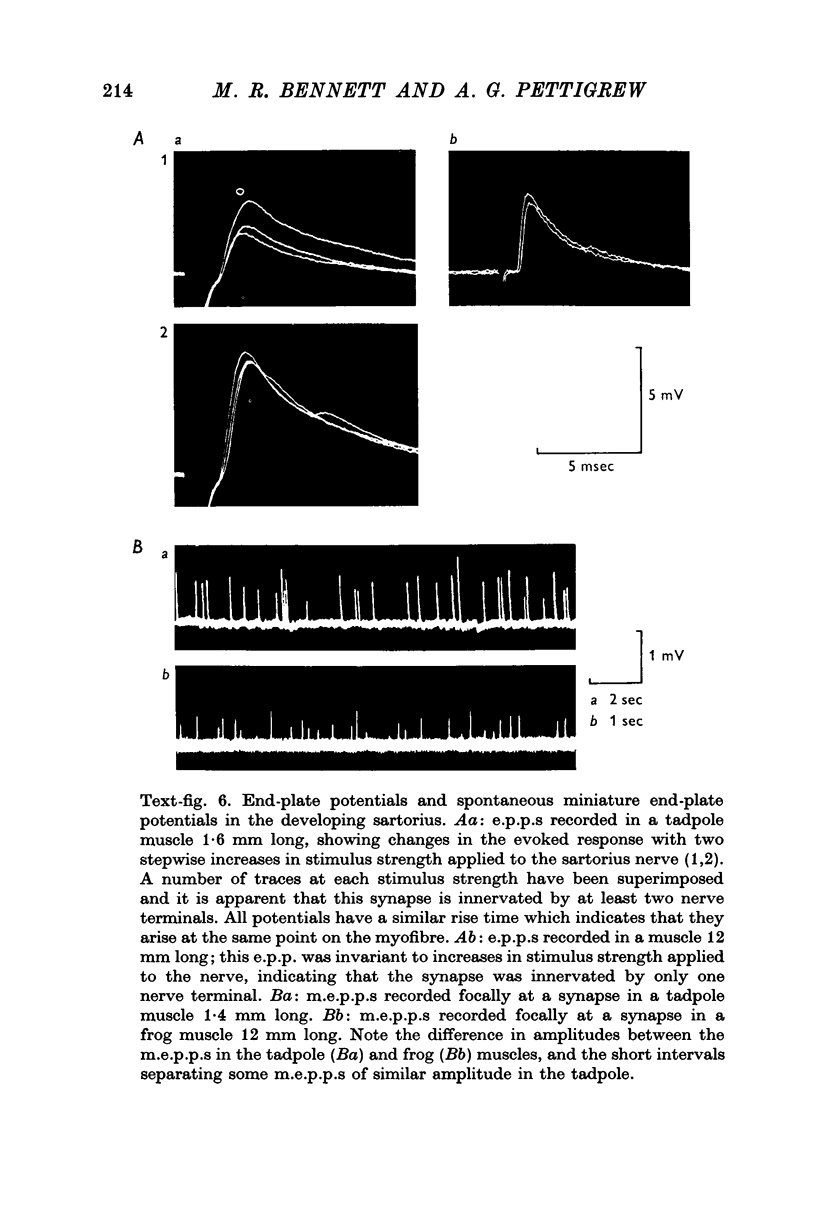
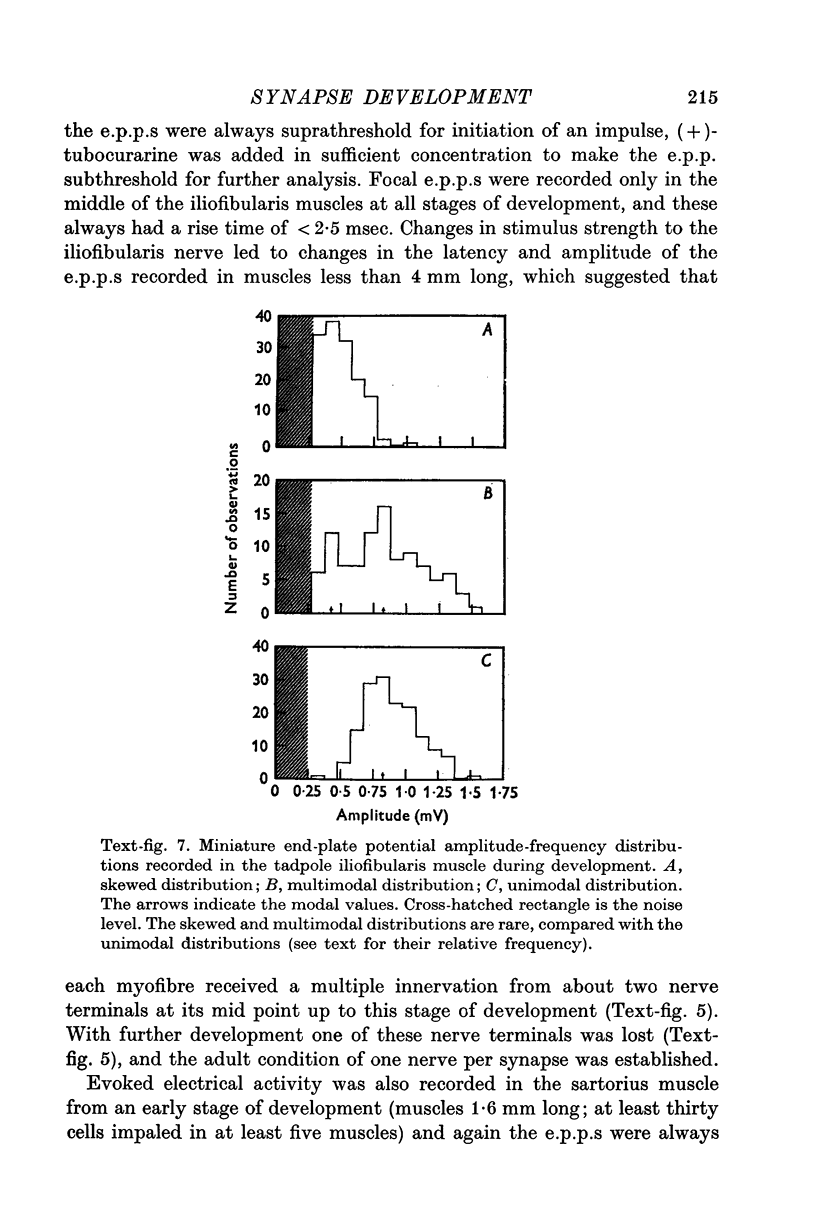
1. A study has been made of the formation of synapses in developing reinnervated and cross-reinnervated amphibian twitch muscles which receive either a focal (iliofibularis) or a distributed (sartorius) innervation from 'en plaque' nerve terminals using histological, ultrastructural and electrophysiological techniques. 2. During the development of the tadpole through metamorphosis to the adult frog, the sartorius myofibres increased in length at about twice the rate of the iliofibularis myofibres, due to a fast rate of growth at their insertions on to the pelvic tendon. 3. The short iliofibularis and sartorius myofibres of young tadpoles (800 mum long) possessed only a single synapse and the iliofibularis myofibres did not receive any further innervation during development. However the sartorius myofibres received further transient innervation on the new muscle laid down during development at the fast growing pelvic insertion, until the distance between the original synapse formed on the myofibres and the synapse at the pelvic end of the muscle was about 12 mm. 4. During development synapses possessed either skewed, multimodal, or unimodal m.e.p.p. amplitude-frequency distributions; the intervals between m.e.p.p.s. were not distributed randomly according to a Poisson process, as m.e.p.p.s. of similar amplitudes tended to be separated by very short intervals; the unit-size e.p.p. had a similar amplitude-frequency distribution as the m.e.p.p.s. if these had a unimodal distribution. 5. Reinnervation or cross-reinnervation of the sartorius and the iliofibularis muscles in adults or at a late stage of development simply reconstituted the normal focal and distributed innervation patterns of the muscles, as found in the control muscles of the contralateral and unoperated legs. 6. These observations on synapse formation in amphibia are consistent with the hypothesis that during development the axon making the initial synaptic contact on the muscle cells induces a property over a length of muscle membrane adjacent to this site which makes it refractory to synapse formation; thus during reinnervation or cross-reinnervation of adult muscles this refractory property constrains synapse formation to these sites.
Full text
PDF










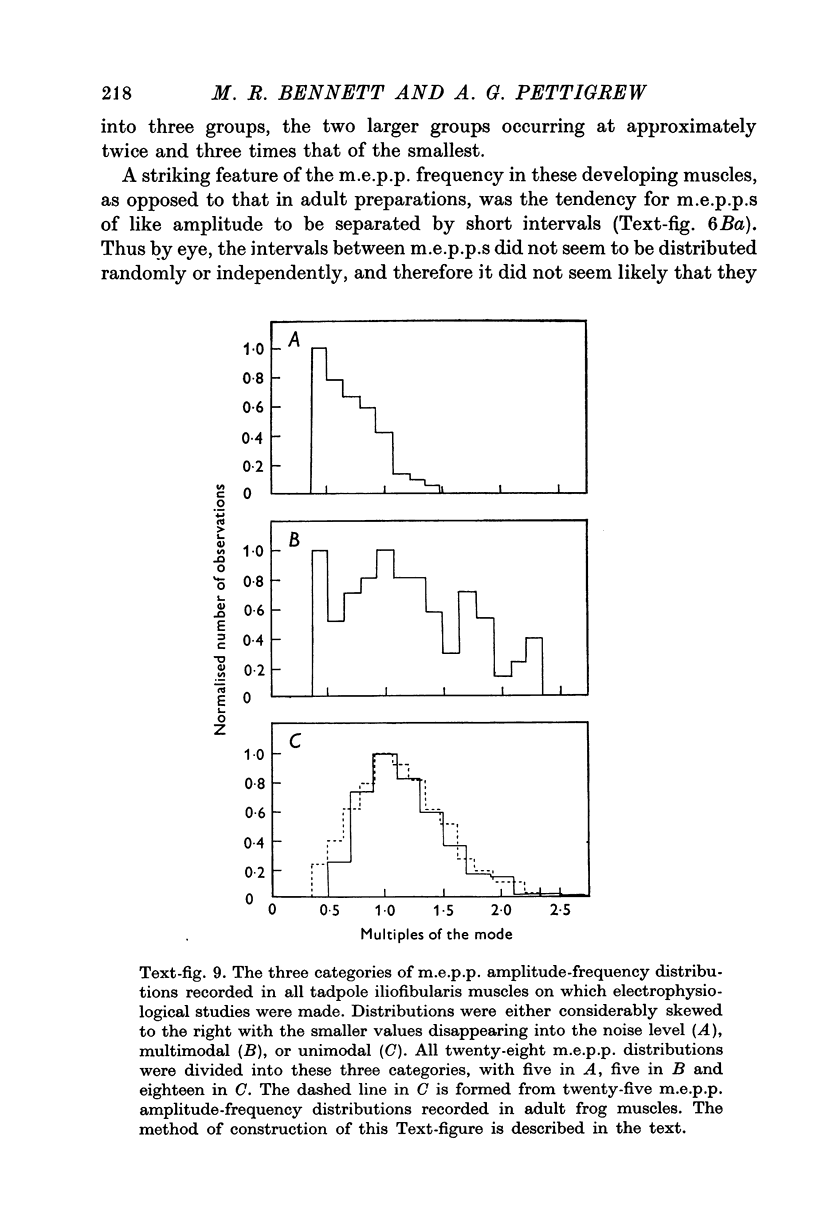




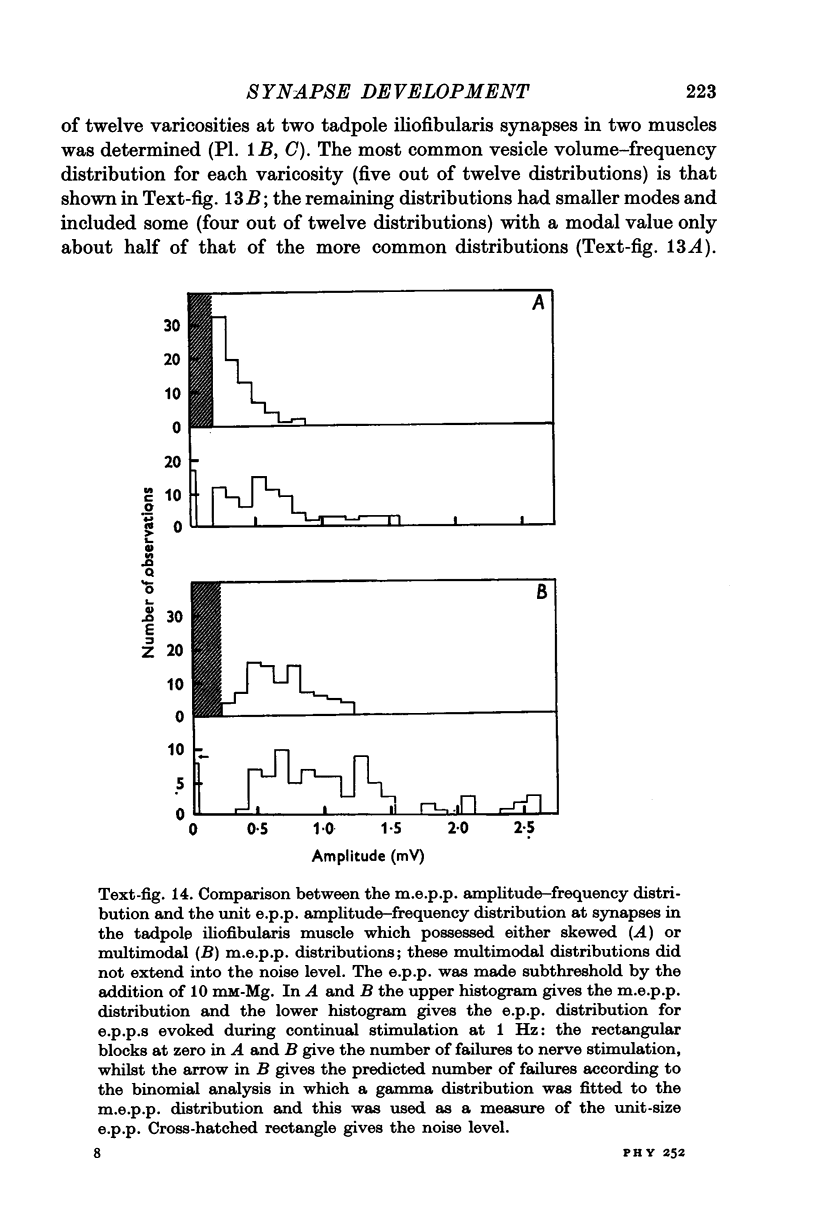



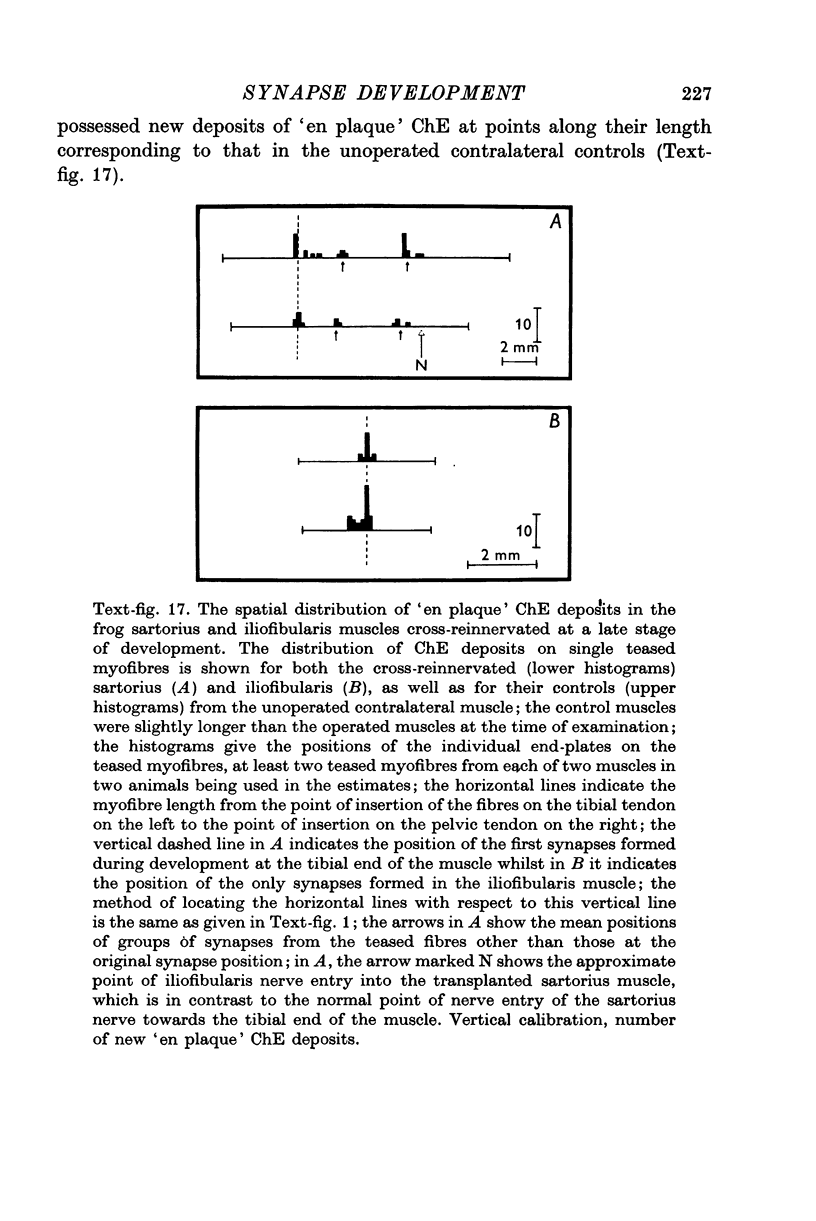














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi A. B., Sassu G. On the fine structure of the motor end-plates during muscular regeneration in Gongylus ocellatus. Acta Anat (Basel) 1973;85(4):580–592. doi: 10.1159/000144097. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Martin A. R., Rahamimoff R. A note on the interaction of spontaneous and evoked release at the frog neuromuscular junction. J Physiol. 1974 Mar;237(2):453–463. doi: 10.1113/jphysiol.1974.sp010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. A statistical analysis of the release of acetylcholine at newly formed synapses in striated muscle. J Physiol. 1974 Apr;238(1):93–107. doi: 10.1113/jphysiol.1974.sp010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Hall R. The effect of calcium ions on the binomial statistic parameters which control acetylcholine release at synapses in striated muscle. J Physiol. 1975 May;247(2):429–446. doi: 10.1113/jphysiol.1975.sp010939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in mammalian striated muscle reinnervated with autonomic preganglionic nerves. J Physiol. 1973 Sep;233(3):501–517. doi: 10.1113/jphysiol.1973.sp010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., McLachlan E. M., Taylor R. S. The formation of synapses in reinnervated mammalian striated muscle. J Physiol. 1973 Sep;233(3):481–500. doi: 10.1113/jphysiol.1973.sp010319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in reinnervated and cross-reinnervated striated muscle during development. J Physiol. 1974 Sep;241(2):547–573. doi: 10.1113/jphysiol.1974.sp010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in striated muscle during development. J Physiol. 1974 Sep;241(2):515–545. doi: 10.1113/jphysiol.1974.sp010670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD G. N. C. An experimental study of tendon growth in the rabbit. J Bone Joint Surg Br. 1950 May;32-B(2):234–243. doi: 10.1302/0301-620X.32B2.234. [DOI] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. Stochastic properties of spontaneous transmitter release at the crayfish neuromuscular junction. J Physiol. 1974 Jan;236(2):363–371. doi: 10.1113/jphysiol.1974.sp010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The intervals between miniature end-plate potentials in the frog are unlikely to be independently or exponentially distributed. J Physiol. 1974 Jan;236(2):327–339. doi: 10.1113/jphysiol.1974.sp010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The stochastic properties of spontaneous quantal release of transmitter at the frog neuromuscular junction. J Physiol. 1974 Jan;236(2):341–361. doi: 10.1113/jphysiol.1974.sp010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradi S., Skoglund S. Observations on the ultrastructure of the initial motor axon segment and dorsal root boutons on the motoneurons in the lumbosacral spinal cord of the cat during postnatal development. Acta Physiol Scand Suppl. 1969;333:53–76. [PubMed] [Google Scholar]
- DIAMOND J., MILEDI R. A study of foetal and new-born rat muscle fibres. J Physiol. 1962 Aug;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The acetylcholine sensitivity in the vicinity of the neuromuscular junction of the frog. Pflugers Arch. 1974 May 6;348(4):273–286. doi: 10.1007/BF00589217. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The spread of acetylcholine sensitivity after denervation of frog skeletal muscle fibers. Pflugers Arch. 1974 May 6;348(4):287–292. doi: 10.1007/BF00589218. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filogamo G., Gabella G. The development of neuro-muscular correlations, in vertebrates. Arch Biol (Liege) 1967;78(1):9–60. [PubMed] [Google Scholar]
- Goldspink G., Tabary C., Tabary J. C., Tardieu C., Tardieu G. Effect of denervation on the adaptation of sarcomere number and muscle extensibility to the functional length of the muscle. J Physiol. 1974 Feb;236(3):733–742. doi: 10.1113/jphysiol.1974.sp010463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Motor innervation of skeletal muscle: multiple innervation of individual muscle fibres and motor unit function. J Physiol. 1954 Nov 29;126(2):293–303. doi: 10.1113/jphysiol.1954.sp005210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F. Spontaneous quantal transmitter release: a statistical analysis and some implications. J Physiol. 1973 Jul;232(1):1–21. doi: 10.1113/jphysiol.1973.sp010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. The effects of nerve stimulation and hemicholinium on synaptic vesicles at the mammalian euromuscular junction. J Physiol. 1970 Mar;207(1):31–50. doi: 10.1113/jphysiol.1970.sp009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITIYAKARA A., ANGEVINE D. M. A STUDY OF THE PATTERN OF POSTEMBRYONIC GROWTH OF M. GRACILIS IN MICE. Dev Biol. 1963 Dec;8:322–340. doi: 10.1016/0012-1606(63)90033-2. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. Pharmacological properties, cholinesterase activity and anatomy of nerve-muscle junctions in vagus-innervated frog sartorius. J Physiol. 1972 Jan;220(1):243–256. doi: 10.1113/jphysiol.1972.sp009704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Liu H. C., Maneely R. B. The development of motor end-plates in the embryonic and regenerative tail of Hemidactylus bowringi (Gray). Acta Anat (Basel) 1968;71(2):249–267. doi: 10.1159/000143189. [DOI] [PubMed] [Google Scholar]
- Lüllmann-Rauch R. The regeneration of neuromuscular junctions during spontaneous re-innervation of the rat diaphragm. Z Zellforsch Mikrosk Anat. 1971;121(4):593–603. doi: 10.1007/BF00560162. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay B., Harrop T. J. An experimental study of the longitudinal growth of skeletal muscle in the rat. Acta Anat (Basel) 1969;72(1):38–49. doi: 10.1159/000143234. [DOI] [PubMed] [Google Scholar]
- Mackay B., Harrop T. J., Muir A. R. The fine structure of the muscle tendon junction in the rat. Acta Anat (Basel) 1969;73(4):588–602. doi: 10.1159/000143318. [DOI] [PubMed] [Google Scholar]
- Manolov S. Initial changes in the neuromuscular synapses of denervated rat diaphragm. Brain Res. 1974 Jan 11;65(2):303–316. doi: 10.1016/0006-8993(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Muhl Z. F., Grimm A. F. Longitudinal growth of striated muscle: a radiographic study. Growth. 1974 Sep;38(3):389–394. [PubMed] [Google Scholar]
- Nickel E., Waser P. G. Elektronenmikroskopische Untersuchungen am Diaphragma der Maus nach einseitiger Phrenikotomie. I. Die degenerierende motorische Endplatte. Z Zellforsch Mikrosk Anat. 1968;88(2):278–296. [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnevi L. O., Conradi S. Ultrastructural evidence for spontaneous elimination of synaptic terminals on spinal motoneurons in the kitten. Brain Res. 1974 Nov 15;80(2):335–339. doi: 10.1016/0006-8993(74)90696-9. [DOI] [PubMed] [Google Scholar]
- Schattenberg P. J. Untersuchungen über das Längenwachstum der Skelettmuskulatur von Fischen. Z Zellforsch Mikrosk Anat. 1973 Oct 26;143(4):587–596. [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Longitudinal growth of striated muscle fibres. J Cell Sci. 1971 Nov;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973 Oct;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]






