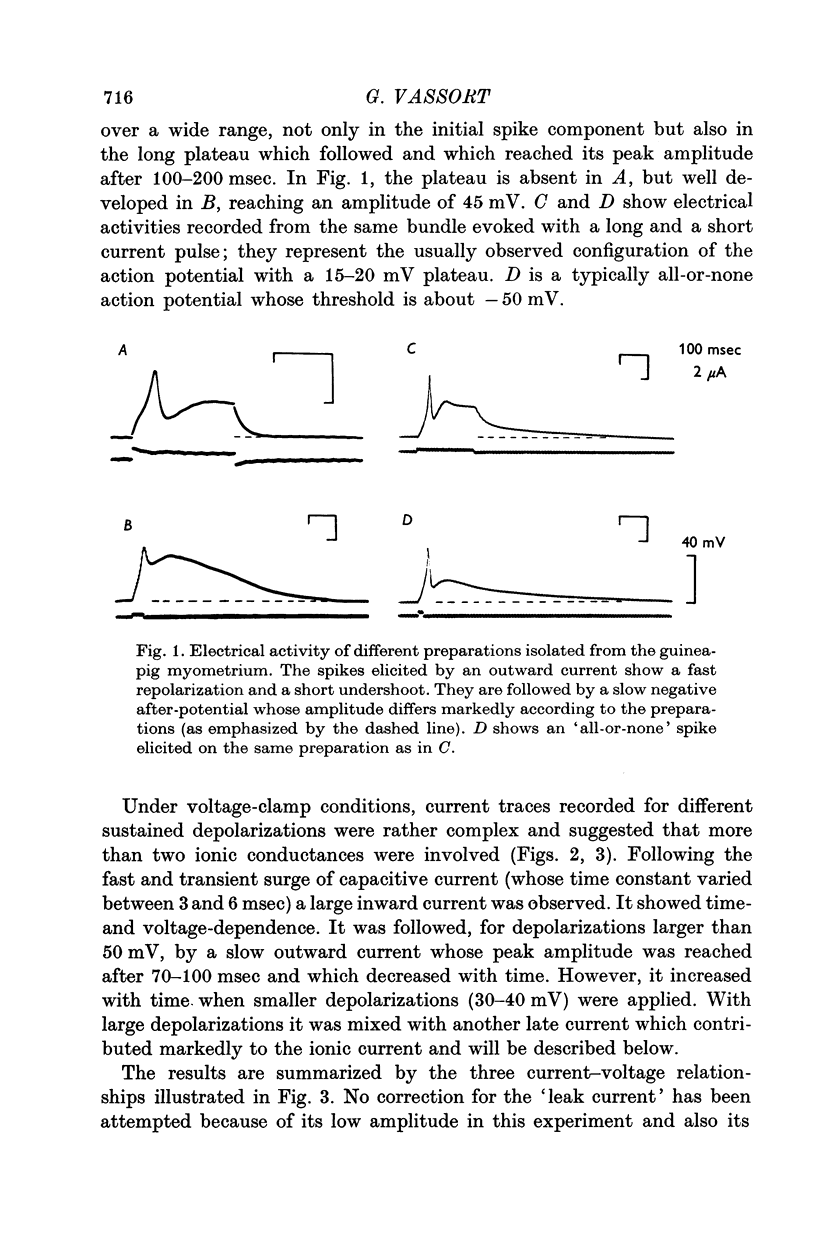
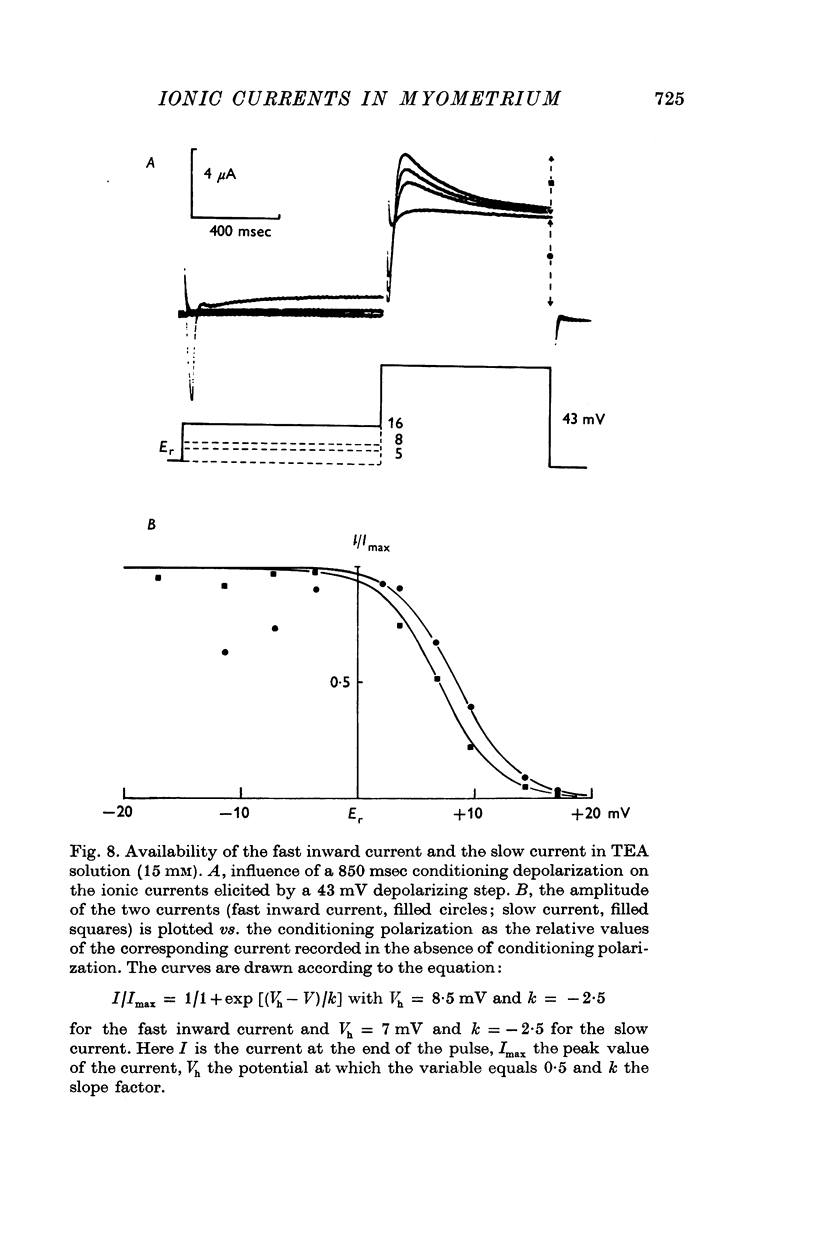
Abstract
Voltage-clamp analysis of ionic transmembrane currents in very small strands of guinea-pig myometrium was carried out with a double sucrose-gap technique. It was found that the electrical activity, consisting of a spike followed by a long plateau, is controlled by, at least, four ionic conductances. (1) A fast inward current is responsible for the spike generation. Its low equilibrium potential accounts, partly, for the low amplitude of the spike. (2) The fast inward current is antagonized by an early outward current which occurs almost simultaneously. This fast outward current is blocked by TEA. Its reversal potential is about -95 mV. A tenfold increase in the external K-concentration shifts the reversal potential by 50 mV. Thus, it is concluded that the initial outward current is carried by K+. (3) A slow current, whose reversal potential ranges from -40 to -10 mV, is responsible for the negative after-potential. Cl-depletion (to one-ninth) does not modify this current while Na-depletion (to one-ninth) decreases its reversal potential by about 20 mV. (4) A late current which shows delayed rectification is elicited by long pulses. Its analysis is made difficult by the change mainly of the K-equilibrium potential suggesting accumulation of K+ outside the cell membrane. (5) The availability of the inward current and of the slow current, determined in TEA solution, shows that both currents are half-inactivated by a 8 mV conditioning depolarization. Using a slope factor of -2-5 or -3 the availability curve fits the experimental values. In normal solution, the availability curve of the initial current appears complex in the hyperpolarization range. The fast outward current, which is partly inactivated at the resting potential, is restored by conditioning hyperpolarization and then antagonizes the Ca inward current more. (6) It is concluded that the fast K-current controls the spike generation and accounts for the fast repolarization of the spike. The fast and transient increase in K-conductance may be the result of a momentary local increase in Ca concentration at the internal surface of the membrane.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y. Effects of changing the ionic environment on passive and active membrane properties of pregnant rat uterus. J Physiol. 1971 Apr;214(1):173–190. doi: 10.1113/jphysiol.1971.sp009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. C., Ramon F., Snyder A. Studies on calcium and sodium in uterine smooth muscle excitation under current-clamp and voltage-clamp conditions. J Gen Physiol. 1971 Sep;58(3):322–339. doi: 10.1085/jgp.58.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSLER E. Conduction, automaticity, and tonus of visceral muscles. Experientia. 1948 Apr 15;4(6):213–218. doi: 10.1007/BF02155366. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. On the nature of the oscillations of the membrane potential (slow waves) produced by acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1971 Jul;216(2):403–418. doi: 10.1113/jphysiol.1971.sp009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Voltage-clamp of potential recorded intracellularly with microelectrodes in smooth muscle. J Physiol. 1975 Jan;244(1):25P–26P. [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. F., Noble S. J. Membrane currents underlying delayed rectification and pace-maker activity in frog atrial muscle. J Physiol. 1969 Oct;204(3):717–736. doi: 10.1113/jphysiol.1969.sp008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Casteels R., Kuriyama H. Membrane potential and ion content in cat and guinea-pig myometrium and the response to adrenaline and noradrenaline. Br J Pharmacol. 1968 Oct;34(2):388–407. doi: 10.1111/j.1476-5381.1968.tb07060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effect of calcium, barium and manganese on the action of adrenaline in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):121–136. doi: 10.1098/rspb.1969.0015. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN F. J., MOORE J. W., GOLDMAN D. E. Current-voltage relations in the lobster giant axon membrane under voltage clamp conditions. J Gen Physiol. 1962 Jul;45:1217–1238. doi: 10.1085/jgp.45.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Lieberman M. Heart: excitation and contraction. Annu Rev Physiol. 1971;33:479–532. doi: 10.1146/annurev.ph.33.030171.002403. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., McCullough J. R. Ionic currents in the uterine smooth muscle. J Physiol. 1975 Mar;246(1):1–36. doi: 10.1113/jphysiol.1975.sp010878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M., Horn L. Voltage clamping of smooth muscle from Taenia coli. Microvasc Res. 1970 Apr;2(2):188–201. doi: 10.1016/0026-2862(70)90007-5. [DOI] [PubMed] [Google Scholar]
- McGuigan J. A. Some limitations of the double sucrose gap, and its use in a study of the slow outward current in mammalian ventricular muscle. J Physiol. 1974 Aug;240(3):775–806. doi: 10.1113/jphysiol.1974.sp010634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Lenfant J. Analyse des réponses électriques de la fibre musculaire lisse d'utérus de ratte: mise en évidence d'un courant lent calcico-sodique. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 18;272(3):436–439. [PubMed] [Google Scholar]
- Mironneau J. Voltage clamp analysis of the ionic currents in uterine smooth muscle using the double sucrose gap method. Pflugers Arch. 1974;352(3):197–120. doi: 10.1007/BF00590485. [DOI] [PubMed] [Google Scholar]
- Mounier Y., Vassort G. Evidence for a transient potassium membrane current dependent on calcium influx in crab muscle fibre. J Physiol. 1975 Oct;251(3):609–625. doi: 10.1113/jphysiol.1975.sp011111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi H. Effects of changes of ionic environment on the negative after-potential of the spike in rat uterine muscle. J Physiol. 1970 Oct;210(3):785–797. doi: 10.1113/jphysiol.1970.sp009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Stämpfli R. Voltage clamp experiments on frog atrial heart muscle fibres with the sucrose gap technique. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;301(2):91–108. doi: 10.1007/BF00362729. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. A new method for measuring membrane potentials with external electrodes. Experientia. 1954 Dec 15;10(12):508–509. doi: 10.1007/BF02166189. [DOI] [PubMed] [Google Scholar]
- Standen N. B. Properties of a calcium channel in snail neurones. Nature. 1974 Jul 26;250(464):340–342. doi: 10.1038/250340a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]


