Abstract
Yersinia enterocolitica maintains three different pathways for type III protein secretion. Each pathway requires the activity of a specific multicomponent apparatus or type III secretion system (TTSS). Two of the TTSSs are categorized as contact-dependent systems which have been shown in a number of different symbiotic and pathogenic bacteria to influence interactions with host organisms by targeting effector proteins into the cytosol of eukaryotic cells. The third TTSS is required for the assembly of flagella and the secretion of the phospholipase YplA, which has been implicated in Y. enterocolitica virulence. In this study, YplA was expressed from a constitutive promoter in strains that contained only a single TTSS. It was determined that each of the three TTSSs is individually sufficient for YplA secretion. Environmental factors such as temperature, calcium availability, and sodium chloride concentration affected the contribution of each system to extracellular protein secretion and, under some conditions, more than one TTSS appeared to operate simultaneously. This suggests that some proteins might normally be exported by more than one TTSS in Y. enterocolitca.
A number of proteins that contribute to virulence are secreted by pathogenic bacteria. The type III mechanism is one way that many gram-negative pathogens have been shown to secrete virulence factors (18). This type of protein secretion is known to require the function of a dedicated apparatus referred to as a type III secretion system (TTSS) that spans the bacterial cell envelope. Proteins secreted by a type III mechanism are transported in a single step by the apparatus without modification. In several cases, these secretion systems have been shown to directly translocate proteins from the bacterial cell to the interior of a eukaryotic cell (18). In the genus Yersinia, the most extensively studied TTSS is encoded by the ysc genes located on the ca. 70-kb plasmid carried by the three species pathogenic for humans and other animals: Yersinia enterocolitica, Y. pestis, and Y. pseudotuberculosis (13-15, 43). In humans, Y. pestis is the causative agent of bubonic and pneumonic forms of the plague. Y. enterocolitica and Y. pseudotuberculosis cause gastrointestinal illnesses. Virulence of all three species requires the Ysc TTSS for the secretion of a set of proteins referred to as Yops (for Yersinia outer proteins). The Yops are a diverse group of proteins called effectors (19). Protein secretion by the Ysc TTSS is stringently controlled to ensure that exported virulence factors are appropriately targeted to the host environment. Using in vitro models of infection, contact-dependent translocation of Yops has been shown to lead to the modification of a number of eukaryotic cellular processes, including the inhibition of phagocytosis by macrophages and polymorphonuclear leukocytes, suppression of T- and B-lymphocyte activation, and alteration of cytokine production by T and B lymphocytes (19). In vitro secretion of Yops by the Ysc TTSS can also be stimulated in the absence of contact with a host cell by growing the bacteria at 37°C in a low calcium medium (35).
Recently, a second TTSS was identified that is encoded by the ysa locus located in the chromosome of Y. enterocolitica (17). The Ysa TTSS has been shown to be required for the production of a set of extracellular proteins called Ysps (for Yersinia secreted proteins). The function of the Ysps is not known, but their export by the Ysa TTSS is believed to be important for Y. enterocolitica virulence since a Ysa TTSS mutant exhibited reduced virulence for orally infected mice (17). Although the host signals required for Ysp secretion by the Ysa TTSS are not known, it has been shown that Ysp secretion occurs in vitro when Y. enterocolitica is grown at 26°C in a high-salt medium (0.49 M NaCl) (17).
A third TTSS that is functionally related to the Ysa and Ysc TTSSs has been shown to operate during the biosynthesis of the bacterial flagellum (30, 38). The core components that form the flagellar apparatus are conserved in all known TTSSs (4). Studies of flagellar biosynthesis in Salmonella enterica serovar Typhimurium have revealed that this system is utilized by the bacterium to transport a subset of flagellar structural subunits from the cytoplasm to the distal end of the organelle during assembly (39). Initially, proteins that form the export machinery assemble to form a base for the entire structure. The export machinery is then used to specifically locate other flagellar subunits to form an intermediate structure (called the hook-basal body) which extends to the outside of the cell (1). The hook-basal body exhibits an architecture that is similar to the architecture of the contact-dependent type III apparatus (5, 27, 51). During the final stages of flagellar biogenesis the hook-basal body serves as both the machinery for the export of filament subunits and the base for filament assembly (30). Coordination of flagellar genes that encode components of the filament, the rotary motor, and the chemosensory system are expressed from σ28-dependent promoters that provide a regulatory mechanism that ensures these components are not synthesized until after the initial flagellar structure is complete (25).
Y. enterocolitica is closely related to Escherichia coli and S. enterica. It has been shown to maintain a similar set of flagellar genes, including those that encode components of the flagellar secretion system (11, 20, 22, 23, 55, 56). The production of flagella by Y. enterocolitica is required for swimming and swarming motility (56). Swimming motility has also been shown to enhance Y. enterocolitica invasion of eukaryotic cells, indicating the flagella contribute to virulence (53). In addition, the secreted virulence factor YplA is exported by the flagellar TTSS (55). YplA is not required for flagellum-dependent motility but is a phospholipase that affects survival of Y. enterocolitica during infection, during which it appears to modulate the host inflammatory response (45, 52). Characterization of the yplA locus revealed that it consists of two open reading frames, designated yplA and yplB, that form an operon (45). The protein encoded by yplA is the extracellular phospholipase. It has been hypothesized that yplB may encode an accessory protein or chaperone required for YplA secretion, but this function has not been established. Located immediately upstream of yplA are −35 and −10 promoter regions that share identity with the consensus sequence of known σ28-dependent promoters and expression of yplA was shown to require the function of fliA (fliA encodes σ28) (45, 46). This indicates that yplA belongs to the flagellar regulon and that YplA is not made until the hook-basal body is complete. This is consistent with the fact that secretion of heterologously produced YplA was blocked in Y. enterocolitica hook-basal body mutants but was not affected in mutants defective for late stages of flagellar assembly (55). The production of several other extracellular proteins, collectively termed Fops (for flagellar outer proteins), also requires the function of the flagellar system. The identity and functions of these proteins have not been defined (55). Secretion of YplA and the other Fops by the flagellar TTSS occurs when Y. enterocolitica is cultured at 26°C in a low-salt growth medium (55).
These three different TTSSs of Y. enterocolitica have each been shown to function in vitro under different conditions. This may indicate that each TTSS is required for secretion of a distinct set of substrates and contributes to distinct stages of infection. Nonetheless, each TTSS is predicted to recognize and transport its respective substrates in a similar manner since these systems all have similar components and are predicted to have similar molecular architectures. In the present study, we evaluated the potential for YplA to be a substrate for each of the three Y. enterocolitica TTSSs. We investigated whether the function of the Ysc and Ysa TTSSs is required for secretion of proteins by the flagellar TTSS. In addition, we have determined that some environmental conditions allow multiple TTSSs to function at the same time.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are described in Table 1. Unless otherwise noted, the E. coli strains were routinely grown at 37°C, and the Y. enterocolitica strains were grown at 26°C in Luria broth (1% tryptone, 0.5% yeast extract, 90 mM NaCl) or on Luria agar (Difco). The medium used for the examination of protein secretion by Yersinia enterocolitica was Luria broth base (L medium; 1% tryptone, 0.5% yeast extract) adjusted to contain NaCl at the final concentrations indicated in the text. Brain heart infusion medium was purchased from Difco (Detroit, Mich.). When necessary, depletion of calcium from the medium was accomplished by the addition of 20 mM sodium oxalate and 20 mM MgCl2. Phospholipase indicator agar (PLA) consisted of MacConkey agar base (Difco) supplemented with 1% Tween 80 and 1 mM CaCl2 (55). Antibiotics were used at the indicated concentrations: chloramphenicol (25 μg/ml for E. coli; 12.5 μg/ml for Y. enterocolitica), kanamycin (50 μg/ml), nalidixic acid (20 μg/ml), and tetracycline (15 μg/ml for E. coli; 7.5 μg/ml for Y. enterocolitica).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotypea | Source or reference |
|---|---|---|
| Strains | ||
| Y. enterocolitica | ||
| JB580v | Serogroup O:8, Nalr ΔyenR (r− m+) | 26 |
| GY460 | ΔflhDC | 56 |
| GY563 | flgA::TnMod-RKm" | -b |
| GY567 | flgB::TnMod-RKm" | -b |
| GY648 | flhB::TnMod-RKm" | -b |
| GY667 | fliE::TnMod-RKm" | -b |
| GY751 | motA::mTn5Km2-lacZYA | -c |
| GY824 | fleB::mTn5Km2-lacZYA | -c |
| YVM139 | flgM::Kan | 29 |
| VK1 | fliA::Str | 29 |
| YEDS10 | yplA-lacZYA yplA+ | 46 |
| YVM356 | yscR::mTn5Km2 | 8 |
| YVM351 | yscU::mTn5Km2 | 8 |
| GY4555 | yscK::TnMod-lacZYA-RKm" | -c |
| GY4412 | ysaI::TnMod-RKm", pGY100 | This study |
| GY4418 | ysaV::TnMod-RKm", pGY100 | This study |
| GY4413 | ysaW::TnMod-RKm", pGY100 | This study |
| GY4415 | ysaT::TnMod-RKm", pGY100 | This study |
| GY4414 | orf1-1::TnMod-RKm", pGY100 | This study |
| GY4416 | orf1-2::TnMod-RKm", pGY100 | This study |
| GY4499 | ysaT::TnMod-RKm" | This study |
| GY4482 | ΔflhDC, ysaT::TnMod-RKm" | This study |
| GY4478 | pYV8081− | This study |
| GY4479 | ΔflhDC, pYV8081− | This study |
| GY4481 | ysaT::TnMod-RKm", pYV8081− | This study |
| GY4492 | ΔflhDC ysaT::TnMod-RKm", pYV8081− | This study |
| E. coli S17-1 λpir | recA thi pro hsdR−M+ RP4::2-Tc::Mu::Km Tn7 λpir | 37 |
| Plasmids | ||
| pGY10 | flhDC locus in pTM100 | 56 |
| pGY22 | ΔflhDC allele in the suicide vector pEP185.2 | 56 |
| pGY100 | Pcat-yplAB derivative of pTM100 | This study |
| pTM100 | mob+, derivative of pACYC184, Cmr Tetr | 33 |
| pTnMod-RKm" | TnMod-RKm" in a delivery vector | 10 |
Cmr, chloramphenicol resistance; Tetr, tetracycline resistance.
S. Petersen and G. M. Young, unpublished data.
K. Venecia and G. M. Young, unpublished data.
Plasmid pGY100 was constructed by subcloning the yplAB locus as a 1.7-kb EcoRI fragment from pDHS32 (55) into the unique EcoRI site of plasmid pTM100 (33). This fragment of DNA contains DNA sequences extending from bp 114 to 1800 of the yplAB region (GenBank accession no. AF067849). The fragment is missing the putative −35 region of the yplA promoter but is oriented downstream of the constitutive Pcat promoter in pTM100. Constitutive expression of cat was confirmed by determining chloramphenicol acetyltransferase levels of Y. enterocolitica pTM100 grown under various conditions as described previously (54).
Preparation of extracellular proteins, SDS-PAGE, and Western blot analysis.
Extracellular proteins were prepared as described with minor modifications (55). Y. enterocolitica was grown overnight in Luria broth and subcultured 1:30 into 5 ml of appropriate medium to induce secretion of Fops, Ysps, or Yops as indicated in the text. A Fop is defined as a protein that is secreted into the culture supernatant under conditions that induce the production of flagella, and its production is controlled by flagellar master regulators (55). A Ysp is defined as a protein secreted into culture supernatants under conditions that induce the Ysa TTSS (17). A Yop is a protein that is secreted into culture supernants under conditions that induce the Ysc TTSS (34). Cultures were grown at 26 or 37°C (as indicated) for 6 h and then used to isolate secreted proteins. At the time of harvesting, the optical density at 600 nm (OD600) of the culture was determined. Bacterial cells were removed by centrifugation in a microcentrifuge at 13,000 rpm for 5 min. The upper two-thirds of the supernatant was removed and centrifuged again. The upper two-thirds of the supernatant was then removed and passed through a 0.22-μm filter. Proteins were concentrated by precipitation with 10% (wt/vol) ice-cold trichloroacetic acid and washed with ice-cold acetone. All samples were resuspended in sample buffer containing 2-mercaptoethanol (31). Resuspension volumes were adjusted according to the OD600 of the cultures so that equivalent amounts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). All samples were heated to 95°C for 5 min and then loaded onto 10% polyacrylamide gels. Proteins were visualized by staining with silver or transferred to nitrocellulose membranes for Western blot analysis (6). Detection of YplA with polyclonal anti-YplA antibody was completed and visualized by an enhanced chemiluminescence method (Amersham) as described previously (55).
Transposon mutagenesis.
Y. enterocolitica pGY100 was mutagenized by conjugation of the plasmid pTnMod-RKm" (10) by using conditions described previously (8). Sixteen separate matings were collected and plated on L agar containing naladixic acid, kanamycin, and tetracycline. The plates were incubated for 48 h at 26°C. Approximately 3,200 colonies were then patched onto high-salt PLA medium (290 mM NaCl) and incubated for 48 h at 26°C to identify strains that exhibited a phospholipase-negative phenotype. Colonies unaffected for YplA secretion appeared pink and had a halo of precipitation, while candidate Ysa TTSS mutants appeared white and lacked a halo of precipitation.
Characterization of transposon insertion sites and DNA sequencing.
Chromosomal DNA was isolated from mutants that contained TnMod-RKm" insertions and digested with EcoRI as described previously (54). The digested DNA was ligated overnight and replicating plasmids were recovered by electroporation of E. coli S17-1 λpir followed by selection for kanamycin resistance. TnMod-RKm" contains oriR6K, which can function in specialized E. coli strains that carry a copy of pir (10). Plasmids were isolated and analyzed by restriction digestion to confirm the integrity of the transposon sequences. The sequence of the chromosomal DNA immediately adjacent to the transposon was then determined by using primers that anneal near the ends of TnMod-RKm" (primer KM1 [5"-CCCCGAGCTCTTAATTAA-3"] and primer KM2 [5"-GAACACTTAACGGCTGAC-3"]). DNA sequence was obtained by using an Applied Biosystems DNA sequencing system and the BigDye terminator cycle sequencing kit (Biosystems) according to the manufacturer's instructions.
Construction of other mutant strains.
The generation of strains that contain the ΔflhDC mutation was done by allelic exchange with pGY22 as described previously (56). To cure strains of plasmid pYV8081, colonies were streaked on L agar that contained 20 mM oxalate and 20 mM MgCl2. Cultures were incubated at 37°C for 48 h, and then large colonies were purified. A complete loss of pYV8081 was confirmed by examination of plasmid preparations by agarose gel electrophoresis and by PCR analysis.
RESULTS
YplA can be secreted by flagellum-dependent and -independent mechanisms.
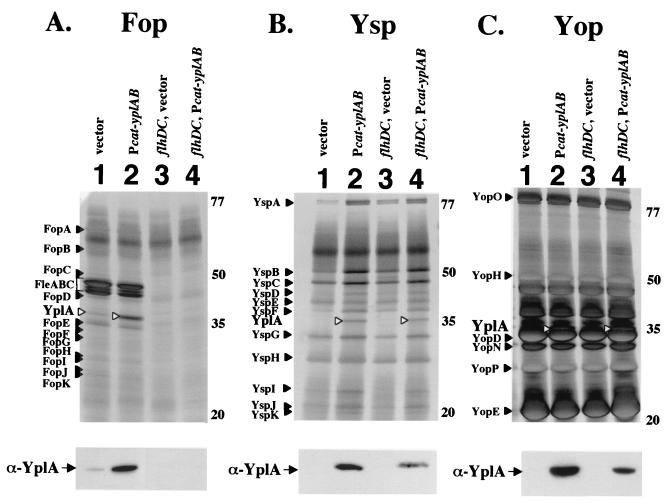
The expression of yplA is controlled by the positive regulatory genes flhDC and fliA that couple regulation to secretion. Previously, it was shown that placing the yplA locus (yplAB) downstream of the Ptac promoter allowed the production of YplA, but it was secreted only from strains that had an intact flagellar TTSS (defined here as the hook-basal body structure) (55). While expression of yplA from Ptac was useful, the amount of YplA produced was not sufficient to allow for efficient visualization by standard SDS-PAGE analysis. To improve upon our ability to study YplA secretion, yplAB was cloned downstream of the constitutively expressed promoter of cat (Pcat), located in the cloning vector pTM100, to generate plasmid pGY100. Introduction of pGY100 into Y. enterocolitica resulted in increased production of secreted YplA to levels that were easily detected by standard protein gel analysis when the organism was cultured at 26°C in a medium that induces the flagellar TTSS (Fig. 1A). Secretion of YplA was confirmed by Western blot analysis of culture supernatants with anti-YplA polyclonal antibody (Fig. 1A). As a negative control, pGY100 was introduced into a flhDC mutant. Even though this strain had an intact chromosomal copy of yplAB and the plasmid encoded Pcat-yplAB, the flhDC mutant did not secrete YplA or other Fop proteins (Fig. 1A). Secretion of YplA was also blocked by mutations affecting flagellar genes such as flgA, flhB, and fliE that encode components of the basal portion of the flagellar export apparatus (data not shown). YplA secretion was not affected by a mutation in motA, which encodes a flagellar component required for rotation of flagella but is not a substrate for, or a component of, the flagellar TTSS (data not shown). Secretion of YplA was not blocked by mutations in fleB and flgM, which encode proteins exported beyond the cell envelope (data not shown). Secretion also was not blocked in a strain that had a mutation in fliA, which should produce a hook-basal body structure but not a flagellar filament, the torque-generating motor, or the chemosensory system. The results of this analysis are similar to and extend those of a previous study that indicated that YplA is secreted by the flagellar TTSS but does not require other flagellum-related functions (55).
FIG. 1.
Analysis of secreted YplA under conditions that induce different type III secretion systems. Extracellular proteins were concentrated from culture supernatants, separated by SDS-PAGE, and stained with silver. The identity of YplA was confirmed by Western blot analysis with polyclonal anti-YplA antibody of a second set of protein gels run under conditions identical to those described in Materials and Methods. Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 1.0 for panels A and B and of 0.5 for panel C. For each panel, labels for Fops, Ysps, and Yops are assigned according to size on the left, and the approximate locations of molecular mass standards (in kilodaltons) are indicated on the right. White arrowheads indicate the location of YplA. Protein detected by Western blot with anti-YplA is indicated by the black arrowheads. (A) Cultures were grown at 26°C in L medium devoid of added NaCl to induce the production of Fops. Lanes: 1, JB580v/pTM100 (vector control); 2, JB580v/pGY100 (Pcat-yplAB); 3, GY460/pTM100 (ΔflhDC); 4, GY460/pGY100 (ΔflhDC, Pcat-yplAB). (B) Cultures were grown at 26°C in L medium plus 290 mM NaCl to induce the production of Ysps. Lanes are as listed for panel A. (C) Cultures were grown at 37°C in L medium plus 90 mM NaCl supplemented with 20 mM sodium oxalate and 20 mM MgCl2 to induce production of Yops. Lanes are as listed for panel A.
The flagellar Ysa and Ysc protein secretion systems are believed to export polypeptides by a similar mechanism. This suggests that some proteins might be recognized as substrates by more than one TTSS in Y. enterocolitica. Consistent with this hypothesis, the export of proteins by multiple TTSS has previously been documented for S. enterica (32). To determine whether YplA could be secreted by one of the flagellum-independent systems, Y. enterocolitica pGY100 and Y. enterocolitica pTM100 (vector control) were cultivated under conditions previously shown to induce the Ysa and Ysc TTSSs to secrete Ysp and Yop proteins, respectively. Culture supernatants were collected, and secreted proteins were examined by SDS-PAGE (Fig. 1B and C). When these strains were grown at 26°C in a medium containing 490 mM NaCl the Ysp proteins were secreted but the growth rate of the culture was slowed by the high salt concentrations (data not shown). This growth effect was eliminated when 290 mM NaCl was added to the medium without affecting the amount of Ysp produced (Fig. 1B). The growth of each strain at 37°C in a medium depleted of calcium resulted in the secretion of Yop proteins (Fig. 1C). In addition, the presence of pGY100 (Pcat-yplAB), but not of pTM100 (vector control), resulted in the secretion of YplA under conditions in which Yops or Ysps are secreted (Fig. 1B, lanes 1 and 2, and 1C, lanes 1 and 2). The identity of YplA was confirmed by Western blot analysis with anti-YplA polyclonal antibody (Fig. 1).
Secretion of YplA under conditions that result in Ysp or Yop production was not affected by a flhDC mutation that blocks production of the flagellar TTSS (Fig. 1B, lanes 3 and 4, and 1C, lanes 3 and 4) or other flagellar mutations including fleB, flgA, flhB, fliA, and motA (data not shown). Likewise, the flagellar defect did not have any impact on the production of Ysps or Yops (Fig. 1B and C). These results indicate that constitutive expression of yplA can lead to YplA secretion by a flagellum-independent mechanism. Flagellum-independent secretion of YplA is likely to require the Ysa and Ysc TTSSs because YplA was exported under conditions where these systems have been shown to function (17, 35). The data also show flagellar mutations do not affect the activity of the Ysa and Ysc TTSSs.
Flagellum-independent secretion of YplA at 26°C in a high-salt medium requires the Ysa type III secretion system.
YplA secretion by Y. enterocolitica pGY100 grown at 26°C in a medium containing high levels of sodium chloride (NaCl) suggested that the Ysa TTSS recognized this protein as a substrate. To test this hypothesis, a genetic screen was developed to identify mutants that were defective for YplA secretion under conditions that induce the Ysa TTSS but maintained the ability to secrete YplA when grown under conditions that induce the flagellar TTSS.
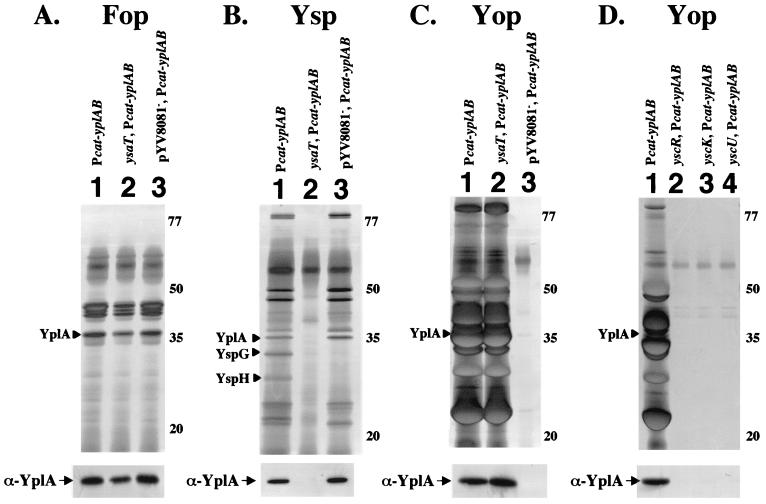
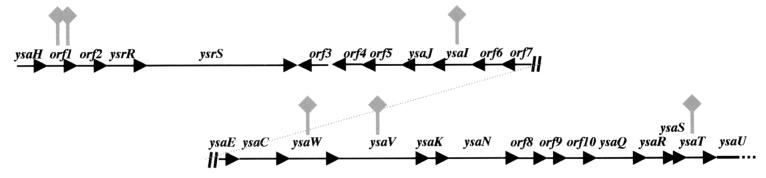
The genetic screen relied on the use of PLA medium, which has previously been shown to be useful for the study of YplA secretion by the flagellar TTSS (55). For this analysis the medium was modified by the addition of NaCl. Colonies of Y. enterocolitica exhibited phospholipase activity on PLA medium with low, but not high, NaCl concentrations (Table 2). In contrast, colonies of Y. enterocolitica pGY100 exhibited phospholipase activity at low and high concentrations of NaCl (Table 2). A flhDC mutation eliminated phospholipase activity by Y. enterocolitica and Y. enterocolitica pGY100 at low NaCl concentrations, but Y. enterocolitica pGY100 retained activity on PLA medium with a high NaCl concentration (Table 2), suggesting that YplA was secreted by the flagellar TTSS at low NaCl concentrations and by the Ysa TTSS at high NaCl concentrations. We hypothesized that mutations which block YplA activity by Y. enterocolitica pGY100 under high-salt conditions would map to the ysa locus. Therefore, Y. enterocolitica pGY100 was subjected to transposon mutagenesis and screened on high-salt PLA medium to identify phospholipase-negative mutants. From this analysis, six candidate mutants (GY4412, GY4413, GY4414, GY4415, GY4416, and GY4418) were identified and further characterized. Each of these mutants was negative for YplA activity on high-salt PLA medium but remained positive on low-salt PLA medium (Table 2). Analysis of protein secretion under Ysa TTSS-inducing conditions revealed that each mutant was defective for production of both Ysps and YplA. However, Fops, Yops and YplA were still secreted under conditions favorable for expression of the flagellar and Ysc TTSS (Fig. 2A and C; data not shown). To map the site of the transposon insertions, the transposon-chromosome junctions were cloned and the DNA sequence immediately adjacent to the transposon was determined. The results from this analysis revealed that each mutant had a transposon insertion located within a 22-kb region of the Y. enterocolitica chromosome known to contain a cluster of genes that encode the Ysa TTSS (Fig. 3 and Table 2). Two of the mutants had transposon insertions that mapped to different sites in orf1 (Fig. 3). The other mutants had transposon insertions that mapped to ysaI, ysaV, ysaW, and ysaT (Fig. 3). This indicated that YplA secretion by Y. enterocolitica grown at 26°C in a high-salt medium required a functional Ysa TTSS. The effect of the mutations was specific to the function of the Ysa TTSS since none of the mutants were affected for Fop or Yop secretion and YplA was secreted under conditions that induce the flagellar or Ysc secretion systems (Fig. 2 and data not shown). In addition, the mutants were motile, indicating these strains are not generally affected for flagellar functions (data not shown).
TABLE 2.
2. YplA phenotype of Y. enterocolitca mutants on PLA medium
| Strain | Relevant genotype | Plasmid | Presence (+) or absence (−) of phospholipase activitya in:
|
|
|---|---|---|---|---|
| Low salt | High salt | |||
| JB580v | Wild type | + | − | |
| GY731 | Vector control | pTM100 | + | − |
| GY728 | Pcat-yplAB | pGY100 | + | + |
| GY460 | ΔflhDC | − | − | |
| GY724 | ΔflhDC | pTM100 | − | − |
| GY722 | ΔflhDC, Pcat-yplAB | pGY100 | − | + |
| GY4412 | ysaI::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
| GY4413 | ysaW::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
| GY4414 | orf1-1::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
| GY4415 | ysaT::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
| GY4416 | orf1-2::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
| GY4418 | ysaV::mTnMod-RKm", Pcat-yplAB | pGY100 | + | − |
It has been shown that YplA export is required for activity to be detected on indicator medium (55). The low-salt medium contained 90 mM sodium chloride, and high-salt medium contained 290 mM sodium chloride. The phospholipase activity was scored as positive for strains that formed colonies surrounded by a zone of precipitation on PLA medium.
FIG. 2.
Analysis of YplA secretion by selected Ysa and Ysc TTSS mutants. Secreted proteins were isolated and analyzed as described for Fig. 1. Proteins separated by SDS-PAGE were visulized by staining with silver, and the identification of YplA was confirmed by Western blot analysis. The location of YplA is indicated on the left, and the approximate locations of molecular mass standards are indicated on the right of each panel. (A) Cultures were grown under conditions that induce Fop production. Lanes: 1, JB580v/pGY100 (wild type, Pcat-yplAB); 2, GY4415/pGY100 (ysaT::mTnMod-RKm", Pcat-yplAB); 3, GY4478/pGY100 (Pcat-yplAB, pYV8081−). (B) Cultures were grown under conditions that induce Ysp production. Lanes are as listed for panel A. (C) Cultures were grown under conditions that induce Yop production. Lanes are as listed for panel A. (D) Cultures were grown under conditions that induce Yop production. Lanes: 1, JB580v/pGY100 (wild type, Pcat-yplAB); 2, YVM356/pGY100 (yscR::mTn5Km2, Pcat-yplAB); 3, GY4555/pGY100 (yscK::mTnMod-lacZYA-RKm", Pcat-yplAB); 4, YVM351/pGY100 (yscU::mTn5Km2, Pcat-yplAB).
FIG. 3.
Schematic diagram of the ysa locus of Y. enterocolitica showing the locations of different transposon insertions isolated in this study. The organization of the ysa locus is based on the published DNA sequence (GenBank accession no. AF005744). The locations of the transposon insertions are indicated by the gray flags.
Secretion of YplA at 37°C and low calcium conditions requires the Ysc type III secretion system.
Secretion of YplA by Y. enterocolitica pGY100 grown at 37°C in calcium-limited medium correlated with Yop production. To determine whether the Ysc TTSS is required for YplA secretion under conditions that induce Yop secretion, Y. enterocolitica was cured of pYV8081, which encodes the Ysc TTSS, resulting in strain GY4478. This strain was then transformed with pGY100 and examined for protein secretion when grown at 37°C in a calcium-limited medium (Fig. 2C, lane 3). GY4478/pGY100 did not secrete YplA or Yops under these conditions. This suggested that export of YplA was due to the function of the Ysc TTSS. Loss of pYV8081 did not affect YplA secretion under conditions that induced the flagellar or Ysa TTSS (Fig. 2A, lane 3, and 2B, lane 3). Specific ysc mutants, with insertion mutations in yscR, yscU, and yscK, carrying pGY100 were also blocked for both Yop and YplA secretion, indicating that the observed effects are due to loss of Ysc TTSS function and not the general result of eliminating pYV8081 (Fig. 2D). The absence of pYV8081 did not appear to affect the production of Fops and there was no general effect on Ysp secretion, with two exceptions. Compared to the wild-type strain, the pYV8081-cured strains did not secrete two Ysps which migrated at apparent molecular masses of ca. 30 and 33 kDa. These proteins have been designated YspG and YspH (Fig. 2B, compare lanes 1 and 3). Production of YspG and YspH was not affected for specific ysc mutants (data not shown). This suggests that some Ysps are encoded by genes located on pYV8081. We are currently investigating this hypothesis.
Mutants defective for Ysa and Ysc TTSS function are not affected for Fop production.
Under standard culture conditions the amount of Fops produced by Y. enterocolitica is quite low, but high expression of the positive regulatory locus flhDC leads to increased Fop levels (55). It did not appear that Fop production was affected by mutations in ysa genes or by loss of pYV8081 (Fig. 2A). However, to further evaluate this possibility, we constructed mutant strains that were defective for both the Ysa and the Ysc TTSSs. The double mutants were constructed by curing strains GY4412, GY4413, GY4414, GY4415, GY4416, and GY4418 of pGY100 and pYV8081. The plasmid-cured strains which have an intact copy of yplA on the chromosome continued to produce wild-type levels of YplA under conditions that induced the flagellar TTSS (described below). In addition, the profile of Fops detected in culture supernatant was not affected when they were overproduced by expressing flhDC from a multicopy plasmid (data not shown). This indicated that flagellar TTSS export of Fop proteins does not require functional Ysc and Ysa TTSSs.
The contribution of the Ysc, Ysa, and flagellar type III secretion systems to extracellular protein secretion is affected by in vitro culture conditions.
Previous studies have described environmental conditions that result in protein export by the Ysc, Ysa, and flagellar TTSSs (17, 35, 55). YplA is already known to be exported by the flagellar TTSS. The results presented above indicated that if yplA is expressed, YplA can be exported by the Ysc and Ysa TTSSs (55). In a previous study, yplA expression was shown to be coordinated with expression of the flagellar genes and repressed under conditions known to favor Ysp and Yop secretion (46). This suggested that YplA is exported only by the flagellar TTSS. However, other conditions may exist that stimulate function of the Ysc, Ysa, or flagellar TTSS. This would provide the opportunity for YplA to be secreted by alternate pathways. Therefore, we decided to determine the effects of a variety of environmental factors on the contribution of the Ysc, Ysa, and flagellar TTSSs to extracellular protein production.
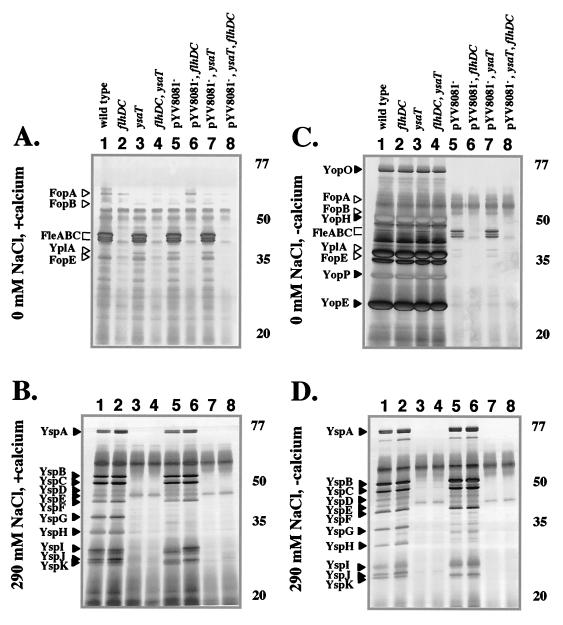
To accomplish this analysis, a set of strains was constructed that were defective for one, two, or all three known TTSSs (Table 1, strain construction described in Materials and Methods). These strains were grown under various environmental conditions, and the profile of proteins secreted into culture media was examined. The three environmental factors considered for this analysis were temperature, sodium chloride levels, and calcium availability because these three factors are known to affect the expression of one or more of the TTSSs. Initially, each strain was examined for protein secretion at 26°C in L medium that contained no or 290 mM sodium chloride (Fig. 4 and data not shown). Under these conditions, Fops were secreted only by strains that had a functional flagellar TTSS and only in the absence of sodium chloride (Fig. 4A, lanes 1, 3, 5, and 7). Mutants that were defective for Ysa and Ysc TTSS function were not affected for Fop secretion (Fig. 4A, lanes 3, 5, and 7). This result supports the conclusion of a previous study that indicated the Fops, including YplA, are exported by the flagellar TTSS (55). The data presented here also exclude the possibility that Fop production is a consequence of flagellar regulatory effects on the Y. enterocolitica contact-dependent TTSS because the profile of Fop proteins was not obviously affected in Ysa and Ysc TTSS mutants.
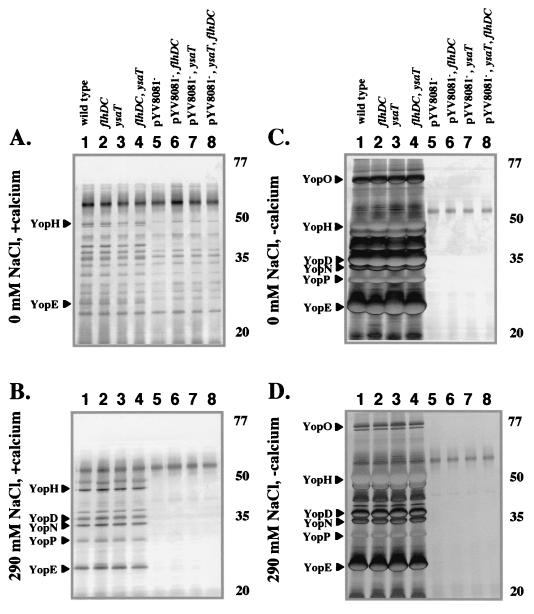
FIG. 4.
Analysis of secreted proteins by selected mutants grown at 26°C. Secreted proteins were isolated from cultures of strains with the indicated mutations grown under different conditions. (A to D) Cultures were grown in L medium containing different amounts of NaCl (+calcium) and medium that was limited for calcium (−calcium) by the addition of 20 mM sodium oxalate and 20 mM MgCl2 as indicated for each panel. The locations of some Fops (white arrowheads) and Ysps and Yops (black arrowheads) are assigned according to size on the left, and the approximate locations of the molecular mass standards (in kilodaltons) are indicated on the right. Lanes: 1, JB580v (wild type); 2, GY460 (ΔflhDC); 3, GY4499 (ysaT::mTnMod-RKm"); 4, GY4482 (ΔflhDC, ysaT::mTnMod-RKm"); 5, GY4478 (pYV8081−); 6, GY4479 (ΔflhDC, pYV8081−); 7, GY4481 (ysaT::mTnMod-RKm", pYV8081−); and 8, GY4492 (ΔflhDC, ysaT::mTnMod-RKm", pYV8081−). Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 1.0.
Secretion of Ysps was observed for strains that had a functional Ysa TTSS when cultured in a medium containing 290 mM sodium chloride (Fig. 4B, lanes 1, 2, 5, and 6). We found that increasing the salt concentration above 290 mM NaCl did not significantly increase the amount of Ysps detected (data not shown). This finding is consistent with the known requirement for high salt concentrations to induce Ysp production. Mutations that inactivated the flagellar TTSS had no affect on Ysp production (Fig. 4B, lanes 2 and 6). As noted above, the absence of pYV8081 resulted in the loss of proteins YspG and YspH, but Ysp production was not otherwise affected (Fig. 4B, lanes 5 and 6).
The contribution of each TTSS to extracellular protein production was then examined when the different TTSS secretion-defective mutants were cultured at 26°C in medium that was calcium limited (Fig. 4C and D). Interestingly, growth in calcium-limited L medium without added NaCl resulted in the production of the Fops and the Yops (Fig. 4C). Under these conditions the Ysa TTSS did not appear to contribute to protein secretion since the profile of proteins secreted into the culture medium was not affected by the ysaT mutation. The presence of the Fops was abolished by a mutation in the flagellar regulatory locus flhDC but was not affected by mutations that affected the Ysa or Ysc TTSS (Fig. 4C). Previously, significant production of the Yops had been observed only when yersiniae were cultured at 37°C. This analysis demonstrated that Yop secretion could also be stimulated at 26°C. The assignment of the secreted proteins as Yops was consistent with the fact that their production was eliminated in strains that were cured of pYV8081, which encodes both the Ysc TTSS and the Yops (Fig. 4C, lanes 5, 6, 7, and 8). In addition, inactivation of both the flagellar TTSS and the Ysa TTSS did not result in any decrease in Yop production (Fig. 4C, lane 4).
The addition of NaCl to calcium-limited culture media at 26°C affected the apparent activity of the three different TTSSs (Fig. 4D). Under these conditions, Fop and Yop production was significantly reduced or not observed at all. In contrast, these conditions resulted in Ysp secretion, and significant levels of proteins were detected in the culture supernatants of strains that retained a functional Ysa TTSS (Fig. 4D, lanes 1, 2, 5, and 6). Inactivation of the flagellar or Ysc TTSS did not inhibit Ysp secretion (Fig. 4D, lanes 2, 5, and 6). However, as described above, the loss of pYV8081 resulted in the loss of YspG and YspH.
When secreted proteins were analyzed from cultures grown at 37°C, the contributions of the three TTSSs were distinctly different (Fig. 5). The effect of the temperature decreased Fop and Ysp production to levels below detection regardless of the medium composition (Fig. 5). In contrast, the production of Yops occurred and was accentuated by calcium limitation (Fig. 5C and D). Under the conditions used in this study, Yop production at 37°C was at least twice that observed at 26°C because half as much sample was required to detect similar levels of protein (compare Fig. 4C to 5C and D). The production of Yops was not significantly affected by changes in the NaCl concentrations, required the Ysc TTSS, and was not influenced by mutations that inactivated the flagellar or Ysa TTSS. This result is consistent with previous studies that have indicated Yops are secreted at high levels at 37°C when calcium is sequestered in the culture medium. However, the data also show that significant levels of Yop secretion, along with Fop secretion, can also occur at 26°C when the culture medium has low levels of calcium and low levels of NaCl.
FIG. 5.
Analysis of secreted proteins by selected mutants grown at 37°C. Secreted proteins were isolated from cultures of strains with the indicated mutations grown under different conditions. (A to D) Cultures were grown in L medium containing different amounts of NaCl (+calcium) and medium limited for calcium (−calcium) by the addition of 20 mM sodium oxalate and 20 mM MgCl2 as indicated for each panel. The locations of some Yops (black arrowheads) are assigned according to size on the left, and the approximate locations of the molecular mass standards (in kilodaltons) are indicated on the right. Lanes: 1, JB580v (wild type); 2, GY460 (ΔflhDC); 3, GY4499 (ysaT::mTnMod-RKm"); 4, GY4482 (ΔflhDC, ysaT::mTnMod-RKm"); 5, GY4478 (pYV8081−); 6, GY4479 (ΔflhDC, pYV8081−); 7, GY4481 (ysaT::mTnMod-RKm", pYV8081−); and 8, GY4492 (ΔflhDC, ysaT::mTnMod-RKm", pYV8081−). Each lane contains the equivalent of 1 ml of culture supernatant at an OD600 of 0.5.
In addition, the observed secretion defects were not due to a particular combination of mutations since analysis of genotypically different Ysc, Ysa, and flagellar TTSS mutants had the same phenotypes (data not shown). Other strains that were examined carried mutations in flagellar genes (flhB or flgA) combined with a ysaV mutation with or without pYV8081. In addition, mutations in flhDC, ysaV, and yscU were combined to form single, double, and triple mutants (data not shown). We also examined TTSS mutants after growth in brain heart infusion medium to provide a comparison of the data generated in this study to other studies that have focused more specifically on Yop production by Yersinia species (data not shown). The results from this analysis also indicated that temperature, salt, and calcium limitation each affects the relative contribution of the three TTSSs to extracellular protein production.
As a control for the experiments described above, we examined the effect of culture conditions on expression of a single-copy yplA-lacZYA transcriptional fusion to determine whether the levels of yplA expression correlated with the observed levels of protein secretion. The data indicated levels of yplA expression correlated with the amount of Fops detected in culture supernatants (data not shown). This is consistent with a previous study on the expression of yplA (46).
DISCUSSION
Secretion of YplA by Y. enterocolitica under standard laboratory conditions requires the function of the flagellar TTSS, but it also appears that the Ysc and Ysa TTSSs can export this protein. In order to examine YplA export by the Ysc and Ysa TTSSs, it was necessary to express yplAB under conditions that had already been established to induce these flagellum-independent systems. Normally, yplA is expressed at 26°C and coordinated with the expression of other flagellar genes (46). By placing yplAB under the control of Pcat it was possible to investigate secretion of YplA under a variety of conditions independent of the constraints of flagellar gene regulation. This allowed us to confirm that YplA was secreted by Y. enterocolitica that had an intact flagellar TTSS (defined here as a hook-basal body structure). YplA was secreted by the wild-type strain and flagellar mutants that produced a hook-basal body complex. This is consistent with the results of an earlier study which utilized a Ptac-yplAB construct, indicating that the observed secretion phenotypes are not a consequence of the particular promoter construct used (55).
When secretion of constitutively expressed YplA was examined under conditions where the Ysa or Ysc TTSS functions, the flagellar TTSS was dispensable. These results revealed the possibility that the two different contact-dependent TTSSs had the capacity to recognize YplA, and mutant analysis provided the corroborating evidence. Transposon mutagenesis and subsequent characterization of mutants deficient for YplA secretion at 26°C under high-salt conditions revealed that these strains were also defective for Ysp secretion. Mapping of the mutations carried by these strains showed that the genetic defects occurred in the ysa locus known to encode the components of the Ysa TTSS (17). The effect of the transposon insertion mutations on the function of the Ysa TTSS was specific since these mutants were not affected for Yop and Fop production or YplA secretion under conditions that induced the Ysc and flagellar TTSSs.
To evaluate the role of the Ysc TTSS in YplA secretion by Y. enterocolitica pGY100, it was necessary to cure the strain of pYV8081. This plasmid contains all of the ysc genes encoding the Ysc secretion apparatus and the genes encoding the Yops (49). As expected, under conditions that normally induce Yop secretion, the loss of pYV8081 eliminated Yop production and YplA secretion. We also tested specific ysc mutants carrying pGY100 and showed that they are blocked for both Yop and YplA secretion, indicating that the observed effects are due to loss of Ysc TTSS function and not the general result of eliminating pYV8081. Inactivation of the Ysc TTSS did not affect Fop production or YplA secretion under conditions that induced the flagellar secretion pathway. YplA was also secreted by a pYV8081-cured derivative of Y. enterocolitica pGY100 under conditions that induced the Ysa TTSS. However, we observed that the loss of pYV8081 did affect the secretion of YspG and YspH but not the other Ysps. Since secretion of other Ysps was not affected and these strains retained the ability to secrete YplA in a Ysa TTSS-dependent fashion when pGY100 was present, we suggest that the genes encoding YspG and YspH are carried on pYV8081. An interesting possibility is that YspG and YspH are identical to two of the Yops. This particular hypothesis is a current focus of our investigations.
The fact that YplA can serve as a substrate of the Ysc, Ysa, or flagellar TTSS indicates that the contribution of these different secretion systems to virulence may be more dynamic than previously expected. For example, this raises the possibility that these systems may, under some conditions, share secretion substrates. To begin to test this possibility, we determined the contribution of the Ysc, Ysa, and flagellar TTSSs to extracellular protein production under a variety of environmental conditions. We found that previously documented conditions shown to induce protein secretion by one system represented conditions that excluded the function of the other two systems (17, 35). However, we also found that other conditions result in more than one TTSS contributing to the profile of proteins recovered from culture supernatants. It is quite notable that at 26°C, the growth of wild-type Y. enterocolitica in a low-salt, low-calcium medium resulted in Fop and Yop production. Previous studies have reported that Yop secretion is induced only at a high temperature (37°C). To our knowledge this is the first observation of significant levels of Yop production for cultures grown at a low temperature. This indicates that a number of environmental factors influence the stringency of temperature regulation on genes encoding the Ysc TTSS and its substrates. From the results of these experiments, we cannot conclude that Fops (including YplA) are transported by the Ysc TTSS or that Yops are transported by the flagellar TTSS during infection. However, this is a distinct possibility that merits further investigation.
Thus far, the flagellar and Ysa TTSSs have only been demonstrated to function at 26°C. This may appear to preclude any role for these systems in vivo, where temperatures are higher. However, a Ysa TTSS mutant has been shown to be less virulent for mice, indicating that the system is expressed in vivo (17). Similarly, a yplA mutant had reduced survival in mice, indicating that flagellum-dependent expression of yplA must occur during an infection (45). This apparent contradiction between the in vivo and in vitro data is not unprecedented. The synthesis of Yst enterotoxin by Y. enterocolitica and the synthesis of invasin by Y. enterocolitica and Y. pseudotuberculosis were originally shown to be important for virulence and occurred at low temperatures but not at high temperatures (3, 7, 21, 42). In each case, subsequent studies revealed that the in vitro production of each of these virulence factors at a high temperature required specific conditions (36, 41). Other virulence factors of Y. enterocolitica are also optimally expressed at low temperatures, including urease and O-antigen outer core (2, 9, 16, 48). A similar scenario has also been described for Vibrio cholerae, which produces much higher levels of cholera toxin in vitro at low temperatures (<30°C) than at a high temperature (37°C) (40, 47). However, there is no doubt that in vivo expression of the ctxAB operon occurs and is important (29). The lack of detection of Fop or Ysp proteins at 37°C in vitro could simply be due to culture conditions not completely mimicking in vivo signals.
It has been suggested that similarities among components that form the TTSS reflect conserved functional and structural features of the components that form the core of these protein secretion systems (4, 18, 30). In support of this idea, several studies focused on the functional properties of flagellum- and contact-dependent TTSSs have revealed common themes leading to the proposal of a unifying model for how substrates are recognized and transported (24). Further support for a unifying model comes from the direct examination of flagellum- and contact-dependent TTSS structures, which has revealed striking images of the protein targeting apparatus with highly conserved architectures (1, 5, 27, 28, 51). The assembly of flagellum- and contact-dependent TTSS structures appears to occur by a similar process (50). Therefore, it might not be surprising that YplA can be a substrate for the Ysc, Ysa, and flagellar TTSSs. Numerous studies have shown that a contact-dependent TTSS can share substrates derived from other bacteria (4, 12, 44) and two different contact-dependent TTSSs of S. enterica have been shown to share some substrates (32). We speculate that the sharing of substrates by different TTSSs of Y. enterocolitica may be important during the course of an infection, and this will be the focus of future studies.
Acknowledgments
We are grateful to Shane Petersen and Krista Venecia for performing some of the experiments described and for providing some strains used in this study. We thank Virginia Miller, Andrew Darwin, and Brian Ahmer for many valuable discussions, strains, and critical review of the manuscript.
This work was supported by University of California start up funds and an Academic Senate Faculty Research Award.
REFERENCES
- 1.Aizawa, S. 1996. Flagellar assembly in Salmonella typhimuium. Mol. Microbiol. 19:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hendy, A., P. Toivanen, and M. Skurnik. 1991. The effect of growth temperature on the biosynthesis of Yersinia enterocolitica O:3 lipopolysaccharide: temperature regulates the transcription of the rfb but not of the rfa region. Microb. Pathog. 10:81-86. [DOI] [PubMed] [Google Scholar]
- 3.Amirmozafari, N., and D. C. Robertson. 1993. Nutritional requirements for the synthesis of heat-stable enterotoxin by Yersinia enterocolitica. Appl. Evirn. Microbiol. 59:3314-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, D. M., D. E. Fouts, A. Collmer, and O. Schneewind. 1999. Reciprocal secretion of proteins by the bacterial type III machines of plant and animal pathogens suggests universal recognition of mRNA targeting signals. Proc. Natl. Acad. Sci. USA 96:12839-12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabaux, P. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 7.Boyce, J. M., E. J. Evans, Jr., D. G. Evans, and H. L. DuPont. 1979. Production of heat-stable, methanol-soluble enterotoxin by Yersinia enterocolitica. Infect. Immun. 25:532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwin, A. J., and V. L. Miller. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol. Microbiol. 32:51-62. [DOI] [PubMed] [Google Scholar]
- 9.de Konig-Ward, T. F., and R. M. Robins-Browne. 1997. A novel mechanism of urease regulation in Yersinia enterocolitica. FEMS Microbiol. Lett. 147:221-226. [DOI] [PubMed] [Google Scholar]
- 10.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: Modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauconnier, A., A. Allaoui, A. Campos, A. Van Elsen, G. Cornelis, and A. Bollen. 1997. Flagellar flhA, flhB, and flhE genes, organized in an operon, cluster upstream of the inv locus in Yersinia enterocolitica. Microbiology 143:3461-3471. [DOI] [PubMed] [Google Scholar]
- 12.Frithz-Lindsten, E., Y. Du, R. Rosqvist, and A. Forsberg. 1997. Intracellular targeting of exoenzyme S of Pseudomonas aeruginosa via type III-dependent translocation induces phagocytosis resistance, cytotoxicity and disruption of actin microfilaments. Mol. Microbiol. 25:1125-1139. [DOI] [PubMed] [Google Scholar]
- 13.Gemski, P., J. R. Lazere, and T. Casey. 1980. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect. Immun. 27:682-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gemski, P., J. R. Lazere, T. Casey, and J. A. Wohlieter. 1980. Presence of a virulence-associated plasmid in Y. pseudotuberculosis. Infect. Immun. 28:1044-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goguen, J. D., J. Yother, and S. C. Straley. 1984. Genetic analysis of the low calcium response in Yersinia pestis Mu d1(Ap lac) insertion mutants. J. Bacteriol. 160:842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gripenberg-Lerche, C., L. Zhang, P. Ahtonen, P. Toivanen, and M. Skurnik. 2000. Construction of urease-negative mutants of Yersinia enterocolitica serotype O:3 and O:8: role of urease in virulence and arthritogenicity. Infect. Immun. 68:942-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller, J. C., S. Carlson, K. J. Pederson, and D. E. Pierson. 2000. A chromosomally encoded type III secretion pathway in Yersinia enterocolitica is important in virulence. Mol. Microbiol. 36:1436-1446. [DOI] [PubMed] [Google Scholar]
- 18.Hueck, C. J. 1998. Type III secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iriarte, M., and G. R. Cornelis. 1999. The 70-kilobase virulence plasmid of yersiniae, p. 91-126. In J. B. Kaper and J. Hacker (ed.), Pathogenicity islands and other mobile genetic elements. American Society for Microbiology, Washington, D.C.
- 20.Iriarte, M., I. Stainier, A. V. Mikulskis, and G. R. Cornelis. 1995. The fliA gene encoding sigma 28 in Yersinia enterocolitica. J. Bacteriol. 177:2299-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isberg, R. R., A. Swain, and S. Falkow. 1988. Analysis of expression and thermoregulation of the Yersinia pseudotuberculosis inv gene with hybrid proteins. Infect. Immun. 56:2133-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapatral, V., and S. A. Minnich. 1995. Co-ordinate, temperature-sensitive regulation of the three Yersinia enterocolitica flagellin genes. Mol. Microbiol. 17:49-56. [DOI] [PubMed] [Google Scholar]
- 23.Kapatral, V., J. W. Olson, J. C. Pepe, V. L. Miller, and S. A. Minnich. 1996. Temperature-dependent regulation of Yersinia enterocolitica class III flagellar genes. Mol. Microbiol. 19:1061-1071. [DOI] [PubMed] [Google Scholar]
- 24.Karlinsey, J. E., J. Lonner, K. L. Brown, and K. T. Hughes. 2000. Translation/secretion coupling by type III secretion systems. Cell 102:487-497. [DOI] [PubMed] [Google Scholar]
- 25.Karlinsey, J. E., S. Tanaka, V. Bettenworth, S. Yamaguchi, W. Boos, S.-I. Aizawa, and K. T. Hughes. 2000. Completion of the hook-basal body complex of the Salmonella typhimurium flagellum is coupled to FlgM secretion and fliC expression. Mol. Microbiol. 37:1220-1231. [DOI] [PubMed] [Google Scholar]
- 26.Kinder, S. A., J. L. Badger, G. O. Bryant, J. C. Pepe, and V. L. Miller. 1993. Cloning of the YenI restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−+ mutant. Gene 136:271-275. [DOI] [PubMed] [Google Scholar]
- 27.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galán, and S.-I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 28.Kubori, T., M. Okumura, N. Kobayashi, D. Nakamura, M. Iwakura, and S.-I. Aizawa. 1997. Purification and characterization of the flagellar hook-basal body complex of Bacillus subtilis. Mol. Microbiol. 24:399-410. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. H., D. L. Hava, M. W. Waldor, and A. Camilli. 1999. Regulation and temporal expression of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 30.Macnab, R. M. 1999. The bacterial flagellum: reversible rotary propellor and type III export apparatus. J. Bacteriol. 181:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Miao, E., C. A. Scherer, R. M. Tsolis, R. A. Kingsley, L. G. Adams, A. J. Baumler, and S. I. Miller. 1999. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol. Microbiol. 34:850-864. [DOI] [PubMed] [Google Scholar]
- 33.Michiels, T., and G. R. Cornelis. 1991. Secretion of hybrid proteins by the Yersinia Yop export system. J. Bacteriol. 173:1677-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michiels, T., J.-C. Vanooteghem, C. Lambert de Rouvroit, B. China, A. Gustin, P. Boudry, and G. Cornelis. 1991. Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J. Bacteriol. 173:4994-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michiels, T., P. Wattiau, R. Brasseur, J.-M. Ruysschaert, and G. Cornelis. 1990. Secretion of Yop proteins by yersiniae. Infect. Immun. 58:2840-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikulskis, A. V., I. Delor, V. Ha Thi, and G. R. Cornelis. 1994. Regulation of the Yersinia enterocolitica enterotoxin yst gene. Influence of growth phase, temperature, osmolarity, pH and bacterial host factors. Mol. Microbiol. 14:905-915. [DOI] [PubMed] [Google Scholar]
- 37.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulaton of outer membrane proteins and virulence determinants in Vibrio cholera requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamino, T., and R. M. Macnab. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minamino, T., and R. M. Macnab. 2000. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052-1064. [DOI] [PubMed] [Google Scholar]
- 40.Parsot, C., and J. J. Mekalanos. 1990. Expression of ToxR, the transcriptional activator of the virulence factors in Vibrio cholerae, is modulated by the heat shock response. Proc. Natl. Acad. Sci. USA 87:9898-9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepe, J. C., J. L. Badger, and V. L. Miller. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol. Microbiol. 11:123-135. [DOI] [PubMed] [Google Scholar]
- 42.Pierson, D., and S. Falkow. 1990. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect. Immun. 58:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portnoy, D. A., S. L. Moseley, and S. Falkow. 1981. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect. Immun. 31:775-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosqvist, R., S. Hakansson, A. Forsberg, and H. Wolf-Watz. 1995. Functional conservation of the secretion and translocation machinery for virulence proteins of yersiniae, salmonellae, and shigellae. EMBO J. 14:4187-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmiel, D. H., E. Wagar, L. Karamanou, D. Weeks, and V. L. Miller. 1998. Phospholipase A of Yersinia enterocolitica contributes to pathogenesis in a mouse model. Infect. Immun. 66:3941-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmiel, D. S., G. M. Young, and V. L. Miller. 2000. The Yersinia enterocolitica phospholipase gene yplA is part of the flagellar regulon. J. Bacteriol. 182:2314-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher, D. A., and K. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skurnik, M., R. Venho, J. A. Bengoechea, and I. Moriyon. 1999. The lipopolysaccharide outer core of Yersinia enterocolitca serotype O:3 is required for virulence and plays a role in outer membrane integrity. Mol. Microbiol. 31:1443-1462. [DOI] [PubMed] [Google Scholar]
- 49.Snellings, N. J., M. Popek, and L. E. Lindler. 2001. Complete DNA sequence of Yersinia enterocolitica serotype O:8 low-calcium-response plasmid-associated replicon. Infect. Immun. 69:4627-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sukhan, A., T. Kubori, J. Wilson, and J. Galan. 2001. Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J. Bacteriol. 183:1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsubokura, M., K. Otsuki, I. Shimohira, and H. Yamamoto. 1979. Production of indirect hemolysin by Yersinia enterocolitica and its properties. Infect. Immun. 25:939-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young, G. M., J. L. Badger, and V. L. Miller. 2000. Motility is required to initiate host cell invasion by Yersinia enterocolitica. Infect. Immun. 68:4323-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young, G. M., and V. L. Miller. 1997. Identification of novel chromosomal loci affecting Yersinia enterocolitica pathogenesis. Mol. Microbiol. 25:319-328. [DOI] [PubMed] [Google Scholar]
- 55.Young, G. M., D. H. Schmiel, and V. L. Miller. 1999. A new pathway for the secretion of virulence factors by bacteria: the flagellar export apparatus functions as a protein-secretion system. Proc. Natl. Acad. Sci. USA 96:6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young, G. M., M. Smith, S. A. Minnich, and V. L. Miller. 1998. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J. Bacteriol. 181:2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]