Abstract
Interactions between rod and cone signals in mudpuppy retinal neurones were investigated by intracellular recording. 2. The mudpuppy retina contains one kind of rod (lambda max = 525 nm) and one kind of cone (lambda max = 572 nm). The responses of receptors can be distinguished on the basis of their spectral sensitivities. 3. Rod and cone responses have different time courses of recovery and absolute sensitivities. Differences between receptor responses can be used to describe inputs to interneurones. 4. There are two spectral classes of horizontal cells: L-type and C-type. L-type cells are hyperpolarized by rods and cones in varying proportion, with some cells receiving little rod input. C-type cells are hyperpolarized by rods and depolarized by cones. 5. Bipolar cell receptive field centres receive input from cones or from rods and cones. There is no correlation between the spectral properties of centre responses and their polarity. 6. Antagonistic surrounds of bipolar cells show cone or rod and cone sensitivity. They are believed to be generated by the L-type horizontal cells. 7. Some bipolar cells exhibit chromatic interactions between cone signals in the centre and rod signals in the surround, which resemble those observed between the signals of different spectral classes of cones in species known to possess colour discrimination. 8. Amacrine and on-off ganglion cells have L-type responses showing both rod and cone sensitivity. 9. It is proposed that interactions between rod and cone signals observed in mudpuppy also exist in primate retina and are at least partially responsible for certain psychophysical observations of rod-cone interactions.
Full text
PDF





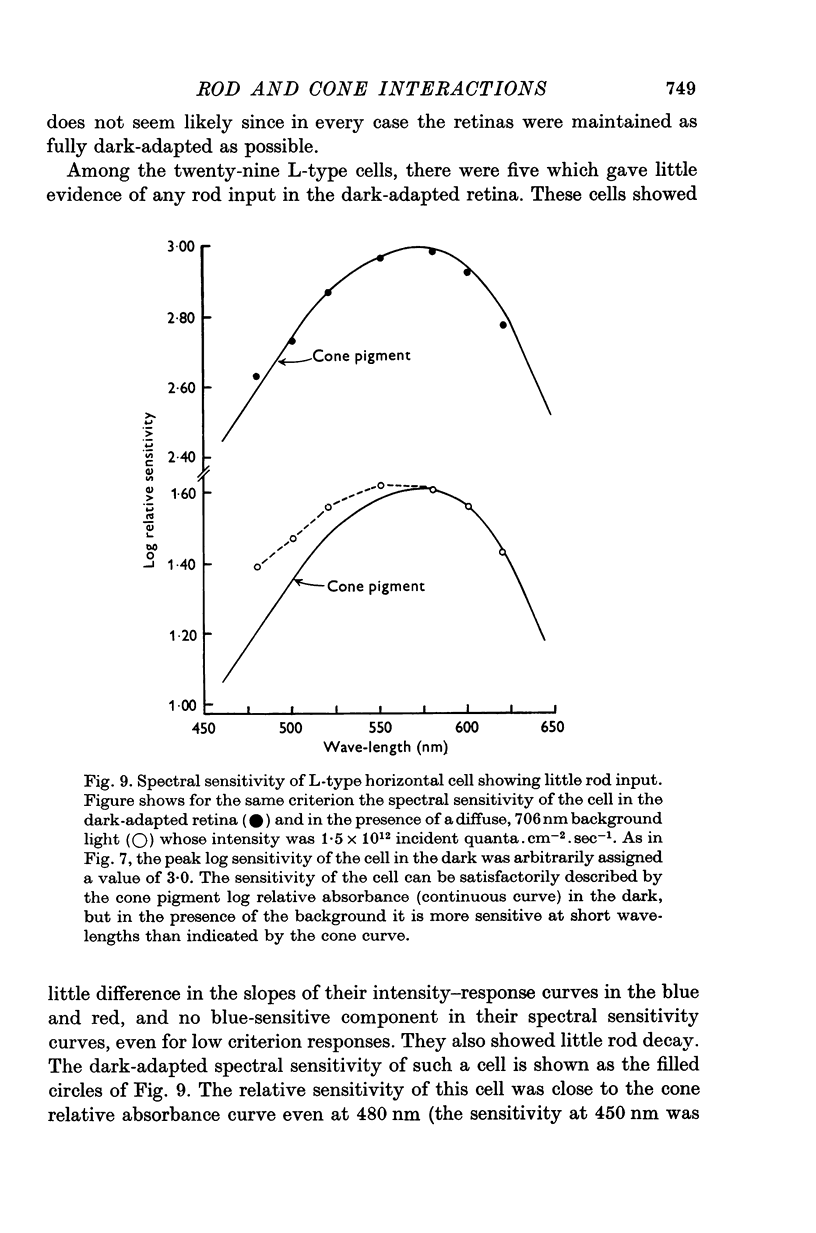

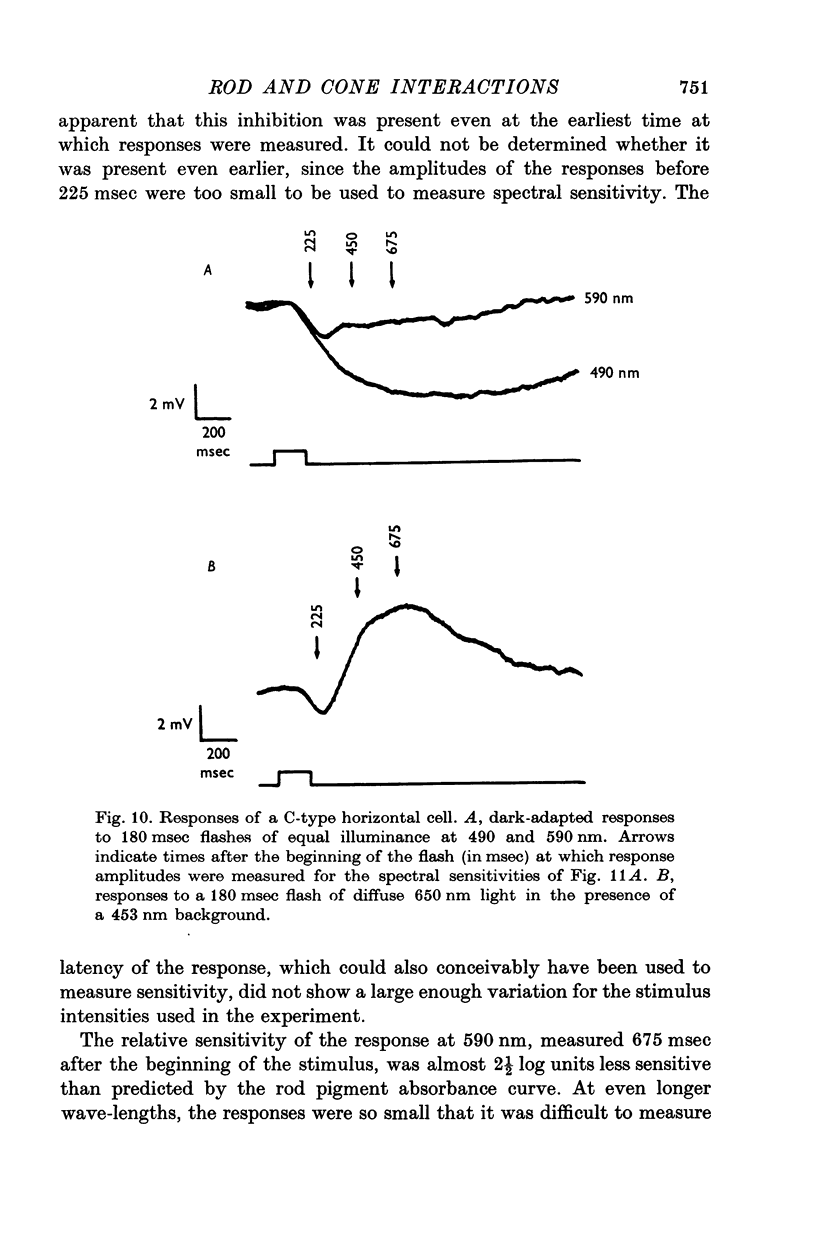
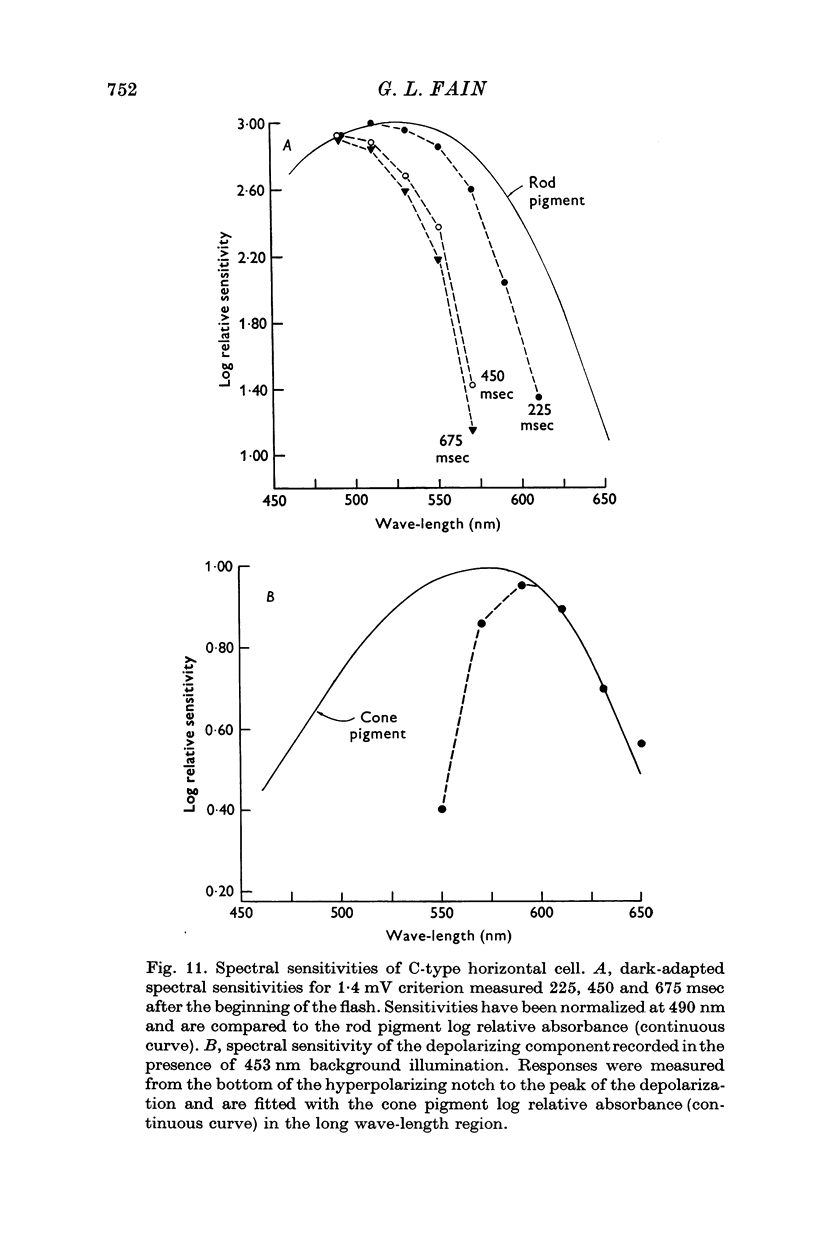

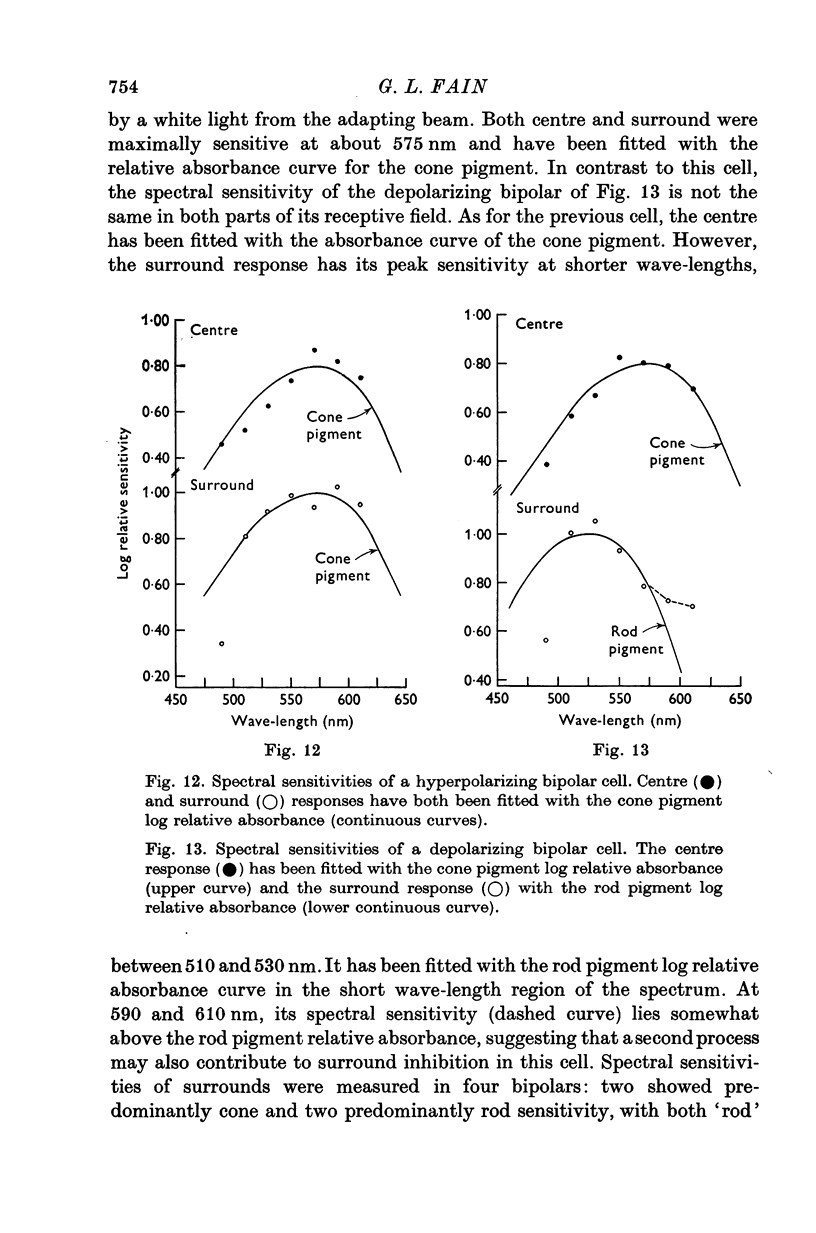









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPERN M. ROD-CONE INDEPENDENCE IN THE AFTER-FLASH EFFECT. J Physiol. 1965 Feb;176:462–472. doi: 10.1113/jphysiol.1965.sp007561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALPERN M., RUSHTON W. A. THE SPECIFICITY OF THE CONE INTERACTION IN THE AFTER-FLASH EFFECT. J Physiol. 1965 Feb;176:473–482. doi: 10.1113/jphysiol.1965.sp007562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN P. K., GIBBONS I. R., WALD G. THE VISUAL CELLS AND VISUAL PIGMENT OF THE MUDPUPPY, NECTURUS. J Cell Biol. 1963 Oct;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN P. K., WALD G. VISUAL PIGMENTS IN HUMAN AND MONKEY RETINAS. Nature. 1963 Oct 5;200:37–43. doi: 10.1038/200037a0. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp R. D. Cone mechanisms initiating response of on-off goldfish optic fibres. Nature. 1974 Jun 14;249(458):668–670. doi: 10.1038/249668a0. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The connections between bipolar cells and photoreceptors in the retina of the domestic cat. J Comp Neurol. 1973 Mar 1;148(1):91–114. doi: 10.1002/cne.901480106. [DOI] [PubMed] [Google Scholar]
- Boycott B. B., Kolb H. The horizontal cells of the rhesus monkey retina. J Comp Neurol. 1973 Mar 1;148(1):115–139. doi: 10.1002/cne.901480107. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Proximal negative response of frog retina. J Neurophysiol. 1970 May;33(3):405–420. doi: 10.1152/jn.1970.33.3.405. [DOI] [PubMed] [Google Scholar]
- CRESCITELLI F. The natural history of visual pigments. Ann N Y Acad Sci. 1959 Nov 12;74(2):230–255. doi: 10.1111/j.1749-6632.1958.tb39548.x. [DOI] [PubMed] [Google Scholar]
- Daw N. W. Colour-coded ganglion cells in the goldfish retina: extension of their receptive fields by means of new stimuli. J Physiol. 1968 Aug;197(3):567–592. doi: 10.1113/jphysiol.1968.sp008575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W. Neurophysiology of color vision. Physiol Rev. 1973 Jul;53(3):571–611. doi: 10.1152/physrev.1973.53.3.571. [DOI] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Fisher S. K., Boycott B. B. Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- Frumkes T. E., Sekuler M. D., Reiss E. H. Rod-cone interaction in human scotopic vision. Science. 1972 Feb 25;175(4024):913–914. doi: 10.1126/science.175.4024.913. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Simon E. J. Interactions leading to horizontal cell responses in the turtle retina. J Physiol. 1974 Jul;240(1):177–198. doi: 10.1113/jphysiol.1974.sp010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Link K. Rod and cone interaction in dark-adapted monkey ganglion cells. J Physiol. 1966 May;184(2):499–510. doi: 10.1113/jphysiol.1966.sp007928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollins M. Brightness contrast at low luminances. Vision Res. 1971 Dec;11(12):1459–1472. doi: 10.1016/0042-6989(71)90066-6. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Famiglietti E. V. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974 Oct 4;186(4158):47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Organization of the outer synaptic layer in the retina of the larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265(872):471–489. doi: 10.1098/rstb.1973.0033. [DOI] [PubMed] [Google Scholar]
- Laties A. M., Liebman P. A., Campbell C. E. Photoreceptor orientation in the primate eye. Nature. 1968 Apr 13;218(5137):172–173. doi: 10.1038/218172a0. [DOI] [PubMed] [Google Scholar]
- Makous W., Boothe R. Cones block signals from rods. Vision Res. 1974 Apr;14(4):285–294. doi: 10.1016/0042-6989(74)90078-9. [DOI] [PubMed] [Google Scholar]
- McCann J. J., Benton J. L. Interaction of the long-wave cones and the rods to produce color sensations. J Opt Soc Am. 1969 Jan;59(1):103–107. doi: 10.1364/josa.59.000103. [DOI] [PubMed] [Google Scholar]
- McKee S. P., Westheimer G. Specificity of cone mechanisms in lateral interaction. J Physiol. 1970 Jan;206(1):117–128. doi: 10.1113/jphysiol.1970.sp009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. J Neurophysiol. 1968 Mar;31(2):268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dowling J. E. Intracellular responses of the Müller (glial) cells of mudpuppy retina: their relation to b-wave of the electroretinogram. J Neurophysiol. 1970 May;33(3):323–341. doi: 10.1152/jn.1970.33.3.323. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Hashimoto Y., Saito T., Tomita T. Physiological and morphological identification of L- and C-type S-potentials in the turtle retina. Vision Res. 1973 Feb;13(2):443–447. doi: 10.1016/0042-6989(73)90121-1. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A comparison of electrical properties of neurons in Necturus retina. J Neurophysiol. 1973 May;36(3):519–535. doi: 10.1152/jn.1973.36.3.519. [DOI] [PubMed] [Google Scholar]
- Niemeyer G., Gouras P. Rod and cone signals in S-potentials of the isolated perfused cat eye. Vision Res. 1973 Aug;13(8):1603–1612. doi: 10.1016/0042-6989(73)90017-5. [DOI] [PubMed] [Google Scholar]
- Ogden T. E. The morphology of retinal neurons of the owl monkey Aotes. J Comp Neurol. 1974 Feb 15;153(4):399–428. doi: 10.1002/cne.901530405. [DOI] [PubMed] [Google Scholar]
- Proenza L. M., Burkhardt D. A. Proximal negative response and retinal sensitivity in the mudpuppy, Necturus maculosus. J Neurophysiol. 1973 May;36(3):502–518. doi: 10.1152/jn.1973.36.3.502. [DOI] [PubMed] [Google Scholar]
- SVAETICHIN G. Spectral response curves from single cones. Acta Physiol Scand Suppl. 1956;39(134):17–46. [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Rod and cone contributions to S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1319–1329. doi: 10.1016/0042-6989(69)90069-8. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H. Rod-cone interaction in S-potentials from the cat retina. Vision Res. 1969 Nov;9(11):1331–1344. doi: 10.1016/0042-6989(69)90070-4. [DOI] [PubMed] [Google Scholar]
- Stell W. K. The structure and relationships of horizontal cells and photoreceptor-bipolar synaptic complexes in goldfish retina. Am J Anat. 1967 Sep;121(2):401–423. doi: 10.1002/aja.1001210213. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner H. G., Macnichol E. F., Wolbarsht M. L. The Response Properties of Single Ganglion Cells in the Goldfish Retina. J Gen Physiol. 1960 Jul 1;43(6):45–62. doi: 10.1085/jgp.43.6.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Adaptation in a vertebrate retina: intracellular recording in Necturus. J Neurophysiol. 1971 Mar;34(2):228–241. doi: 10.1152/jn.1971.34.2.228. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1970 May;33(3):342–350. doi: 10.1152/jn.1970.33.3.342. [DOI] [PubMed] [Google Scholar]
- Westheimer G. Rod-cone independence for sensitizing interaction in the human retina. J Physiol. 1970 Jan;206(1):109–116. doi: 10.1113/jphysiol.1970.sp009000. [DOI] [PMC free article] [PubMed] [Google Scholar]


